Abstract
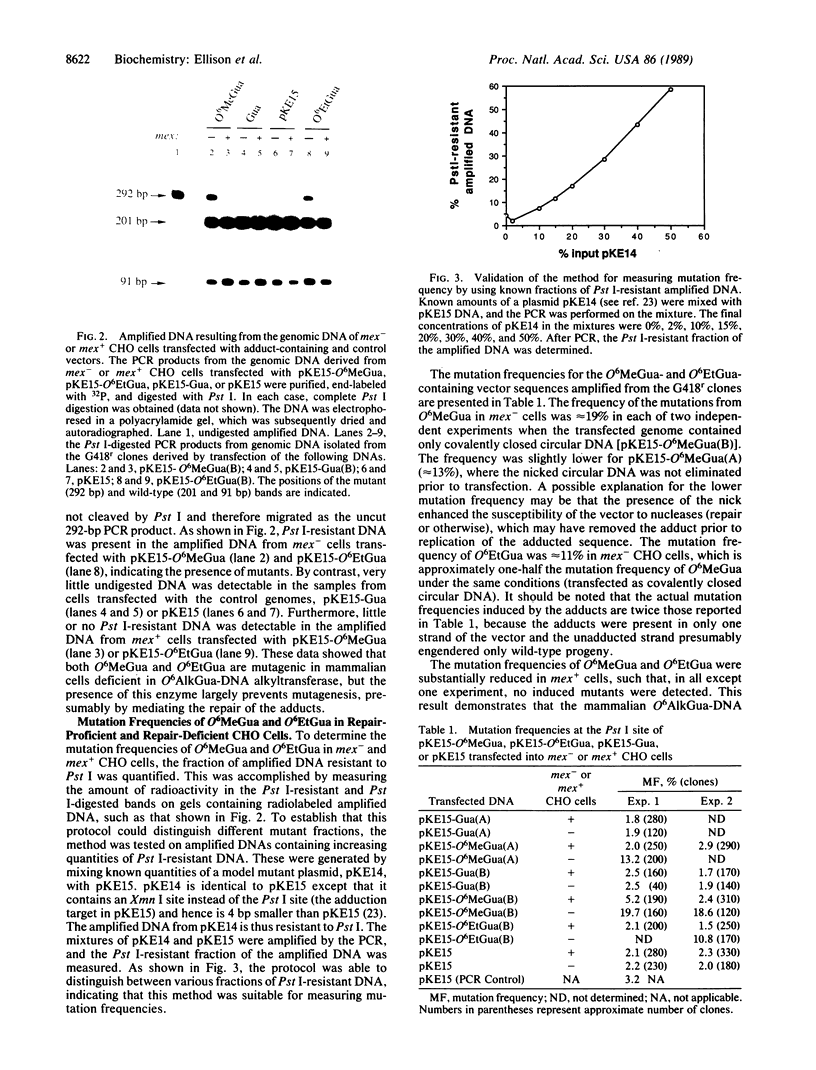
A plasmid was constructed in which a single guanine residue was replaced with either O6-methylguanine or O6-ethylguanine, two of the DNA adducts formed by carcinogenic alkylating agents. The vectors were introduced in parallel into a pair of Chinese hamster ovary cells, in which one member of the pair was deficient in the repair enzyme O6-alkylguanine-DNA alkyltransferase (mex-) and the other was proficient in this activity (mex+). The vectors integrated into and replicated within the respective host genomes. After intrachromosomal replication, the DNA sequence in the vicinity of the originally adducted site of each integrated vector was amplified from the host genome by using the polymerase chain reaction and was analyzed for mutations. High levels of mutation were observed from the O6-methylguanine- and O6-ethylguanine-containing vectors replicated in mex- cells (approximately 19% for O6-methylguanine and approximately 11% for O6-ethylguanine). DNA sequencing revealed the induced mutations to be almost exclusively G----A transitions. By contrast, little or no mutagenesis was detected when the adducted vectors were introduced into mex+ cells, indicating the significant role of the O6-alkylguanine-DNA alkyltransferase in the repair of O6-methylguanine and O6-ethylguanine in these mammalian cells.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott P. J., Saffhill R. DNA synthesis with methylated poly(dC-dG) templates. Evidence for a competitive nature to miscoding by O(6)-methylguanine. Biochim Biophys Acta. 1979 Mar 28;562(1):51–61. doi: 10.1016/0005-2787(79)90125-4. [DOI] [PubMed] [Google Scholar]
- Ashman C. R., Davidson R. L. DNA base sequence changes induced by ethyl methanesulfonate in a chromosomally integrated shuttle vector gene in mouse cells. Somat Cell Mol Genet. 1987 Sep;13(5):563–568. doi: 10.1007/BF01534497. [DOI] [PubMed] [Google Scholar]
- Basu A. K., Essigmann J. M. Site-specifically modified oligodeoxynucleotides as probes for the structural and biological effects of DNA-damaging agents. Chem Res Toxicol. 1988 Jan-Feb;1(1):1–18. doi: 10.1021/tx00001a001. [DOI] [PubMed] [Google Scholar]
- Bhanot O. S., Ray A. The in vivo mutagenic frequency and specificity of O6-methylguanine in phi X174 replicative form DNA. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7348–7352. doi: 10.1073/pnas.83.19.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bignami M., Terlizzese M., Zijno A., Calcagnile A., Frosina G., Abbondandolo A., Dogliotti E. Cytotoxicity, mutations and SCEs induced by methylating agents are reduced in CHO cells expressing an active mammalian O6-methylguanine-DNA methyltransferase gene. Carcinogenesis. 1987 Oct;8(10):1417–1421. doi: 10.1093/carcin/8.10.1417. [DOI] [PubMed] [Google Scholar]
- Chambers R. W., Sledziewska-Gojska E., Hirani-Hojatti S., Borowy-Borowski H. uvrA and recA mutations inhibit a site-specific transition produced by a single O6-methylguanine in gene G of bacteriophage phi X174. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7173–7177. doi: 10.1073/pnas.82.21.7173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coulondre C., Miller J. H. Genetic studies of the lac repressor. IV. Mutagenic specificity in the lacI gene of Escherichia coli. J Mol Biol. 1977 Dec 15;117(3):577–606. doi: 10.1016/0022-2836(77)90059-6. [DOI] [PubMed] [Google Scholar]
- Ding R., Ghosh K., Eastman A., Bresnick E. DNA-mediated transfer and expression of a human DNA repair gene that demethylates O6-methylguanine. Mol Cell Biol. 1985 Nov;5(11):3293–3296. doi: 10.1128/mcb.5.11.3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuBridge R. B., Calos M. P. Recombinant shuttle vectors for the study of mutation in mammalian cells. Mutagenesis. 1988 Jan;3(1):1–9. doi: 10.1093/mutage/3.1.1. [DOI] [PubMed] [Google Scholar]
