Abstract
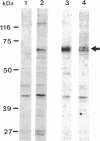
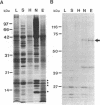
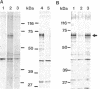
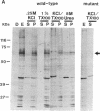
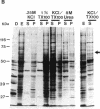
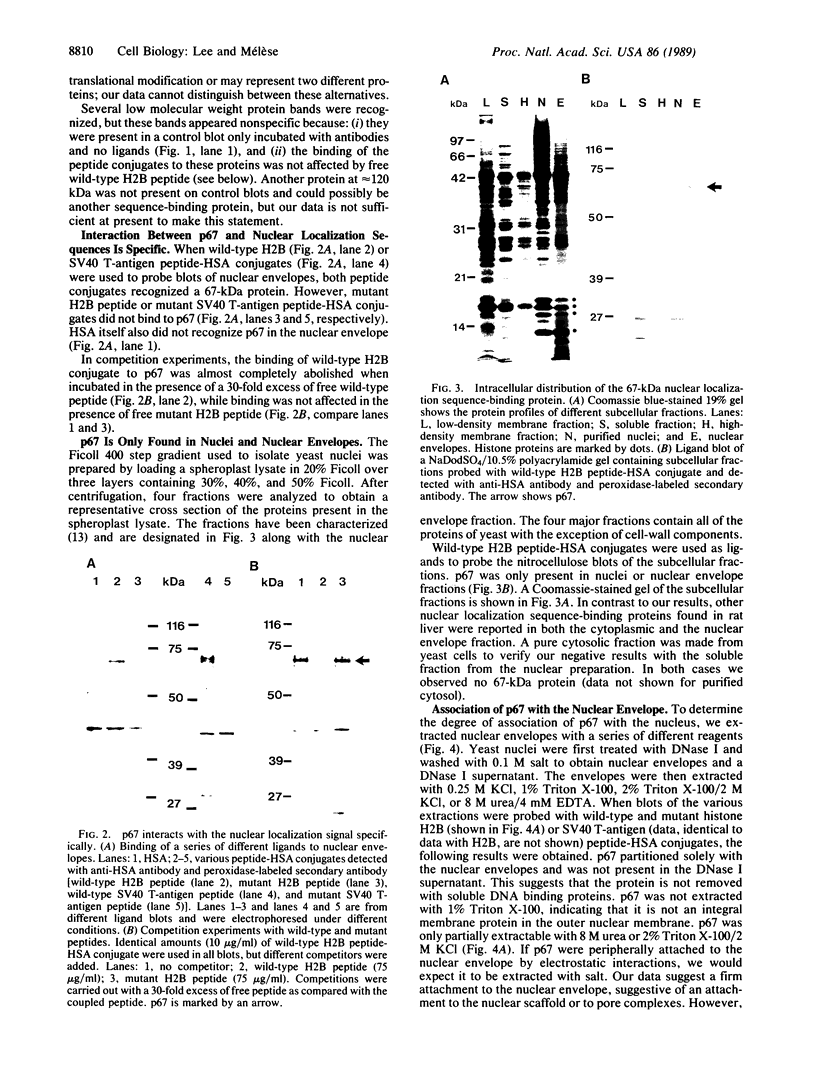
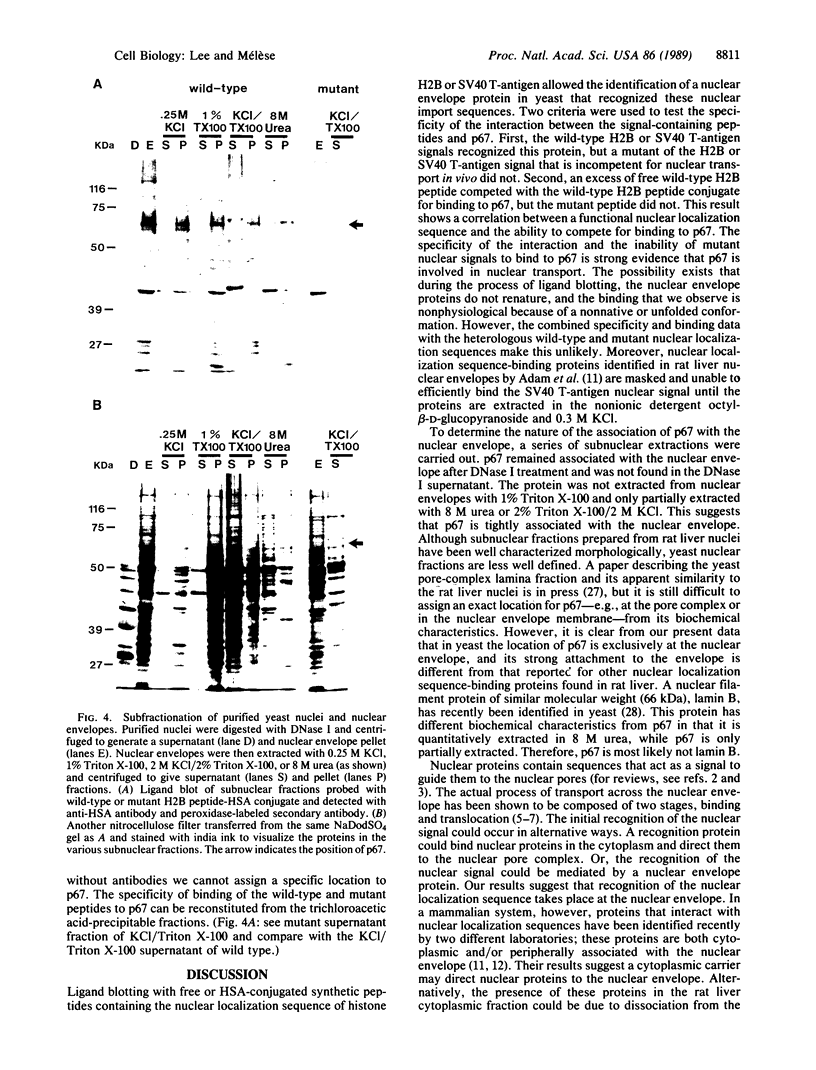
Nuclear proteins contain specific regions that are required for entry into the nucleus. Using ligand blotting, we have shown that a 67-kDa yeast nuclear envelope protein (p67) recognizes synthetic peptides containing the yeast histone H2B or simian virus 40 large tumor antigen nuclear localization sequence. Both free peptide and peptide conjugated to human serum albumin are recognized. The interaction between p67 and the nuclear localization sequences is specific; neither a mutant peptide that is incompetent for nuclear transport in vivo nor HSA can interact with p67 on blots. Moreover, although the wild-type peptide competes for binding to p67, the mutant peptides do not. p67 appears to be located at the nuclear envelope and is not present in other subcellular fractions. The nuclear localization sequence-binding protein is not extracted from the nuclear envelope with nonionic detergents and only partially extracted with high-salt buffer or 8 M urea, suggestive of a tight association with the nuclear envelope. Together our results are consistent with a role for p67 in nuclear transport.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adam S. A., Lobl T. J., Mitchell M. A., Gerace L. Identification of specific binding proteins for a nuclear location sequence. Nature. 1989 Jan 19;337(6204):276–279. doi: 10.1038/337276a0. [DOI] [PubMed] [Google Scholar]
- Aris J. P., Blobel G. Identification and characterization of a yeast nucleolar protein that is similar to a rat liver nucleolar protein. J Cell Biol. 1988 Jul;107(1):17–31. doi: 10.1083/jcb.107.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker D., Hicke L., Rexach M., Schleyer M., Schekman R. Reconstitution of SEC gene product-dependent intercompartmental protein transport. Cell. 1988 Jul 29;54(3):335–344. doi: 10.1016/0092-8674(88)90196-1. [DOI] [PubMed] [Google Scholar]
