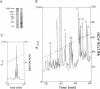
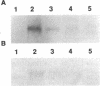
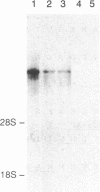
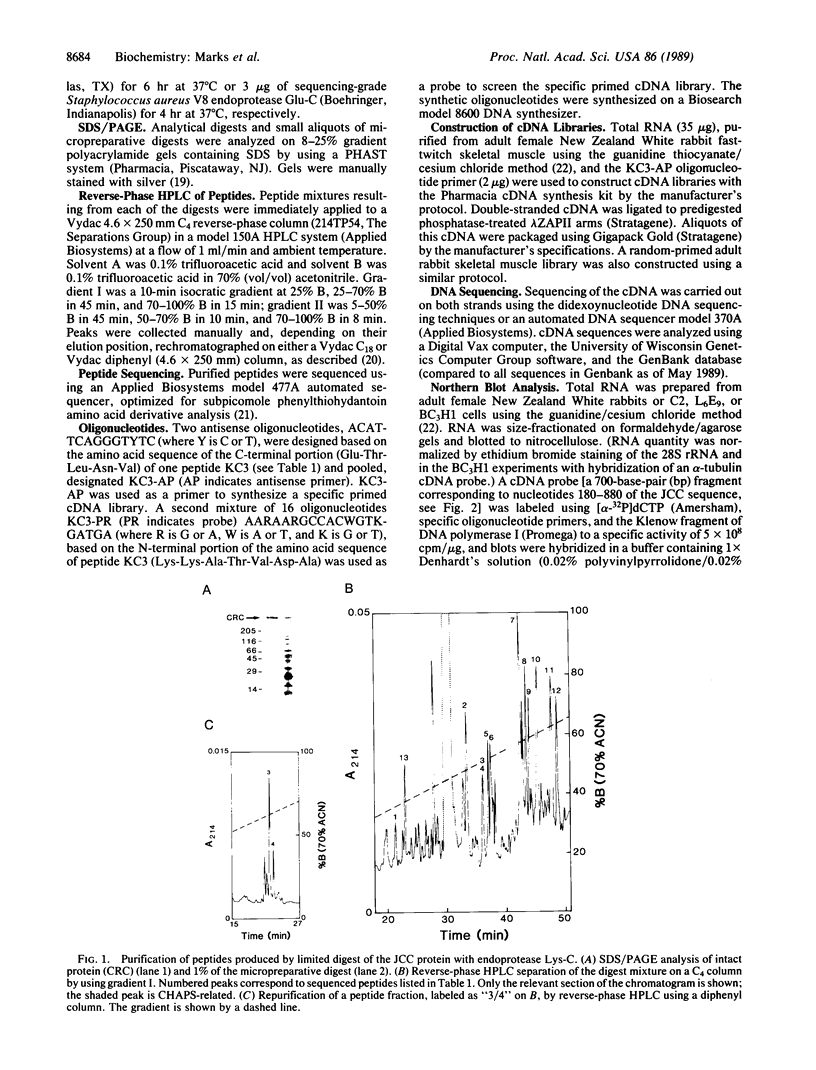
Abstract
Major progress has been made in elucidating the calcium release mechanism involved in excitation-contraction coupling. The ryanodine receptor of sarcoplasmic reticulum has been isolated and found to be morphologically identical to the foot structure, which is involved in the junctional association of terminal cisternae with the transverse tubule. The foot structure also contains the calcium release channel itself. For this reason, we refer to the foot structure as the junctional channel complex (JCC). The JCC consists of an oligomer of a single high molecular weight protein. Although progress has been made in characterizing important aspects of the structure and function of the JCC, further understanding of the JCC protein subunit awaits the molecular cloning of the JCC. We report on the isolation of cDNA clones encoding portions of the JCC from rabbit fast-twitch skeletal muscle and its tissue distribution and expression. The large size and lack of solubility of the JCC protein posed particular challenges to cloning this molecule. Among these was the necessity to develop techniques for partially digesting the JCC protein subunit with endoproteases in the presence of detergent. With this approach we obtained partial amino acid sequences from regions of the JCC and designed oligonucleotide primers and probes to synthesize and screen cDNA libraries. The rabbit skeletal muscle JCC mRNA encodes an approximately 16-kilobase mRNA present in skeletal, heart, and aortic smooth muscle, as determined by RNA blot analysis with a 700-base-pair cDNA probe. Whereas the JCC mRNA appears to be relatively abundant in adult rabbit fast-twitch skeletal muscle, it is much less abundant in heart and smooth muscle. The JCC mRNA in BC3H1 (a myoblast cell line) is reversibly regulated by growth factors in a manner similar to muscle-specific contractile protein genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caffrey J. M., Brown A. M., Schneider M. D. Mitogens and oncogenes can block the induction of specific voltage-gated ion channels. Science. 1987 May 1;236(4801):570–573. doi: 10.1126/science.2437651. [DOI] [PubMed] [Google Scholar]
- Catterall W. A. Structure and function of voltage-sensitive ion channels. Science. 1988 Oct 7;242(4875):50–61. doi: 10.1126/science.2459775. [DOI] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Inui M. Biochemistry and biophysics of excitation-contraction coupling. Annu Rev Biophys Biophys Chem. 1989;18:333–364. doi: 10.1146/annurev.bb.18.060189.002001. [DOI] [PubMed] [Google Scholar]
- Fleischer S., Ogunbunmi E. M., Dixon M. C., Fleer E. A. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franzini-Armstrong C., Nunzi G. Junctional feet and particles in the triads of a fast-twitch muscle fibre. J Muscle Res Cell Motil. 1983 Apr;4(2):233–252. doi: 10.1007/BF00712033. [DOI] [PubMed] [Google Scholar]
