Abstract
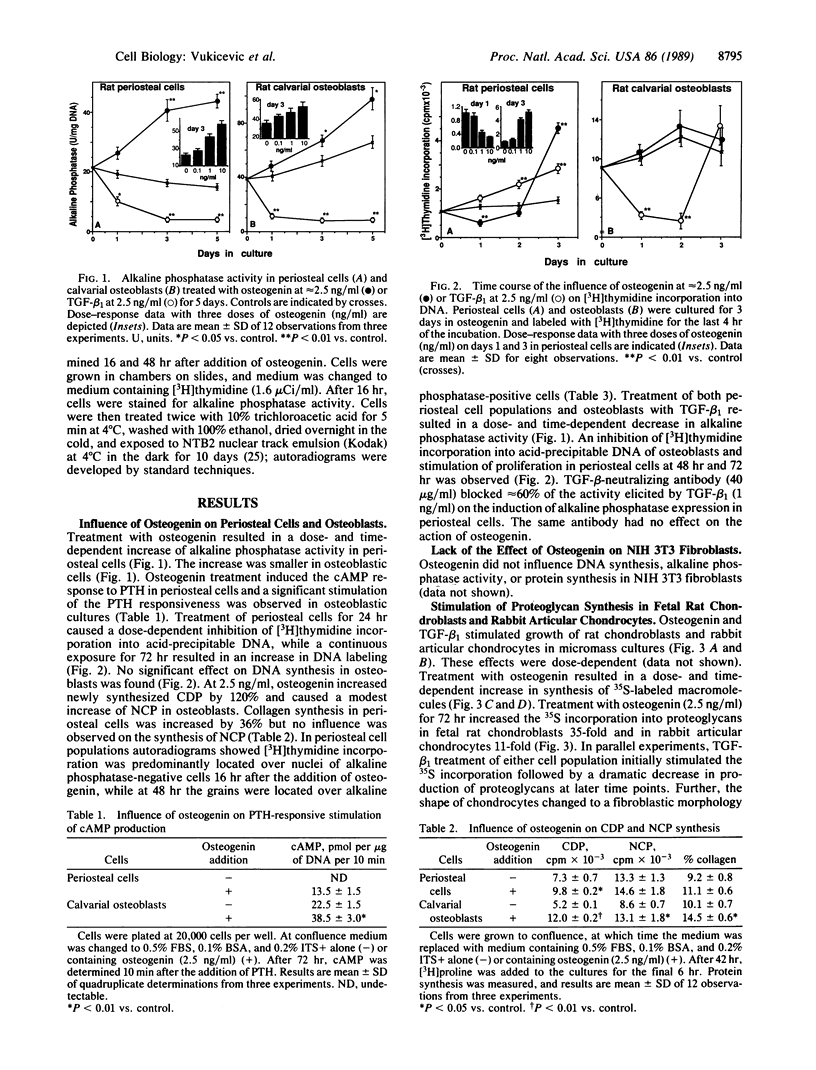
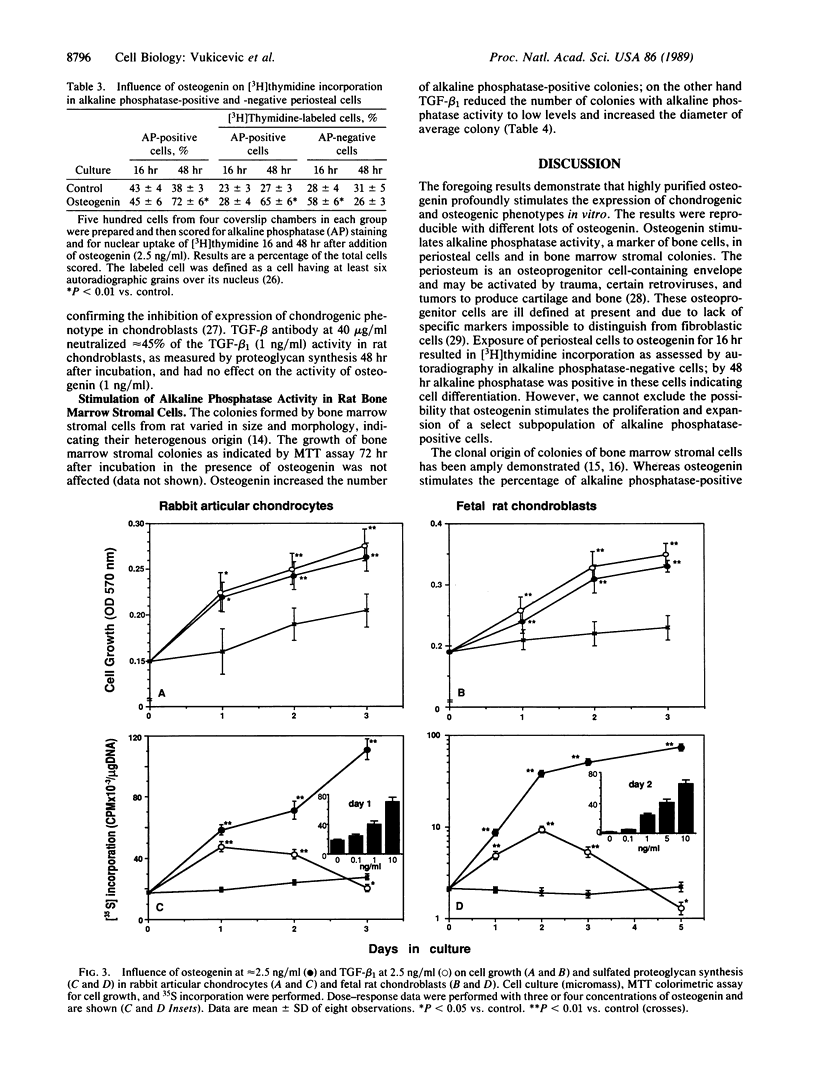
Osteogenin was recently purified and the amino acid sequences of tryptic peptides were determined. Osteogenin in conjunction with insoluble collagenous bone matrix induces cartilage and bone formation in vivo. To understand the mechanism of action of osteogenin, we examined its influence on periosteal cells, osteoblasts, fibroblasts, chondrocytes, and bone marrow stromal cells in vitro. Osteogenin stimulated alkaline phosphatase activity and collagen synthesis in periosteal cells. The cAMP response to parathyroid hormone in periosteal cells was increased by osteogenin. In primary cultures of calvarial osteoblasts, osteogenin stimulated alkaline phosphatase activity, the cAMP response to parathyroid hormone, and the synthesis of collagenous and noncollagenous proteins; however, cell proliferation was not affected. Osteogenin increased the production of sulfated proteoglycans in fetal rat chondroblasts and in rabbit articular chondrocytes. The present experiments demonstrate the significant influence of osteogenin in the stimulation of osteogenic and chondrogenic phenotypes in vitro.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bellows C. G., Aubin J. E., Heersche J. N., Antosz M. E. Mineralized bone nodules formed in vitro from enzymatically released rat calvaria cell populations. Calcif Tissue Int. 1986 Mar;38(3):143–154. doi: 10.1007/BF02556874. [DOI] [PubMed] [Google Scholar]
- Ecarot-Charrier B., Glorieux F. H., van der Rest M., Pereira G. Osteoblasts isolated from mouse calvaria initiate matrix mineralization in culture. J Cell Biol. 1983 Mar;96(3):639–643. doi: 10.1083/jcb.96.3.639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedenstein A. J., Chailakhyan R. K., Gerasimov U. V. Bone marrow osteogenic stem cells: in vitro cultivation and transplantation in diffusion chambers. Cell Tissue Kinet. 1987 May;20(3):263–272. doi: 10.1111/j.1365-2184.1987.tb01309.x. [DOI] [PubMed] [Google Scholar]
