Abstract
The presence of recipient lymphocytes in grafts is thought to equate with rejection. Thus, we wished to follow the fate of lymphocytes after transplant of the small bowel. Three complete small-bowel transplants, two with the liver from the same donor also transplanted, were done successfully. Patients were immunosuppressed with FK 506. 5 to 11% of lymphocytes in the recipients’ peripheral blood were of donor origin during the early postoperative period when there were no clinical signs of graft-versus-host disease. However, donor cells were no longer detectable after 12 to 54 days. Serial biopsy specimens of the grafted small bowel showed progressive replacement of lymphocytes in the lamina propria by those of the recipient’s HLA phenotype. Lymphoid repopulation was complete after 10 to 12 weeks but the epithelial cells of the intestine remained those of the donor. The patients are on enteral alimentation after 5, 6, and 8 months with histopathologically normal or nearly normal intestines. Re-examination of assumptions about the rejection of intestinal grafts and strategies for its prevention are required following these observations.
Little is known about the fate and function of lymphocytes in intestinal grafts, partly because long-term survival after transplant of the small intestine has been difficult to achieve. The first successful complete small-intestine transplant in man1 was accomplished with continuous intravenous infusion of cyclosporin. The patient briefly had donor lymphocytes in peripheral blood during the early postoperative phase and at the same time had symptoms of graft-versus-host disease (GVHD). We have followed the fate of host and donor lymphocytes in three patients treated with FK 506—one after small-bowel transplant and two after combined liver-intestine grafting.
Patient 1 lost the entire small bowel and most of the colon 5 months before transplant after a gun shot wound of the superior mesenteric artery; liver function was normal. Patients 2 and 3 had had total small-bowel resection several years earlier because of necrotising enterocolitis and thrombosis of the superior mesenteric artery, respectively, and both had liver failure following parenteral hyperalimentation. All grafts received arterial blood from the aorta, and intestinal venous outflow was through the liver of patient 1 or through the liver grafts of patients 2 and 3. FK 506 for immunosuppression was given intravenously at first (0·1 mg/kg per day) and later enterally (0·3 mg/kg per day in divided doses). Maintenance doses of FK 506 were lower. Prednisolone was given initially and later stopped (patients 2 and 3) or reduced (patient 1). Patients were maintained on intravenous nutrition for at least 2 months before starting jejunostomy and, ultimately, oral feeding. Patients 1 and 2 have had normal gastrointestinal continuity restored, and patient 3 is still being fed through a nasogastric tube with its tip advanced into the graft jejunum.
Peripheral blood lymphocytes were isolated with ‘Ficoll-Hypaque’ (Pharmacia LKB) and stored in liquid nitrogen until tested. Lymphocytes were added to monoclonal antibodies (One Lambda Inc, Los Angeles), fixed in 2% paraformaldehyde-phosphate buffer solution, and identified by flow cytometry (‘FACScan’, Becton Dickinson). Monoclonal antibodies were also used for immunocytochemical identification of lymphocyte HLA phenotypes in biopsy specimens obtained through jejunal and ileal stomas.
During the first week after transplant, between 5 and 11 % of the circulating lymphocytes of all patients were of the donor type (figure). However, after 12–54 days donor lymphocytes were no longer detectable. The proportion of donor lymphocytes in the lamina propria of patient 1 decreased from 80% at 21 days post-transplant to 30% after 35 days and 0% after 70 days (figure). Jejunal and ileal biopsies showed the same pattern of change. Donor lymphocytes were replaced by host lymphocytes in patients 2 and 3 after 77 and 84 days, respectively.
Figure.
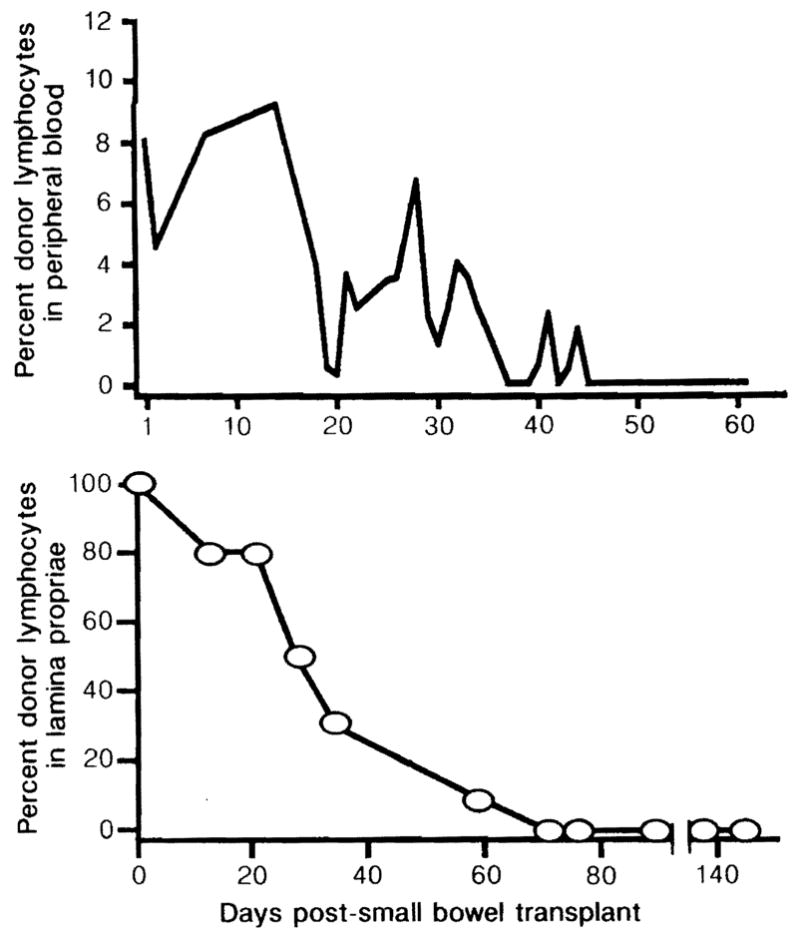
Proportion of donor lymphocytes in peripheral blood and lamina propria after small-bowel transplant (patient 1).