- Hymel L., Inui M., Fleischer S., Schindler H. Purified ryanodine receptor of skeletal muscle sarcoplasmic reticulum forms Ca2+-activated oligomeric Ca2+ channels in planar bilayers. Proc Natl Acad Sci U S A. 1988 Jan;85(2):441–445. doi: 10.1073/pnas.85.2.441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hymel L., Schindler H., Inui M., Fleischer S. Reconstitution of purified cardiac muscle calcium release channel (ryanodine receptor) in planar bilayers. Biochem Biophys Res Commun. 1988 Apr 15;152(1):308–314. doi: 10.1016/s0006-291x(88)80715-0. [DOI] [PubMed] [Google Scholar]
- Imagawa T., Smith J. S., Coronado R., Campbell K. P. Purified ryanodine receptor from skeletal muscle sarcoplasmic reticulum is the Ca2+-permeable pore of the calcium release channel. J Biol Chem. 1987 Dec 5;262(34):16636–16643. [PubMed] [Google Scholar]
- Inui M., Fleischer S. Purification of Ca2+ release channel (ryanodine receptor) from heart and skeletal muscle sarcoplasmic reticulum. Methods Enzymol. 1988;157:490–505. doi: 10.1016/0076-6879(88)57098-2. [DOI] [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Isolation of the ryanodine receptor from cardiac sarcoplasmic reticulum and identity with the feet structures. J Biol Chem. 1987 Nov 15;262(32):15637–15642. [PubMed] [Google Scholar]
- Inui M., Saito A., Fleischer S. Purification of the ryanodine receptor and identity with feet structures of junctional terminal cisternae of sarcoplasmic reticulum from fast skeletal muscle. J Biol Chem. 1987 Feb 5;262(4):1740–1747. [PubMed] [Google Scholar]
- Inui M., Wang S., Saito A., Fleischer S. Characterization of junctional and longitudinal sarcoplasmic reticulum from heart muscle. J Biol Chem. 1988 Aug 5;263(22):10843–10850. [PubMed] [Google Scholar]
- Lai F. A., Erickson H. P., Rousseau E., Liu Q. Y., Meissner G. Purification and reconstitution of the calcium release channel from skeletal muscle. Nature. 1988 Jan 28;331(6154):315–319. doi: 10.1038/331315a0. [DOI] [PubMed] [Google Scholar]
- Lathrop B., Olson E., Glaser L. Control by fibroblast growth factor of differentiation in the BC3H1 muscle cell line. J Cell Biol. 1985 May;100(5):1540–1547. doi: 10.1083/jcb.100.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLennan D. H., Brandl C. J., Korczak B., Green N. M. Amino-acid sequence of a Ca2+ + Mg2+-dependent ATPase from rabbit muscle sarcoplasmic reticulum, deduced from its complementary DNA sequence. Nature. 1985 Aug 22;316(6030):696–700. doi: 10.1038/316696a0. [DOI] [PubMed] [Google Scholar]
- Merril C. R., Goldman D., Sedman S. A., Ebert M. H. Ultrasensitive stain for proteins in polyacrylamide gels shows regional variation in cerebrospinal fluid proteins. Science. 1981 Mar 27;211(4489):1437–1438. doi: 10.1126/science.6162199. [DOI] [PubMed] [Google Scholar]
- Munson R., Jr, Caldwell K. L., Glaser L. Multiple controls for the synthesis of muscle-specific proteins in BC3H1 cells. J Cell Biol. 1982 Feb;92(2):350–356. doi: 10.1083/jcb.92.2.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson E. N., Caldwell K. L., Gordon J. I., Glaser L. Regulation of creatine phosphokinase expression during differentiation of BC3H1 cells. J Biol Chem. 1983 Feb 25;258(4):2644–2652. [PubMed] [Google Scholar]