- Gerstenfeld L. C., Chipman S. D., Kelly C. M., Hodgens K. J., Lee D. D., Landis W. J. Collagen expression, ultrastructural assembly, and mineralization in cultures of chicken embryo osteoblasts. J Cell Biol. 1988 Mar;106(3):979–989. doi: 10.1083/jcb.106.3.979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hefley T., Cushing J., Brand J. S. Enzymatic isolation of cells from bone: cytotoxic enzymes of bacterial collagenase. Am J Physiol. 1981 May;240(5):C234–C238. doi: 10.1152/ajpcell.1981.240.5.C234. [DOI] [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Luyten F. P., Cunningham N. S., Ma S., Muthukumaran N., Hammonds R. G., Nevins W. B., Woods W. I., Reddi A. H. Purification and partial amino acid sequence of osteogenin, a protein initiating bone differentiation. J Biol Chem. 1989 Aug 15;264(23):13377–13380. [PubMed] [Google Scholar]
- Luyten F. P., Hascall V. C., Nissley S. P., Morales T. I., Reddi A. H. Insulin-like growth factors maintain steady-state metabolism of proteoglycans in bovine articular cartilage explants. Arch Biochem Biophys. 1988 Dec;267(2):416–425. doi: 10.1016/0003-9861(88)90047-1. [DOI] [PubMed] [Google Scholar]
- Majeska R. J., Rodan G. A. Alkaline phosphatase inhibition by parathyroid hormone and isoproterenol in a clonal rat osteosarcoma cell line. Possible mediation by cyclic AMP. Calcif Tissue Int. 1982 Jan;34(1):59–66. doi: 10.1007/BF02411210. [DOI] [PubMed] [Google Scholar]
- McCarthy T. L., Centrella M., Canalis E. Further biochemical and molecular characterization of primary rat parietal bone cell cultures. J Bone Miner Res. 1988 Aug;3(4):401–408. doi: 10.1002/jbmr.5650030406. [DOI] [PubMed] [Google Scholar]
- McCarthy T. L., Centrella M., Canalis E. Regulatory effects of insulin-like growth factors I and II on bone collagen synthesis in rat calvarial cultures. Endocrinology. 1989 Jan;124(1):301–309. doi: 10.1210/endo-124-1-301. [DOI] [PubMed] [Google Scholar]
- Miller S. C., Bowman B. M. Medullary bone osteogenesis following estrogen administration to mature male Japanese quail. Dev Biol. 1981 Oct 15;87(1):52–63. doi: 10.1016/0012-1606(81)90060-9. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Muthukumaran N., Ma S., Reddi A. H. Dose-dependence of and threshold for optimal bone induction by collagenous bone matrix and osteogenin-enriched fraction. Coll Relat Res. 1988 Sep;8(5):433–441. doi: 10.1016/s0174-173x(88)80016-5. [DOI] [PubMed] [Google Scholar]
- Nijweide P. J., Mulder R. J. Identification of osteocytes in osteoblast-like cell cultures using a monoclonal antibody specifically directed against osteocytes. Histochemistry. 1986;84(4-6):342–347. doi: 10.1007/BF00482961. [DOI] [PubMed] [Google Scholar]
