Abstract
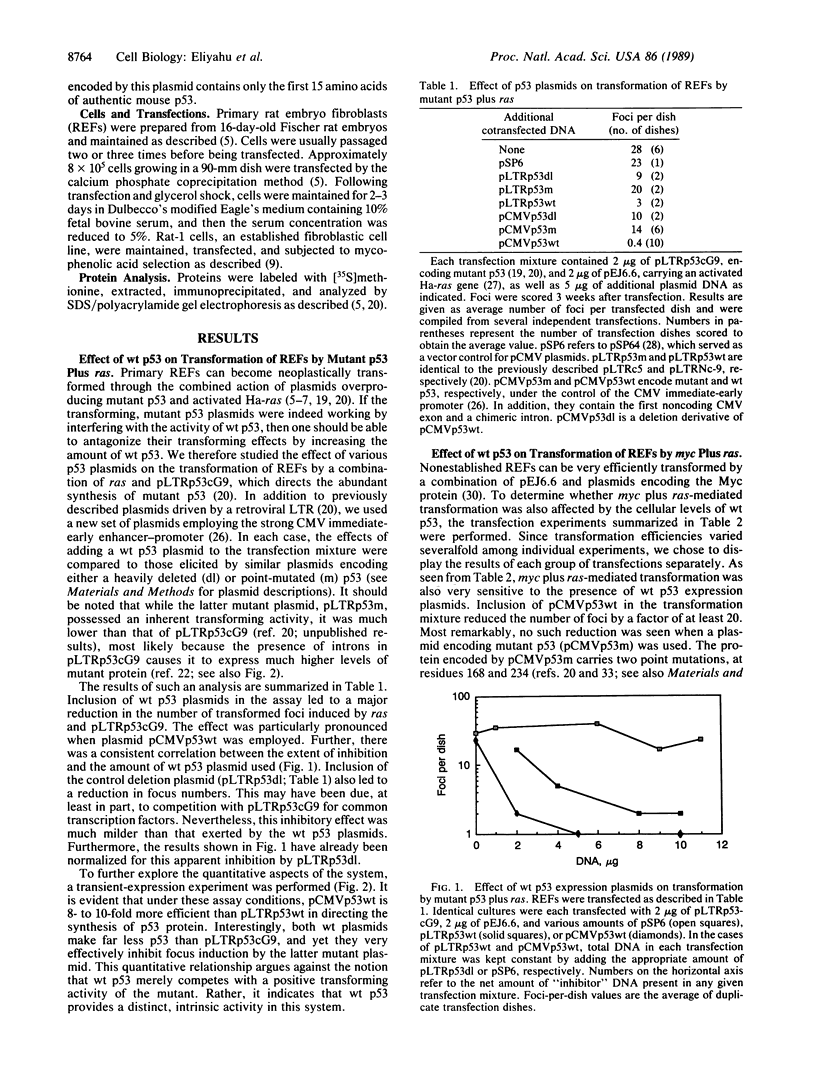
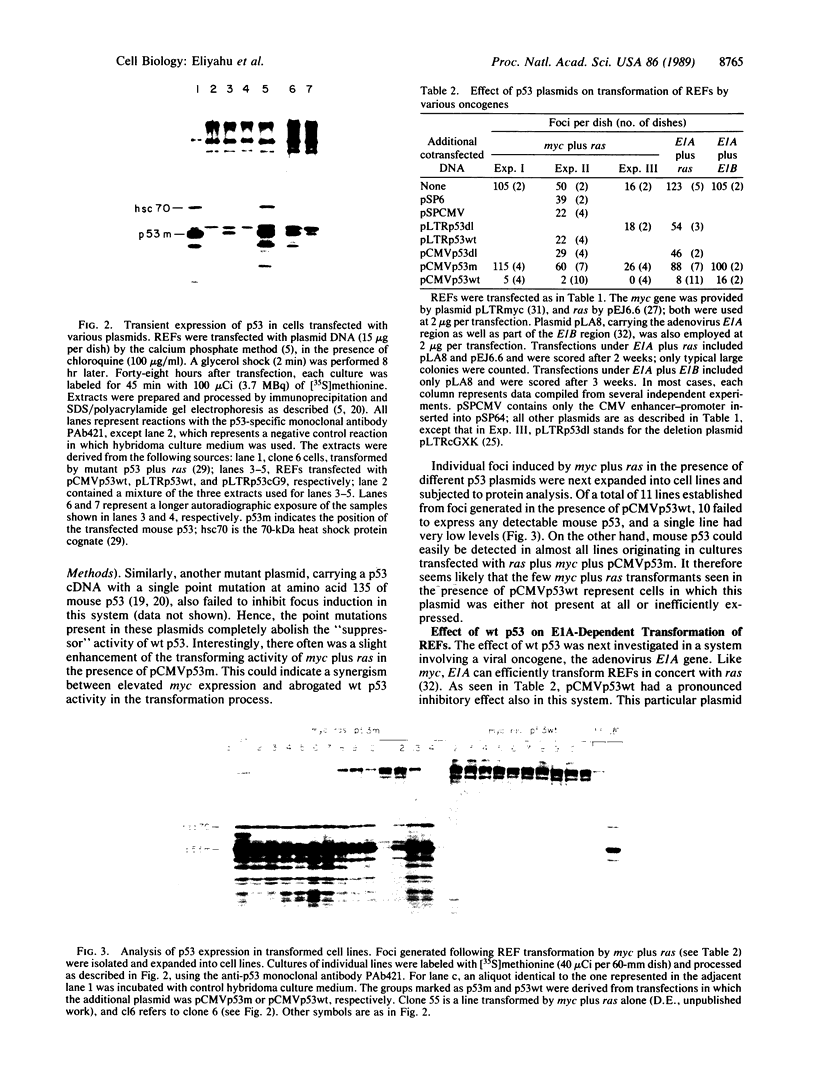
Mutant forms of the p53 cellular tumor antigen elicit neoplastic transformation in vitro. Recent evidence indicated that loss of normal p53 expression is a frequent event in certain types of tumors, raising the possibility that such loss provides transformed cells with a selective growth advantage. Thus, it was conceivable that the mutants might contribute to transformation by abrogating normal p53 function. We therefore studied the effect of plasmids encoding wild-type (wt) p53 on the ability of primary rat embryo fibroblasts to be transformed by a combination of mutant p53 and ras. It was found that wt p53 plasmids indeed caused a marked reduction in the number of transformed foci. Furthermore, wt p53 plasmids also suppressed the induction of transformed foci by combinations of bona fide oncogenes, such as myc plus ras or adenovirus E1A plus ras. On the other hand, plasmids carrying mutations in the p53 coding region totally failed to inhibit oncogene-mediated focus induction and often even slightly stimulated it. Hence, such mutations completely abolished the activity of wt p53 that is responsible for the "suppressor" effect. The latter fact is of special interest, since similar mutations in p53 are often observed in human and rodent tumors. The inhibitory effect of p53 was most pronounced when early-passage cells were used as targets, whereas established cell lines were less sensitive. These data support the notions that wt p53 expression may be restrictive to neoplastic progression and that p53 inactivation may play a crucial role in tumorigenesis.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai N., Nomura D., Yokota K., Wolf D., Brill E., Shohat O., Rotter V. Immunologically distinct p53 molecules generated by alternative splicing. Mol Cell Biol. 1986 Sep;6(9):3232–3239. doi: 10.1128/mcb.6.9.3232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker S. J., Fearon E. R., Nigro J. M., Hamilton S. R., Preisinger A. C., Jessup J. M., vanTuinen P., Ledbetter D. H., Barker D. F., Nakamura Y. Chromosome 17 deletions and p53 gene mutations in colorectal carcinomas. Science. 1989 Apr 14;244(4901):217–221. doi: 10.1126/science.2649981. [DOI] [PubMed] [Google Scholar]
- Ben David Y., Prideaux V. R., Chow V., Benchimol S., Bernstein A. Inactivation of the p53 oncogene by internal deletion or retroviral integration in erythroleukemic cell lines induced by Friend leukemia virus. Oncogene. 1988 Aug;3(2):179–185. [PubMed] [Google Scholar]