- Bowen B., Steinberg J., Laemmli U. K., Weintraub H. The detection of DNA-binding proteins by protein blotting. Nucleic Acids Res. 1980 Jan 11;8(1):1–20. doi: 10.1093/nar/8.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chelsky D., Ralph R., Jonak G. Sequence requirements for synthetic peptide-mediated translocation to the nucleus. Mol Cell Biol. 1989 Jun;9(6):2487–2492. doi: 10.1128/mcb.9.6.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher P. A., Berrios M., Blobel G. Isolation and characterization of a proteinaceous subnuclear fraction composed of nuclear matrix, peripheral lamina, and nuclear pore complexes from embryos of Drosophila melanogaster. J Cell Biol. 1982 Mar;92(3):674–686. doi: 10.1083/jcb.92.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Maroulakou I., Blobel G. Lamin A, lamin B, and lamin B receptor analogues in yeast. J Cell Biol. 1989 Jun;108(6):2069–2082. doi: 10.1083/jcb.108.6.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgatos S. D., Weber K., Geisler N., Blobel G. Binding of two desmin derivatives to the plasma membrane and the nuclear envelope of avian erythrocytes: evidence for a conserved site-specificity in intermediate filament-membrane interactions. Proc Natl Acad Sci U S A. 1987 Oct;84(19):6780–6784. doi: 10.1073/pnas.84.19.6780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerace L., Burke B. Functional organization of the nuclear envelope. Annu Rev Cell Biol. 1988;4:335–374. doi: 10.1146/annurev.cb.04.110188.002003. [DOI] [PubMed] [Google Scholar]
- Goldfarb D. S., Gariépy J., Schoolnik G., Kornberg R. D. Synthetic peptides as nuclear localization signals. Nature. 1986 Aug 14;322(6080):641–644. doi: 10.1038/322641a0. [DOI] [PubMed] [Google Scholar]
