Abstract
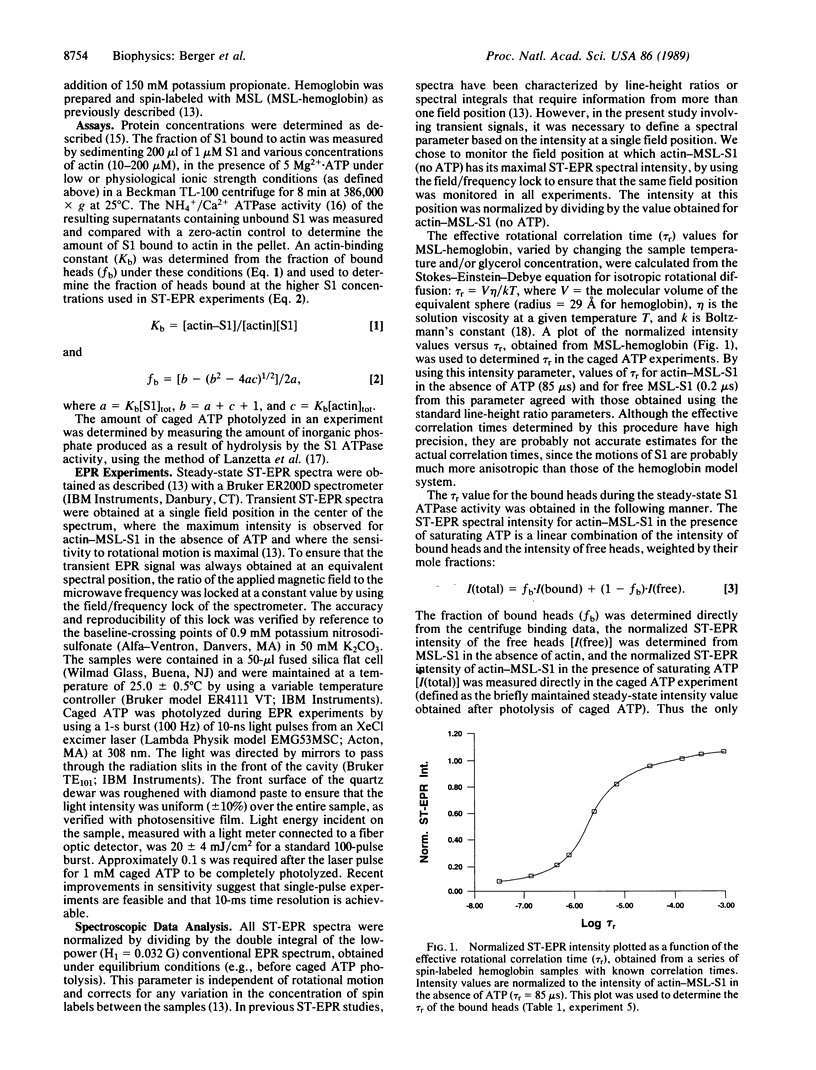
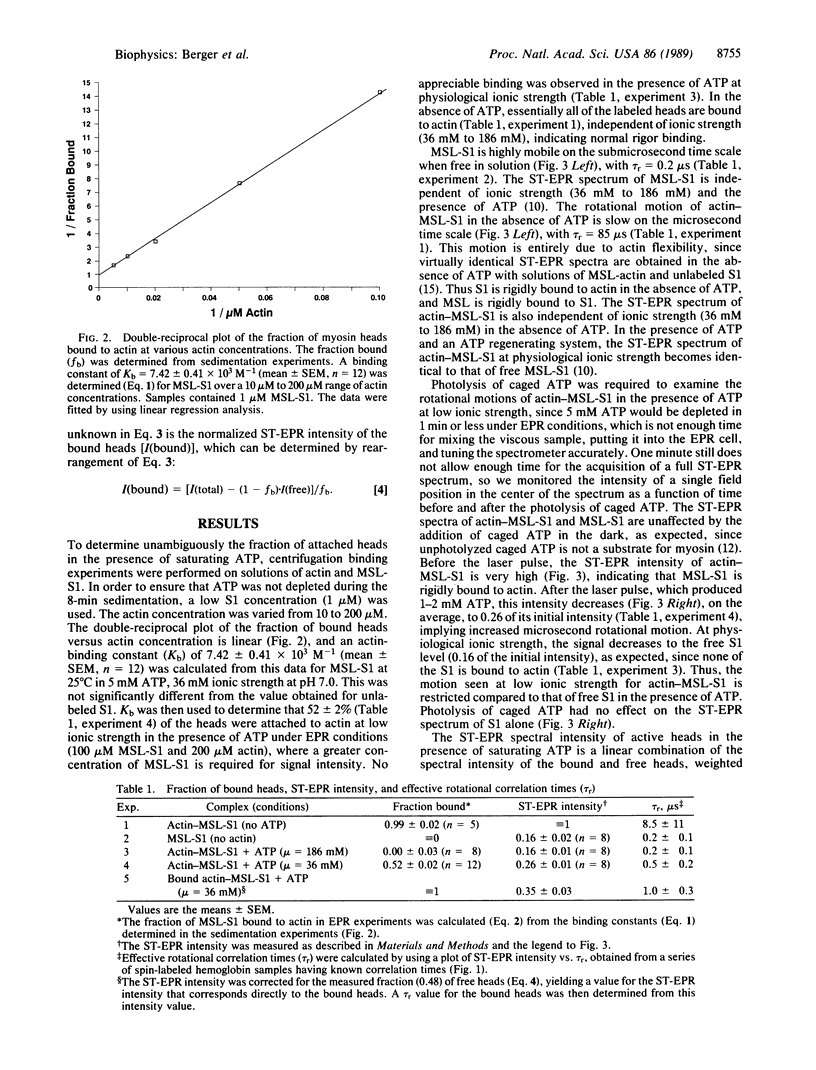
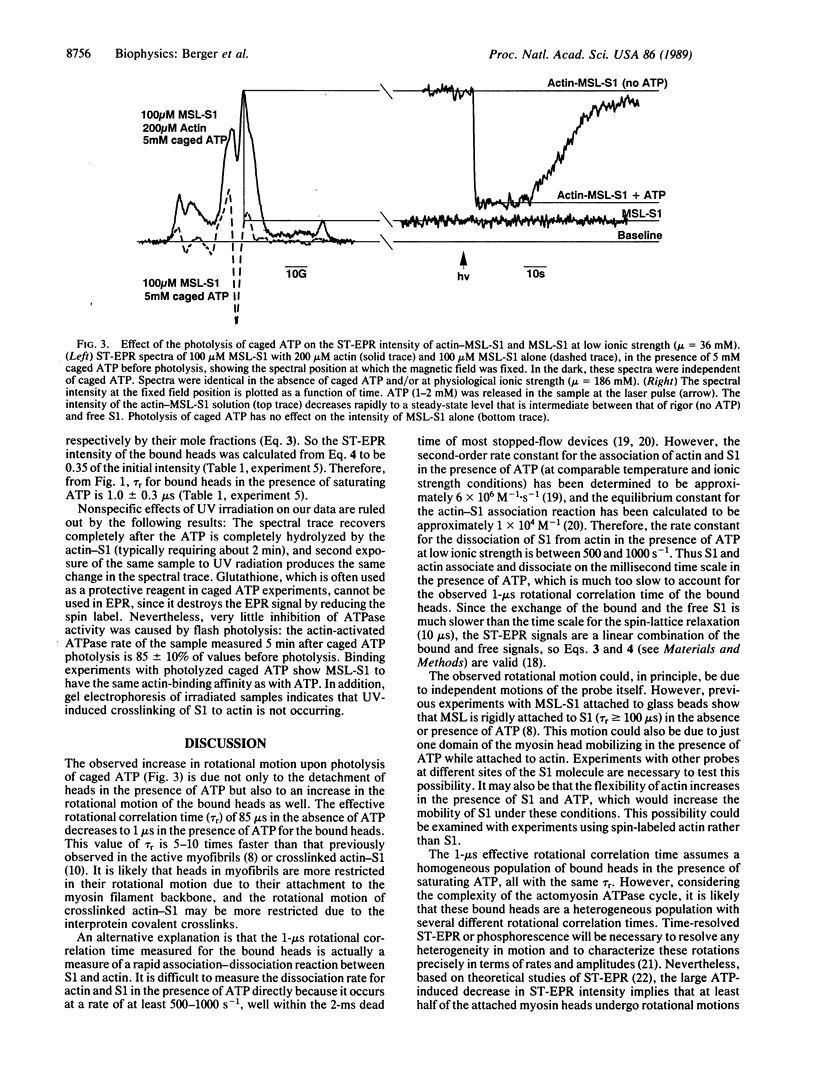
To test the proposal that ATPase activity is coupled to the rotation of muscle cross-bridges (myosin heads attached to actin), we have used saturation-transfer EPR to detect the rotational motion of spin-labeled myosin heads (subfragment 1; S1) bound to actin following the photolysis of caged ATP (a photoactivatable analog of ATP). In order to ensure that most of the heads were bound to actin in the presence of ATP, solutions contained high (200 microns) actin concentrations and were of low (36 mM) ionic strength. Sedimentation measurements indicated that 52 +/- 2% of the spin-labeled heads were attached in the steady state of ATP hydrolysis during EPR measurements. Five millimolar caged ATP was added to the actin-S1 solution in an EPR cell in the dark, with no effect on the intense saturation-transfer EPR signal, implying a rigid actin-S1 complex. A laser pulse produced 1 mM ATP, which decreased the signal rapidly to a brief steady-state level that indicated only slightly less rotational mobility than that of free heads. After correcting for the fraction of free heads, we conclude that the bound heads have an effective rotational correlation time of 1.0 +/- 0.3 microseconds, which is about 100 times shorter (faster) than that in the absence of ATP. To our knowledge, this is the first direct evidence that myosin heads undergo rotational motion when bound to actin during the ATPase cycle. It is likely that similar cross-bridge rotations occur during muscle contraction.