- Bienz B., Zakut-Houri R., Givol D., Oren M. Analysis of the gene coding for the murine cellular tumour antigen p53. EMBO J. 1984 Sep;3(9):2179–2183. doi: 10.1002/j.1460-2075.1984.tb02110.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boshart M., Weber F., Jahn G., Dorsch-Häsler K., Fleckenstein B., Schaffner W. A very strong enhancer is located upstream of an immediate early gene of human cytomegalovirus. Cell. 1985 Jun;41(2):521–530. doi: 10.1016/s0092-8674(85)80025-8. [DOI] [PubMed] [Google Scholar]
- Chow V., Ben-David Y., Bernstein A., Benchimol S., Mowat M. Multistage Friend erythroleukemia: independent origin of tumor clones with normal or rearranged p53 cellular oncogenes. J Virol. 1987 Sep;61(9):2777–2781. doi: 10.1128/jvi.61.9.2777-2781.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford L. The 53,000-dalton cellular protein and its role in transformation. Int Rev Exp Pathol. 1983;25:1–50. [PubMed] [Google Scholar]
- Eliyahu D., Goldfinger N., Pinhasi-Kimhi O., Shaulsky G., Skurnik Y., Arai N., Rotter V., Oren M. Meth A fibrosarcoma cells express two transforming mutant p53 species. Oncogene. 1988 Sep;3(3):313–321. [PubMed] [Google Scholar]
- Eliyahu D., Michalovitz D., Oren M. Overproduction of p53 antigen makes established cells highly tumorigenic. Nature. 1985 Jul 11;316(6024):158–160. doi: 10.1038/316158a0. [DOI] [PubMed] [Google Scholar]
- Eliyahu D., Raz A., Gruss P., Givol D., Oren M. Participation of p53 cellular tumour antigen in transformation of normal embryonic cells. Nature. 1984 Dec 13;312(5995):646–649. doi: 10.1038/312646a0. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Levine A. J. The p53 proto-oncogene can act as a suppressor of transformation. Cell. 1989 Jun 30;57(7):1083–1093. doi: 10.1016/0092-8674(89)90045-7. [DOI] [PubMed] [Google Scholar]
- Finlay C. A., Hinds P. W., Tan T. H., Eliyahu D., Oren M., Levine A. J. Activating mutations for transformation by p53 produce a gene product that forms an hsc70-p53 complex with an altered half-life. Mol Cell Biol. 1988 Feb;8(2):531–539. doi: 10.1128/mcb.8.2.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Functional inactivation of genes by dominant negative mutations. Nature. 1987 Sep 17;329(6136):219–222. doi: 10.1038/329219a0. [DOI] [PubMed] [Google Scholar]
