Abstract
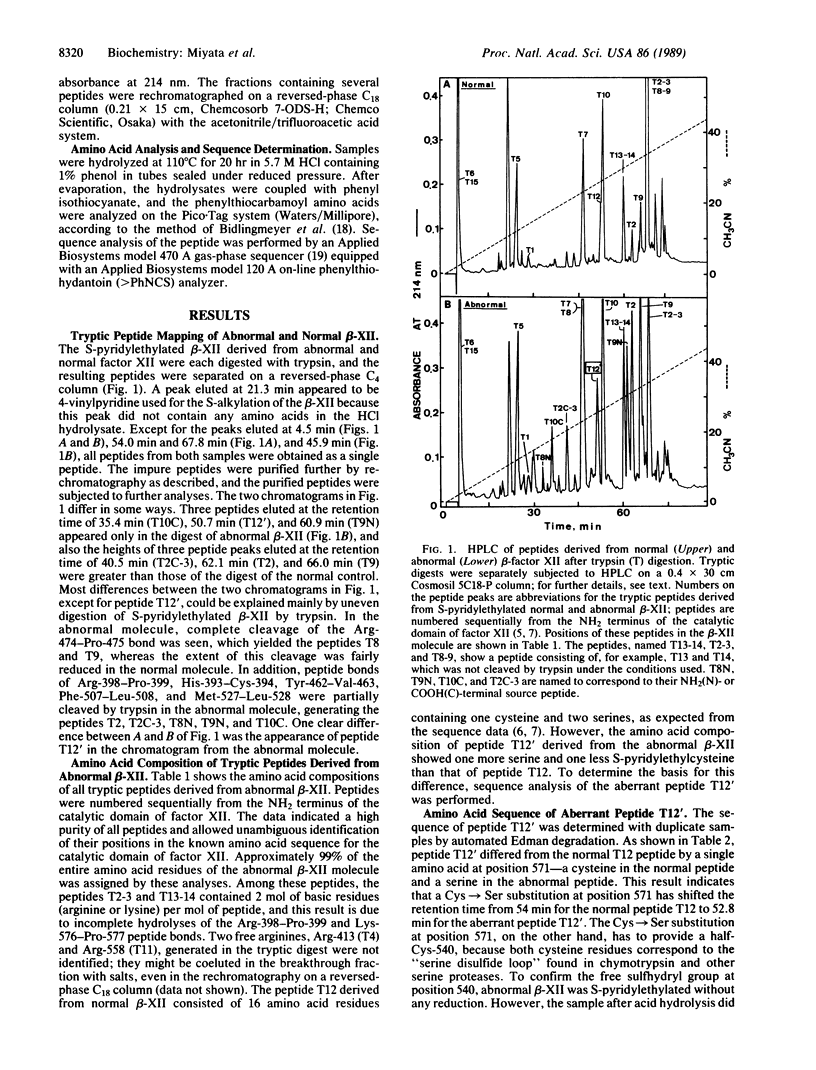
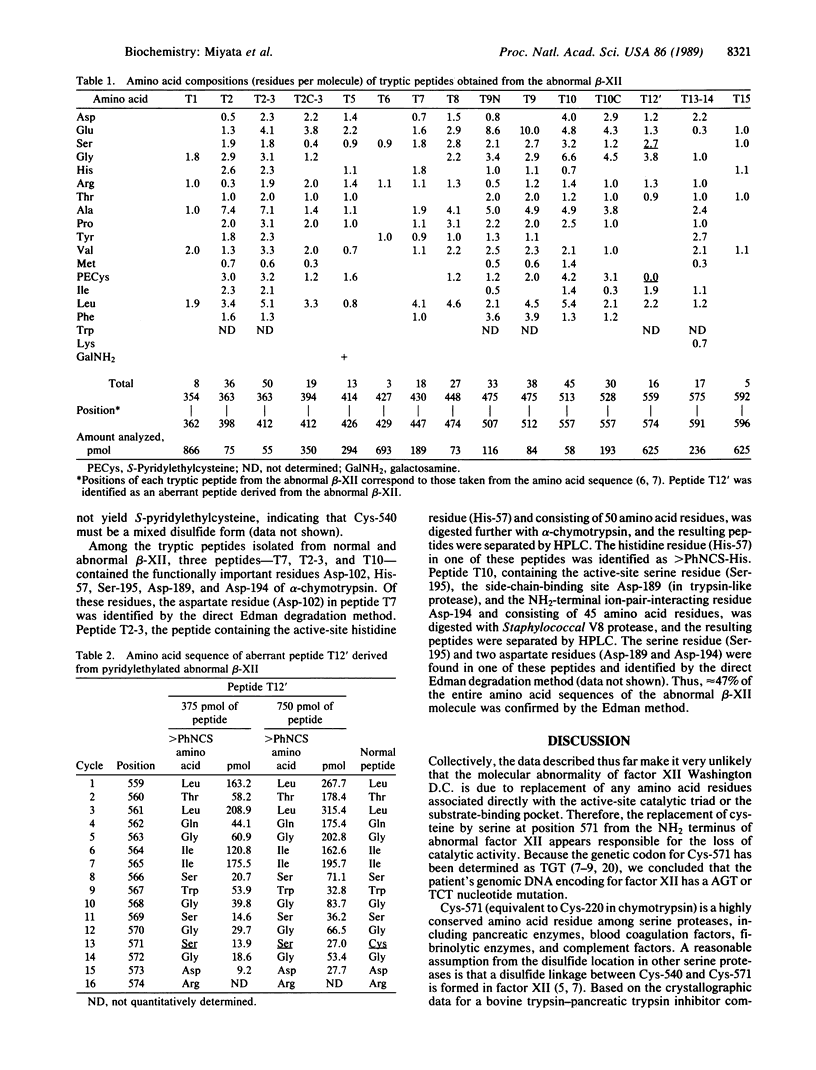
Structural studies on a congenital abnormal coagulation factor XII (Hageman factor), factor XII Washington D.C., have been performed to identify the defect responsible for its lack of procoagulant activity. Amino acid sequence analysis of a tryptic peptide isolated from the abnormal factor XII indicated that Cys-571 (equivalent to Cys-220 in the chymotrypsin numbering system) had been replaced by serine. No other substitutions in the active-site triad--namely, His-393, Asp-442, and Ser-544--were found. We propose that the Cys-571----Ser replacement found in this factor XII variant destroys the formation of the disulfide linkage between Cys-540 and Cys-571, giving rise to an altered conformation of the active-site serine residue or the secondary substrate-binding site and, thus, leads to the loss of enzyme activity.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bidlingmeyer B. A., Cohen S. A., Tarvin T. L. Rapid analysis of amino acids using pre-column derivatization. J Chromatogr. 1984 Dec 7;336(1):93–104. doi: 10.1016/s0378-4347(00)85133-6. [DOI] [PubMed] [Google Scholar]
- Cochrane C. G., Griffin J. H. The biochemistry and pathophysiology of the contact system of plasma. Adv Immunol. 1982;33:241–306. doi: 10.1016/s0065-2776(08)60837-8. [DOI] [PubMed] [Google Scholar]
- Colman R. W. Surface-mediated defense reactions. The plasma contact activation system. J Clin Invest. 1984 May;73(5):1249–1253. doi: 10.1172/JCI111326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cool D. E., Edgell C. J., Louie G. V., Zoller M. J., Brayer G. D., MacGillivray R. T. Characterization of human blood coagulation factor XII cDNA. Prediction of the primary structure of factor XII and the tertiary structure of beta-factor XIIa. J Biol Chem. 1985 Nov 5;260(25):13666–13676. [PubMed] [Google Scholar]