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnett V. A., Thomas D. D. Microsecond rotational motion of spin-labeled myosin heads during isometric muscle contraction. Saturation transfer electron paramagnetic resonance. Biophys J. 1989 Sep;56(3):517–523. doi: 10.1016/S0006-3495(89)82698-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner B., Schoenberg M., Chalovich J. M., Greene L. E., Eisenberg E. Evidence for cross-bridge attachment in relaxed muscle at low ionic strength. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7288–7291. doi: 10.1073/pnas.79.23.7288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chalovich J. M., Eisenberg E. Inhibition of actomyosin ATPase activity by troponin-tropomyosin without blocking the binding of myosin to actin. J Biol Chem. 1982 Mar 10;257(5):2432–2437. [PMC free article] [PubMed] [Google Scholar]
- Cooke R., Crowder M. S., Thomas D. D. Orientation of spin labels attached to cross-bridges in contracting muscle fibres. Nature. 1982 Dec 23;300(5894):776–778. doi: 10.1038/300776a0. [DOI] [PubMed] [Google Scholar]
- Eads T. M., Thomas D. D., Austin R. H. Microsecond rotational motions of eosin-labeled myosin measured by time-resolved anisotropy of absorption and phosphorescence. J Mol Biol. 1984 Oct 15;179(1):55–81. doi: 10.1016/0022-2836(84)90306-1. [DOI] [PubMed] [Google Scholar]
- Greene L. E., Eisenberg E. Formation of a ternary complex: actin, 5'-adenylyl imidodiphosphate, and the subfragments of myosin. Proc Natl Acad Sci U S A. 1978 Jan;75(1):54–58. doi: 10.1073/pnas.75.1.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUXLEY A. F., NIEDERGERKE R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954 May 22;173(4412):971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- HUXLEY H., HANSON J. Changes in the cross-striations of muscle during contraction and stretch and their structural interpretation. Nature. 1954 May 22;173(4412):973–976. doi: 10.1038/173973a0. [DOI] [PubMed] [Google Scholar]