- Hinds P., Finlay C., Levine A. J. Mutation is required to activate the p53 gene for cooperation with the ras oncogene and transformation. J Virol. 1989 Feb;63(2):739–746. doi: 10.1128/jvi.63.2.739-746.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J. R., Chumakov P., Addison C., Stürzbecher H. W., Wade-Evans A. Two distinct regions of the murine p53 primary amino acid sequence are implicated in stable complex formation with simian virus 40 T antigen. J Virol. 1988 Oct;62(10):3903–3906. doi: 10.1128/jvi.62.10.3903-3906.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Chumakov P., Currie G. A. The cellular oncogene p53 can be activated by mutagenesis. 1985 Oct 31-Nov 6Nature. 317(6040):816–818. doi: 10.1038/317816a0. [DOI] [PubMed] [Google Scholar]
- Jenkins J. R., Rudge K., Currie G. A. Cellular immortalization by a cDNA clone encoding the transformation-associated phosphoprotein p53. Nature. 1984 Dec 13;312(5995):651–654. doi: 10.1038/312651a0. [DOI] [PubMed] [Google Scholar]
- Kaczmarek L., Oren M., Baserga R. Co-operation between the p53 protein tumor antigen and platelet-poor plasma in the induction of cellular DNA synthesis. Exp Cell Res. 1986 Jan;162(1):268–272. doi: 10.1016/0014-4827(86)90445-3. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Lübbert M., Miller C. W., Crawford L., Koeffler H. P. p53 in chronic myelogenous leukemia. Study of mechanisms of differential expression. J Exp Med. 1988 Mar 1;167(3):873–886. doi: 10.1084/jem.167.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda H., Miller C., Koeffler H. P., Battifora H., Cline M. J. Rearrangement of the p53 gene in human osteogenic sarcomas. Proc Natl Acad Sci U S A. 1987 Nov;84(21):7716–7719. doi: 10.1073/pnas.84.21.7716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melton D. A., Krieg P. A., Rebagliati M. R., Maniatis T., Zinn K., Green M. R. Efficient in vitro synthesis of biologically active RNA and RNA hybridization probes from plasmids containing a bacteriophage SP6 promoter. Nucleic Acids Res. 1984 Sep 25;12(18):7035–7056. doi: 10.1093/nar/12.18.7035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer W. E., Nelson D., DeLeo A. B., Old L. J., Baserga R. Microinjection of monoclonal antibody to protein p53 inhibits serum-induced DNA synthesis in 3T3 cells. Proc Natl Acad Sci U S A. 1982 Oct;79(20):6309–6312. doi: 10.1073/pnas.79.20.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moav B., Horowitz M., Cohen J. B., Rechavi G., Eliyahu E., Oren M., Givol D. Structure and activity of the translocated c-myc in mouse plasmacytoma XRPC-24. Gene. 1986;48(2-3):297–300. doi: 10.1016/0378-1119(86)90089-2. [DOI] [PubMed] [Google Scholar]
- Mowat M., Cheng A., Kimura N., Bernstein A., Benchimol S. Rearrangements of the cellular p53 gene in erythroleukaemic cells transformed by Friend virus. Nature. 1985 Apr 18;314(6012):633–636. doi: 10.1038/314633a0. [DOI] [PubMed] [Google Scholar]
