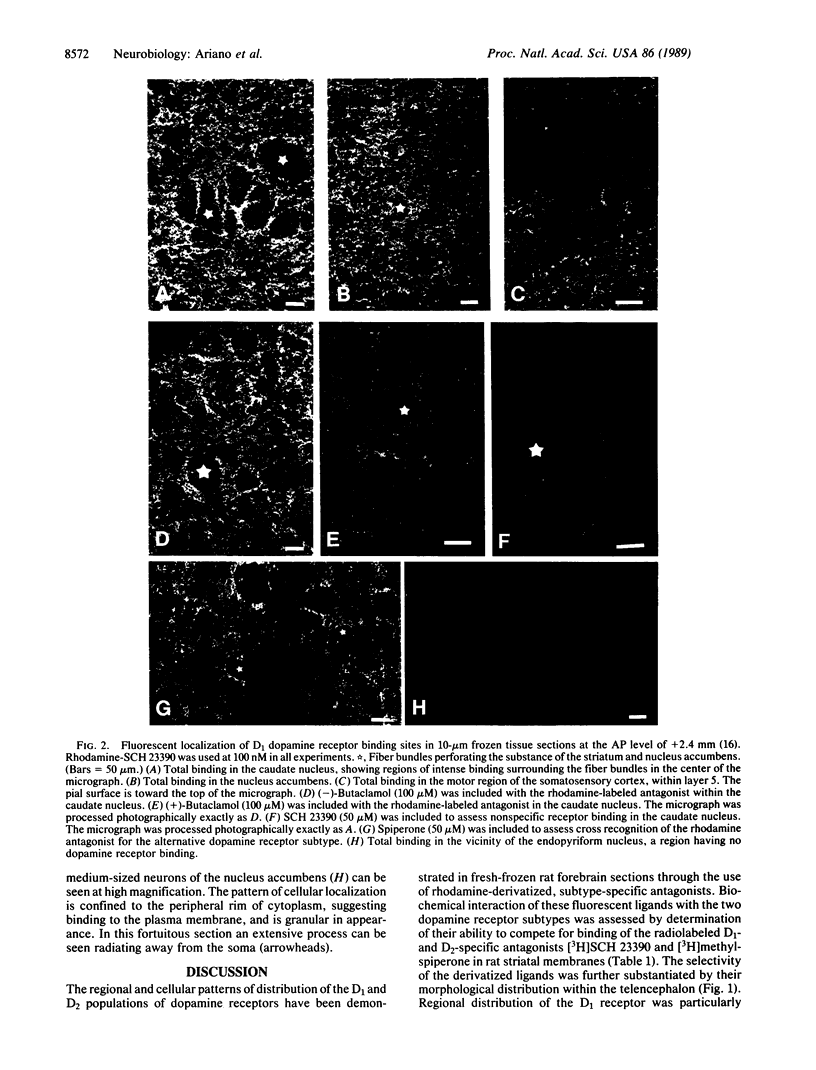
Abstract
The regional and cellular localization of the two subtypes of dopamine receptors, D1 and D2, have been ascertained in rat forebrain by use of fluorescent dopaminergic antagonist ligands. (R,S)-5-(4'-aminophenyl)-8-chloro-2,3,4,5-tetrahydro-3- methyl-[1H]-3-benzazepin-7-ol, the 4'-amino derivative of the high-affinity D1-specific antagonist SCH 23390, and the D2 selective antagonist N-(p-aminophenethyl)spiperone were chemically derivatized using the fluorescent compound tetramethylrhodamine. The modification of these antagonist ligands has allowed the specific, cellular resolution of the D1 and D2 receptor binding sites in intact, highly organized regions of forebrain slices in a very rapid experimental time frame. The regional localization of receptors labeled by the fluorescent probes is in agreement with previous receptor autoradiography studies. Moreover, the specific cellular binding patterns for both receptors can now be compared and contrasted to one another in the same tissue by using these fluorescent ligands. D1 receptor sites are most evident within the striatum and exhibit regions of intense "patch" fluorescence corresponding to receptor reactivity in cells and their processes. The distribution of D1 receptor binding is highly analogous to the pattern of dopamine terminal histofluorescence in the caudate nucleus. D2 receptor sites are less prevalent overall and may be localized to a subpopulation of the D1 fluorescent neurons in the caudate nucleus and nucleus accumbens regions.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altar C. A., Marien M. R. Picomolar affinity of 125I-SCH 23982 for D1 receptors in brain demonstrated with digital subtraction autoradiography. J Neurosci. 1987 Jan;7(1):213–222. doi: 10.1523/JNEUROSCI.07-01-00213.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ariano M. A. Comparison of dopamine binding sites in the rat superior cervical ganglion and caudate nucleus. Brain Res. 1987 Sep 22;421(1-2):245–254. doi: 10.1016/0006-8993(87)91294-7. [DOI] [PubMed] [Google Scholar]
- Ariano M. A., Kenny S. L. Neurotransmitter receptor autoradiography in immunohistochemically identified neurons. J Neurosci Methods. 1985 Oct;15(1):49–61. doi: 10.1016/0165-0270(85)90061-5. [DOI] [PubMed] [Google Scholar]
- Ariano M. A. Striatal D1 dopamine receptor distribution following chemical lesion of the nigrostriatal pathway. Brain Res. 1988 Mar 8;443(1-2):204–214. doi: 10.1016/0006-8993(88)91614-9. [DOI] [PubMed] [Google Scholar]
- Ariano M. A., Ufkes S. K. Cyclic nucleotide distribution within rat striatonigral neurons. Neuroscience. 1983 May;9(1):23–29. doi: 10.1016/0306-4522(83)90043-x. [DOI] [PubMed] [Google Scholar]
- Boyson S. J., McGonigle P., Molinoff P. B. Quantitative autoradiographic localization of the D1 and D2 subtypes of dopamine receptors in rat brain. J Neurosci. 1986 Nov;6(11):3177–3188. doi: 10.1523/JNEUROSCI.06-11-03177.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creese I., Sibley D. R., Hamblin M. W., Leff S. E. The classification of dopamine receptors: relationship to radioligand binding. Annu Rev Neurosci. 1983;6:43–71. doi: 10.1146/annurev.ne.06.030183.000355. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Barone P., Sidhu A., Wamsley J. K., Chase T. N. Quantitative autoradiographic localization of D-1 dopamine receptors in the rat brain: use of the iodinated ligand [125I]SCH 23982. Neurosci Lett. 1986 Aug 4;68(3):261–266. doi: 10.1016/0304-3940(86)90499-4. [DOI] [PubMed] [Google Scholar]