- Cool D. E., MacGillivray R. T. Characterization of the human blood coagulation factor XII gene. Intron/exon gene organization and analysis of the 5'-flanking region. J Biol Chem. 1987 Oct 5;262(28):13662–13673. [PubMed] [Google Scholar]
- Fujikawa K., Heimark R. L., Kurachi K., Davie E. W. Activation of bovine factor XII (Hageman factor) by plasma kallikrein. Biochemistry. 1980 Apr 1;19(7):1322–1330. doi: 10.1021/bi00548a010. [DOI] [PubMed] [Google Scholar]
- Fujikawa K., McMullen B. A. Amino acid sequence of human beta-factor XIIa. J Biol Chem. 1983 Sep 25;258(18):10924–10933. [PubMed] [Google Scholar]
- Hermodson M. A., Ericsson L. H., Neurath H., Walsh K. A. Determination of the amino acid sequence of porcine trypsin by sequenator aalysis. Biochemistry. 1973 Aug 14;12(17):3146–3153. doi: 10.1021/bi00741a002. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Huber R., Kukla D., Bode W., Schwager P., Bartels K., Deisenhofer J., Steigemann W. Structure of the complex formed by bovine trypsin and bovine pancreatic trypsin inhibitor. II. Crystallographic refinement at 1.9 A resolution. J Mol Biol. 1974 Oct 15;89(1):73–101. doi: 10.1016/0022-2836(74)90163-6. [DOI] [PubMed] [Google Scholar]
- Kirby E. P., McDevitt P. J. The binding of bovine factor XII to kaolin. Blood. 1983 Apr;61(4):652–659. [PubMed] [Google Scholar]
- McMullen B. A., Fujikawa K. Amino acid sequence of the heavy chain of human alpha-factor XIIa (activated Hageman factor). J Biol Chem. 1985 May 10;260(9):5328–5341. [PubMed] [Google Scholar]
- Que B. G., Davie E. W. Characterization of a cDNA coding for human factor XII (Hageman factor). Biochemistry. 1986 Apr 8;25(7):1525–1528. doi: 10.1021/bi00355a009. [DOI] [PubMed] [Google Scholar]
- RATNOFF O. D., COLOPY J. E. A familial hemorrhagic trait associated with a deficiency of a clot-promoting fraction of plasma. J Clin Invest. 1955 Apr;34(4):602–613. doi: 10.1172/JCI103109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratnoff O. D., Saito H. Surface-mediated reactions. Curr Top Hematol. 1979;2:1–57. [PubMed] [Google Scholar]