- Nijweide P. J., van Iperen-van Gent A. S., Kawilarang-de Haas E. W., van der Plas A., Wassenaar A. M. Bone formation and calcification by isolated osteoblastlike cells. J Cell Biol. 1982 May;93(2):318–323. doi: 10.1083/jcb.93.2.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijweide P. J., van der Plas A., Scherft J. P. Biochemical and histological studies on various bone cell preparations. Calcif Tissue Int. 1981;33(5):529–540. doi: 10.1007/BF02409485. [DOI] [PubMed] [Google Scholar]
- Owen M. E., Cavé J., Joyner C. J. Clonal analysis in vitro of osteogenic differentiation of marrow CFU-F. J Cell Sci. 1987 Jun;87(Pt 5):731–738. doi: 10.1242/jcs.87.5.731. [DOI] [PubMed] [Google Scholar]
- Peterkofsky B., Diegelmann R. Use of a mixture of proteinase-free collagenases for the specific assay of radioactive collagen in the presence of other proteins. Biochemistry. 1971 Mar 16;10(6):988–994. doi: 10.1021/bi00782a009. [DOI] [PubMed] [Google Scholar]
- Rath N. C., Reddi A. H. Collagenous bone matrix is a local mitogen. Nature. 1979 Apr 26;278(5707):855–857. doi: 10.1038/278855a0. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Anderson W. A. Collagenous bone matrix-induced endochondral ossification hemopoiesis. J Cell Biol. 1976 Jun;69(3):557–572. doi: 10.1083/jcb.69.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. B. Hormone-dependent haematopoiesis in fibroblast-transformation ossicles. Nature. 1976 Oct 7;263(5577):514–515. doi: 10.1038/263514a0. [DOI] [PubMed] [Google Scholar]
- Reddi A. H., Huggins C. Biochemical sequences in the transformation of normal fibroblasts in adolescent rats. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1601–1605. doi: 10.1073/pnas.69.6.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodan S. B., Wesolowski G., Thomas K., Rodan G. A. Growth stimulation of rat calvaria osteoblastic cells by acidic fibroblast growth factor. Endocrinology. 1987 Dec;121(6):1917–1923. doi: 10.1210/endo-121-6-1917. [DOI] [PubMed] [Google Scholar]
- Rosen D. M., Stempien S. A., Thompson A. Y., Seyedin S. M. Transforming growth factor-beta modulates the expression of osteoblast and chondroblast phenotypes in vitro. J Cell Physiol. 1988 Mar;134(3):337–346. doi: 10.1002/jcp.1041340304. [DOI] [PubMed] [Google Scholar]