- Haselgrove J. C., Huxley H. E. X-ray evidence for radial cross-bridge movement and for the sliding filament model in actively contracting skeletal muscle. J Mol Biol. 1973 Jul 15;77(4):549–568. doi: 10.1016/0022-2836(73)90222-2. [DOI] [PubMed] [Google Scholar]
- Huxley A. F., Simmons R. M. Proposed mechanism of force generation in striated muscle. Nature. 1971 Oct 22;233(5321):533–538. doi: 10.1038/233533a0. [DOI] [PubMed] [Google Scholar]
- Huxley H. E., Kress M. Crossbridge behaviour during muscle contraction. J Muscle Res Cell Motil. 1985 Apr;6(2):153–161. doi: 10.1007/BF00713057. [DOI] [PubMed] [Google Scholar]
- Huxley H. E. The mechanism of muscular contraction. Science. 1969 Jun 20;164(3886):1356–1365. doi: 10.1126/science.164.3886.1356. [DOI] [PubMed] [Google Scholar]
- Kishino A., Yanagida T. Force measurements by micromanipulation of a single actin filament by glass needles. Nature. 1988 Jul 7;334(6177):74–76. doi: 10.1038/334074a0. [DOI] [PubMed] [Google Scholar]
- Lanzetta P. A., Alvarez L. J., Reinach P. S., Candia O. A. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979 Nov 15;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- McCray J. A., Herbette L., Kihara T., Trentham D. R. A new approach to time-resolved studies of ATP-requiring biological systems; laser flash photolysis of caged ATP. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7237–7241. doi: 10.1073/pnas.77.12.7237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulhern S. A., Eisenberg E. Interaction of spin-labeled and N-(iodacetylaminoethyl)-5-naphthylamine-1-sulfonic acid SH1-blocked heavy meromyosin and myosin with actin and adenosine triphosphate. Biochemistry. 1978 Oct 17;17(21):4419–4425. doi: 10.1021/bi00614a010. [DOI] [PubMed] [Google Scholar]