- Saito H., Scialla S. J. Isolation and properties of an abnormal Hageman factor (Factor XII) molecule in a cross-reacting material-positive hageman trait plasma. J Clin Invest. 1981 Oct;68(4):1028–1035. doi: 10.1172/JCI110325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., Scott J. G., Movat H. Z., Scialla S. J. Molecular heterogeneity of Hageman trait (factor XII deficiency): evidence that two of 49 subjects are cross-reacting material positive (CRM+). J Lab Clin Med. 1979 Aug;94(2):256–265. [PubMed] [Google Scholar]
- Segal D. M., Powers J. C., Cohen G. H., Davies D. R., Wilcox P. E. Substrate binding site in bovine chymotrypsin A-gamma. A crystallographic study using peptide chloromethyl ketones as site-specific inhibitors. Biochemistry. 1971 Sep 28;10(20):3728–3738. doi: 10.1021/bi00796a014. [DOI] [PubMed] [Google Scholar]
- Steitz T. A., Henderson R., Blow D. M. Structure of crystalline alpha-chymotrypsin. 3. Crystallographic studies of substrates and inhibitors bound to the active site of alpha-chymotrypsin. J Mol Biol. 1969 Dec 14;46(2):337–348. doi: 10.1016/0022-2836(69)90426-4. [DOI] [PubMed] [Google Scholar]
- Takahashi I., Saito H. A rapid purification with high recovery of factor XII (Hageman factor) on immunoaffinity column: application to an abnormal clotting factor XII (factor XIITORONTO). J Biochem. 1988 Apr;103(4):641–643. doi: 10.1093/oxfordjournals.jbchem.a122321. [DOI] [PubMed] [Google Scholar]
- Tans G., Griffin J. H. Properties of sulfatides in factor-XII-dependent contact activation. Blood. 1982 Jan;59(1):69–75. [PubMed] [Google Scholar]
- Tripodi M., Citarella F., Guida S., Galeffi P., Fantoni A., Cortese R. cDNA sequence coding for human coagulation factor XII (Hageman). Nucleic Acids Res. 1986 Apr 11;14(7):3146–3146. doi: 10.1093/nar/14.7.3146. [DOI] [PMC free article] [PubMed] [Google Scholar]