- Dimova R., Vuillet J., Seite R. Study of the rat neostriatum using a combined Golgi-electron microscope technique and serial sections. Neuroscience. 1980;5(9):1581–1596. doi: 10.1016/0306-4522(80)90022-6. [DOI] [PubMed] [Google Scholar]
- Dubois A., Savasta M., Curet O., Scatton B. Autoradiographic distribution of the D1 agonist [3H]SKF 38393, in the rat brain and spinal cord. Comparison with the distribution of D2 dopamine receptors. Neuroscience. 1986 Sep;19(1):125–137. doi: 10.1016/0306-4522(86)90010-2. [DOI] [PubMed] [Google Scholar]
- Enjalbert A., Sladeczek F., Guillon G., Bertrand P., Shu C., Epelbaum J., Garcia-Sainz A., Jard S., Lombard C., Kordon C. Angiotensin II and dopamine modulate both cAMP and inositol phosphate productions in anterior pituitary cells. Involvement in prolactin secretion. J Biol Chem. 1986 Mar 25;261(9):4071–4075. [PubMed] [Google Scholar]
- Gehlert D. R., Dawson T. M., Filloux F. M., Sanna E., Hanbauer I., Wamsley J. K. Evidence that [3H]forskolin binding in the substantia nigra is intrinsic to a striatal-nigral projection: an autoradiographic study of rat brain. Neurosci Lett. 1987 Jan 14;73(2):114–118. doi: 10.1016/0304-3940(87)90003-6. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R., Herkenham M., Thibault J. The neostriatal mosaic: II. Patch- and matrix-directed mesostriatal dopaminergic and non-dopaminergic systems. J Neurosci. 1987 Dec;7(12):3915–3934. doi: 10.1523/JNEUROSCI.07-12-03915.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic. I. Compartmental organization of projections from the striatum to the substantia nigra in the rat. J Comp Neurol. 1985 Jun 22;236(4):454–476. doi: 10.1002/cne.902360404. [DOI] [PubMed] [Google Scholar]
- Gerfen C. R. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984 Oct 4;311(5985):461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- Joyce J. N., Marshall J. F. Striatal topography of D-2 receptors correlates with indexes of cholinergic neuron localization. Neurosci Lett. 1985 Jan 7;53(1):127–131. doi: 10.1016/0304-3940(85)90108-9. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Lacey M. G., Mercuri N. B., North R. A. Dopamine acts on D2 receptors to increase potassium conductance in neurones of the rat substantia nigra zona compacta. J Physiol. 1987 Nov;392:397–416. doi: 10.1113/jphysiol.1987.sp016787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monsma F. J., Jr, Barton A. C., Kang H. C., Brassard D. L., Haugland R. P., Sibley D. R. Characterization of novel fluorescent ligands with high affinity for D1 and D2 dopaminergic receptors. J Neurochem. 1989 May;52(5):1641–1644. doi: 10.1111/j.1471-4159.1989.tb09220.x. [DOI] [PubMed] [Google Scholar]
- Spano P. F., Trabucchi M., Di Chiara G. Localization of nigral dopamine-sensitive adenylate cyclase on neurons originating from the corpus striatum. Science. 1977 Jun 17;196(4296):1343–1345. doi: 10.1126/science.17159. [DOI] [PubMed] [Google Scholar]