- Pate E., Cooke R. Energetics of the actomyosin bond in the filament array of muscle fibers. Biophys J. 1988 Apr;53(4):561–573. doi: 10.1016/S0006-3495(88)83136-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squier T. C., Thomas D. D. Methodology for increased precision in saturation transfer electron paramagnetic resonance studies of rotational dynamics. Biophys J. 1986 Apr;49(4):921–935. doi: 10.1016/S0006-3495(86)83720-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein L. A., Schwarz R. P., Jr, Chock P. B., Eisenberg E. Mechanism of actomyosin adenosine triphosphatase. Evidence that adenosine 5'-triphosphate hydrolysis can occur without dissociation of the actomyosin complex. Biochemistry. 1979 Sep 4;18(18):3895–3909. doi: 10.1021/bi00585a009. [DOI] [PubMed] [Google Scholar]
- Svensson E. C., Thomas D. D. ATP induces microsecond rotational motions of myosin heads crosslinked to actin. Biophys J. 1986 Nov;50(5):999–1002. doi: 10.1016/S0006-3495(86)83541-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Ishiwata S., Seidel J. C., Gergely J. Submillisecond rotational dynamics of spin-labeled myosin heads in myofibrils. Biophys J. 1980 Dec;32(3):873–889. doi: 10.1016/S0006-3495(80)85023-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. D., Seidel J. C., Gergely J. Rotational dynamics of spin-labeled F-actin in the sub-millisecond time range. J Mol Biol. 1979 Aug 15;132(3):257–273. doi: 10.1016/0022-2836(79)90259-6. [DOI] [PubMed] [Google Scholar]
- Toyoshima Y. Y., Kron S. J., McNally E. M., Niebling K. R., Toyoshima C., Spudich J. A. Myosin subfragment-1 is sufficient to move actin filaments in vitro. Nature. 1987 Aug 6;328(6130):536–539. doi: 10.1038/328536a0. [DOI] [PubMed] [Google Scholar]
- White H. D., Taylor E. W. Energetics and mechanism of actomyosin adenosine triphosphatase. Biochemistry. 1976 Dec 28;15(26):5818–5826. doi: 10.1021/bi00671a020. [DOI] [PubMed] [Google Scholar]