Our finding of recipient lymphocyte repopulation of chronically functioning intestinal grafts, and similar results in rats treated with FK 506,2 indicate that presence of recipient lymphocytes in the graft does not necessarily equate with rejection.3 What do we know about the fate and function of the lymphoid component of intestinal or mutlivisceral grafts? In non-immunosuppressed dogs receiving multivisceral transplants the intestine was rejected and there was histopathological evidence of GVHD within 1 week.4 Subsequently, the consequences of GVHD (parent to F1 hybrid) were separated from those of rejection (F1 to parent) in inbred rats.5 The design of these experiments rendered either the recipient or intestinal graft vulnerable to immunological attack by the other, whereas under clinical circumstances both immune systems are activated. It is unlikely that alteration of the graft lymphoid system could be achieved more efficiently by pretransplant manipulations (such as irradiation or antilymphocyte globulin) than occurred with the use of potent immunosuppression in our patients and in rats.2 Donor pretreatment was not used in the three cases we report, nor in a fourth with only 7 weeks of follow-up. GVHD was not encountered. Histocompatible bone-marrow transplant experiments in rats show that FK 506 given prophylactically prevents GVHD.6 Established GVHD has been reversed with FK 506 in human bone-marrow recipients.7 This may be important if, as seems likely, the migrated donor lymphocytes have survived and are diffusely established in recipient tissue.
Lymphoreticular repopulation is not a unique feature of intestinal or multivisceral grafts. The macrophage system in human liver grafts switches completely to that of the recipient within 100 days.8 Fung et al9,10 have described graft lymphoid tissues in transition after human heart-lung and liver transplant. Thus, lymphoreticular repopulation probably occurs with successful transplant of any lymphoid-containing graft. Whether this contributes to graft acceptance should be the subject for future inquiry.
Acknowledgments
Supported by the University of Pittsburgh Pathology Education, and Research Fund, project grant DK 29961 from the National Institutes of Health, and by a grant from the Veterans Administration.
References
- 1.Grant D, Wall W, Mimeault R, et al. Successful small-bowel/liver transplantation. Lancet. 1990;335:181–84. doi: 10.1016/0140-6736(90)90275-a. [DOI] [PubMed] [Google Scholar]
- 2.Murase N, Demetris AJ, Matsuzaki T, et al. Long-term survival in rats after multivisceral versus isolated small bowel allotransplantation under FK 506. Surgery. (in press) [PMC free article] [PubMed] [Google Scholar]
- 3.Ingham Clark CL, Cunningham AJ, Crane PW, Wood RFM, Lear PA. Lymphocyte infiltration patterns in rat small-bowel transplants. Transplant Proc. 1990;22:2460. [PubMed] [Google Scholar]
- 4.Starzl TE, Kaupp HA, Jr, Brock DR, Butz GW, Jr, Linman JW. Homotransplantation of multiple visceral organs. Am J Surg. 1962;103:219–29. doi: 10.1016/0002-9610(62)90491-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monchik GJ, Russell PS. Transplantation of the small bowel in the rat: technical and immunologic considerations. Surgery. 1971;70:693–702. [PubMed] [Google Scholar]
- 6.Markus PM, Cai X, Ming W, Demetris AJ, Starzl TE, Fung JJ. Prevention of graft-versus-host disease following allogeneic bone marrow transplantation in rats using FK 506. Transplantation. doi: 10.1097/00007890-199110000-00002. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tzakis AG, Fung JJ, Todo S, Reyes J, Green M, Starzl TE. Use of FK 506 in pediatric patients. Transplant Proc. 1991;23:924–27. [PMC free article] [PubMed] [Google Scholar]
- 8.Gouw ASH, Houthoff JH, Huitema S, Beelen JM, Gips CH, Poppema S. Expression of major histocompatibility complex antigens and replacement of donor cells by recipient ones in human liver grafts. Transplantation. 1987;43:291–96. doi: 10.1097/00007890-198702000-00025. [DOI] [PubMed] [Google Scholar]
- 9.Fung JJ, Zeevi A, Kaufman C, et al. Interactions between bronchoalveolar lymphocytes and macrophages in heart-lung transplant recipients. Hum Immunol. 1985;14:287–94. doi: 10.1016/0198-8859(85)90236-8. [DOI] [PubMed] [Google Scholar]
- 10.Fung JJ, Zeevi A, Demetris AJ, et al. Origin of lymph node derived lymphocytes in human hepatic allografts. Clin Transplant. 1989;3:316–24. [PMC free article] [PubMed] [Google Scholar]