- Munroe D. G., Rovinski B., Bernstein A., Benchimol S. Loss of a highly conserved domain on p53 as a result of gene deletion during Friend virus-induced erythroleukemia. Oncogene. 1988 Jun;2(6):621–624. [PubMed] [Google Scholar]
- Oren M. The p53 cellular tumor antigen: gene structure, expression and protein properties. Biochim Biophys Acta. 1985 Nov 12;823(1):67–78. doi: 10.1016/0304-419x(85)90015-0. [DOI] [PubMed] [Google Scholar]
- Parada L. F., Land H., Weinberg R. A., Wolf D., Rotter V. Cooperation between gene encoding p53 tumour antigen and ras in cellular transformation. Nature. 1984 Dec 13;312(5995):649–651. doi: 10.1038/312649a0. [DOI] [PubMed] [Google Scholar]
- Pinhasi-Kimhi O., Michalovitz D., Ben-Zeev A., Oren M. Specific interaction between the p53 cellular tumour antigen and major heat shock proteins. Nature. 1986 Mar 13;320(6058):182–184. doi: 10.1038/320182a0. [DOI] [PubMed] [Google Scholar]
- Robbins K. C., Devare S. G., Reddy E. P., Aaronson S. A. In vivo identification of the transforming gene product of simian sarcoma virus. Science. 1982 Dec 10;218(4577):1131–1133. doi: 10.1126/science.6293053. [DOI] [PubMed] [Google Scholar]
- Rotter V., Wolf D. Biological and molecular analysis of p53 cellular-encoded tumor antigen. Adv Cancer Res. 1985;43:113–141. doi: 10.1016/s0065-230x(08)60944-6. [DOI] [PubMed] [Google Scholar]
- Rovinski B., Benchimol S. Immortalization of rat embryo fibroblasts by the cellular p53 oncogene. Oncogene. 1988 May;2(5):445–452. [PubMed] [Google Scholar]
- Ruley H. E. Adenovirus early region 1A enables viral and cellular transforming genes to transform primary cells in culture. Nature. 1983 Aug 18;304(5927):602–606. doi: 10.1038/304602a0. [DOI] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Shohat O., Greenberg M., Reisman D., Oren M., Rotter V. Inhibition of cell growth mediated by plasmids encoding p53 anti-sense. Oncogene. 1987;1(3):277–283. [PubMed] [Google Scholar]
- Vogelstein B., Fearon E. R., Kern S. E., Hamilton S. R., Preisinger A. C., Nakamura Y., White R. Allelotype of colorectal carcinomas. Science. 1989 Apr 14;244(4901):207–211. doi: 10.1126/science.2565047. [DOI] [PubMed] [Google Scholar]
- Wolf D., Harris N., Rotter V. Reconstitution of p53 expression in a nonproducer Ab-MuLV-transformed cell line by transfection of a functional p53 gene. Cell. 1984 Aug;38(1):119–126. doi: 10.1016/0092-8674(84)90532-4. [DOI] [PubMed] [Google Scholar]
- Zakut-Houri R., Oren M., Bienz B., Lavie V., Hazum S., Givol D. A single gene and a pseudogene for the cellular tumour antigen p53. Nature. 1983 Dec 8;306(5943):594–597. doi: 10.1038/306594a0. [DOI] [PubMed] [Google Scholar]