- Sampath T. K., Muthukumaran N., Reddi A. H. Isolation of osteogenin, an extracellular matrix-associated, bone-inductive protein, by heparin affinity chromatography. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7109–7113. doi: 10.1073/pnas.84.20.7109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott B. L. Thymidine-3H electron microscope radioautography of osteogenic cells in the fetal rat. J Cell Biol. 1967 Oct;35(1):115–126. doi: 10.1083/jcb.35.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmons D. J., Kent G. N., Jilka R. L., Scott D. M., Fallon M., Cohn D. V. Formation of bone by isolated, cultured osteoblasts in millipore diffusion chambers. Calcif Tissue Int. 1982 May;34(3):291–294. doi: 10.1007/BF02411253. [DOI] [PubMed] [Google Scholar]
- Urist M. R. Bone: formation by autoinduction. Science. 1965 Nov 12;150(3698):893–899. doi: 10.1126/science.150.3698.893. [DOI] [PubMed] [Google Scholar]
- Vukicević S., Marusić A., Stavljenić A., Cicak N., Vogel M., Krempien B. Talc granulomatosis in the rat: the relationship between osteoblast insufficiency and adjacent bone marrow hyperplasia. Exp Hematol. 1988 Oct;16(9):735–740. [PubMed] [Google Scholar]
- Wong G. L., Cohn D. V. Target cells in bone for parathormone and calcitonin are different: enrichment for each cell type by sequential digestion of mouse calvaria and selective adhesion to polymeric surfaces. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3167–3171. doi: 10.1073/pnas.72.8.3167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wozney J. M., Rosen V., Celeste A. J., Mitsock L. M., Whitters M. J., Kriz R. W., Hewick R. M., Wang E. A. Novel regulators of bone formation: molecular clones and activities. Science. 1988 Dec 16;242(4885):1528–1534. doi: 10.1126/science.3201241. [DOI] [PubMed] [Google Scholar]
- Włodarski K. H. Normal and heterotopic periosteum. Clin Orthop Relat Res. 1989 Apr;(241):265–277. [PubMed] [Google Scholar]
- Yanagishita M., Midura R. J., Hascall V. C. Proteoglycans: isolation and purification from tissue cultures. Methods Enzymol. 1987;138:279–289. doi: 10.1016/0076-6879(87)38023-1. [DOI] [PubMed] [Google Scholar]
- Zanetti N. C., Solursh M. Induction of chondrogenesis in limb mesenchymal cultures by disruption of the actin cytoskeleton. J Cell Biol. 1984 Jul;99(1 Pt 1):115–123. doi: 10.1083/jcb.99.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]