- Saito A., Inui M., Radermacher M., Frank J., Fleischer S. Ultrastructure of the calcium release channel of sarcoplasmic reticulum. J Cell Biol. 1988 Jul;107(1):211–219. doi: 10.1083/jcb.107.1.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider M. F., Chandler W. K. Voltage dependent charge movement of skeletal muscle: a possible step in excitation-contraction coupling. Nature. 1973 Mar 23;242(5395):244–246. doi: 10.1038/242244a0. [DOI] [PubMed] [Google Scholar]
- Schubert D., Harris A. J., Devine C. E., Heinemann S. Characterization of a unique muscle cell line. J Cell Biol. 1974 May;61(2):398–413. doi: 10.1083/jcb.61.2.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. S., Imagawa T., Ma J., Fill M., Campbell K. P., Coronado R. Purified ryanodine receptor from rabbit skeletal muscle is the calcium-release channel of sarcoplasmic reticulum. J Gen Physiol. 1988 Jul;92(1):1–26. doi: 10.1085/jgp.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standaert M. L., Schimmel S. D., Pollet R. J. The development of insulin receptors and responses in the differentiating nonfusing muscle cell line BC3H-1. J Biol Chem. 1984 Feb 25;259(4):2337–2345. [PubMed] [Google Scholar]
- Strauch A. R., Offord J. D., Chalkley R., Rubenstein P. A. Characterization of actin mRNA levels during BC3H1 cell differentiation. J Biol Chem. 1986 Jan 15;261(2):849–855. [PubMed] [Google Scholar]
- Tada M., Yamamoto T., Tonomura Y. Molecular mechanism of active calcium transport by sarcoplasmic reticulum. Physiol Rev. 1978 Jan;58(1):1–79. doi: 10.1152/physrev.1978.58.1.1. [DOI] [PubMed] [Google Scholar]
- Takeshima H., Nishimura S., Matsumoto T., Ishida H., Kangawa K., Minamino N., Matsuo H., Ueda M., Hanaoka M., Hirose T. Primary structure and expression from complementary DNA of skeletal muscle ryanodine receptor. Nature. 1989 Jun 8;339(6224):439–445. doi: 10.1038/339439a0. [DOI] [PubMed] [Google Scholar]
- Tanabe T., Beam K. G., Powell J. A., Numa S. Restoration of excitation-contraction coupling and slow calcium current in dysgenic muscle by dihydropyridine receptor complementary DNA. Nature. 1988 Nov 10;336(6195):134–139. doi: 10.1038/336134a0. [DOI] [PubMed] [Google Scholar]
- Taubman M. B., Smith C. W., Izumo S., Grant J. W., Endo T., Andreadis A., Nadal-Ginard B. The expression of sarcomeric muscle-specific contractile protein genes in BC3H1 cells: BC3H1 cells resemble skeletal myoblasts that are defective for commitment to terminal differentiation. J Cell Biol. 1989 May;108(5):1799–1806. doi: 10.1083/jcb.108.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tempst P., Woo D. D., Teplow D. B., Aebersold R., Hood L. E., Kent S. B. Microscale structure analysis of a high-molecular-weight, hydrophobic membrane glycoprotein fraction with platelet-derived growth factor-dependent kinase activity. J Chromatogr. 1986 May 30;359:403–412. doi: 10.1016/0021-9673(86)80094-2. [DOI] [PubMed] [Google Scholar]
- Wagenknecht T., Grassucci R., Frank J., Saito A., Inui M., Fleischer S. Three-dimensional architecture of the calcium channel/foot structure of sarcoplasmic reticulum. Nature. 1989 Mar 9;338(6211):167–170. doi: 10.1038/338167a0. [DOI] [PubMed] [Google Scholar]