- DuBridge R. B., Tang P., Hsia H. C., Leong P. M., Miller J. H., Calos M. P. Analysis of mutation in human cells by using an Epstein-Barr virus shuttle system. Mol Cell Biol. 1987 Jan;7(1):379–387. doi: 10.1128/mcb.7.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eckert K. A., Ingle C. A., Klinedinst D. K., Drinkwater N. R. Molecular analysis of mutations induced in human cells by N-ethyl-N-nitrosourea. Mol Carcinog. 1988;1(1):50–56. doi: 10.1002/mc.2940010111. [DOI] [PubMed] [Google Scholar]
- Ellison K. S., Dogliotti E., Essigmann J. M. Construction of a shuttle vector containing a single O6-methylguanine: a probe for mutagenesis in mammalian cells. Mutat Res. 1989 Mar-May;220(2-3):93–100. doi: 10.1016/0165-1110(89)90014-6. [DOI] [PubMed] [Google Scholar]
- Gerchman L. L., Ludlum D. B. The properties of O 6 -methylguanine in templates for RNA polymerase. Biochim Biophys Acta. 1973 May 10;308(2):310–316. doi: 10.1016/0005-2787(73)90160-3. [DOI] [PubMed] [Google Scholar]
- Goth-Goldstein R. Inability of Chinese hamster ovary cells to excise O6-alkylguanine. Cancer Res. 1980 Jul;40(7):2623–2624. [PubMed] [Google Scholar]
- Hill-Perkins M., Jones M. D., Karran P. Site-specific mutagenesis in vivo by single methylated or deaminated purine bases. Mutat Res. 1986 Sep;162(2):153–163. doi: 10.1016/0027-5107(86)90081-3. [DOI] [PubMed] [Google Scholar]
- Lebkowski J. S., Miller J. H., Calos M. P. Determination of DNA sequence changes induced by ethyl methanesulfonate in human cells, using a shuttle vector system. Mol Cell Biol. 1986 May;6(5):1838–1842. doi: 10.1128/mcb.6.5.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loechler E. L., Green C. L., Essigmann J. M. In vivo mutagenesis by O6-methylguanine built into a unique site in a viral genome. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6271–6275. doi: 10.1073/pnas.81.20.6271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehta J. R., Ludlum D. B. Synthesis and properties of O6-methyldeoxyguanylic acid and its copolymers with deoxycytidylic acid. Biochim Biophys Acta. 1978 Dec 21;521(2):770–778. doi: 10.1016/0005-2787(78)90316-7. [DOI] [PubMed] [Google Scholar]
- Miller E. C. Some current perspectives on chemical carcinogenesis in humans and experimental animals: Presidential Address. Cancer Res. 1978 Jun;38(6):1479–1496. [PubMed] [Google Scholar]
- Miller J. H. Mutational specificity in bacteria. Annu Rev Genet. 1983;17:215–238. doi: 10.1146/annurev.ge.17.120183.001243. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Richardson K. K., Richardson F. C., Crosby R. M., Swenberg J. A., Skopek T. R. DNA base changes and alkylation following in vivo exposure of Escherichia coli to N-methyl-N-nitrosourea or N-ethyl-N-nitrosourea. Proc Natl Acad Sci U S A. 1987 Jan;84(2):344–348. doi: 10.1073/pnas.84.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumar S., Notario V., Martin-Zanca D., Barbacid M. Induction of mammary carcinomas in rats by nitroso-methylurea involves malignant activation of H-ras-1 locus by single point mutations. Nature. 1983 Dec 15;306(5944):658–661. doi: 10.1038/306658a0. [DOI] [PubMed] [Google Scholar]
- Zarbl H., Sukumar S., Arthur A. V., Martin-Zanca D., Barbacid M. Direct mutagenesis of Ha-ras-1 oncogenes by N-nitroso-N-methylurea during initiation of mammary carcinogenesis in rats. 1985 May 30-Jun 5Nature. 315(6018):382–385. doi: 10.1038/315382a0. [DOI] [PubMed] [Google Scholar]