- Green N., Alexander H., Olson A., Alexander S., Shinnick T. M., Sutcliffe J. G., Lerner R. A. Immunogenic structure of the influenza virus hemagglutinin. Cell. 1982 Mar;28(3):477–487. doi: 10.1016/0092-8674(82)90202-1. [DOI] [PubMed] [Google Scholar]
- Jentoft N., Dearborn D. G. Labeling of proteins by reductive methylation using sodium cyanoborohydride. J Biol Chem. 1979 Jun 10;254(11):4359–4365. [PubMed] [Google Scholar]
- Kalderon D., Richardson W. D., Markham A. F., Smith A. E. Sequence requirements for nuclear location of simian virus 40 large-T antigen. Nature. 1984 Sep 6;311(5981):33–38. doi: 10.1038/311033a0. [DOI] [PubMed] [Google Scholar]
- Kalderon D., Roberts B. L., Richardson W. D., Smith A. E. A short amino acid sequence able to specify nuclear location. Cell. 1984 Dec;39(3 Pt 2):499–509. doi: 10.1016/0092-8674(84)90457-4. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lanford R. E., Kanda P., Kennedy R. C. Induction of nuclear transport with a synthetic peptide homologous to the SV40 T antigen transport signal. Cell. 1986 Aug 15;46(4):575–582. doi: 10.1016/0092-8674(86)90883-4. [DOI] [PubMed] [Google Scholar]
- Markland W., Smith A. E., Roberts B. L. Signal-dependent translocation of simian virus 40 large-T antigen into rat liver nuclei in a cell-free system. Mol Cell Biol. 1987 Dec;7(12):4255–4265. doi: 10.1128/mcb.7.12.4255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreland R. B., Langevin G. L., Singer R. H., Garcea R. L., Hereford L. M. Amino acid sequences that determine the nuclear localization of yeast histone 2B. Mol Cell Biol. 1987 Nov;7(11):4048–4057. doi: 10.1128/mcb.7.11.4048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson M., Silver P. Context affects nuclear protein localization in Saccharomyces cerevisiae. Mol Cell Biol. 1989 Feb;9(2):384–389. doi: 10.1128/mcb.9.2.384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newmeyer D. D., Forbes D. J. Nuclear import can be separated into distinct steps in vitro: nuclear pore binding and translocation. Cell. 1988 Mar 11;52(5):641–653. doi: 10.1016/0092-8674(88)90402-3. [DOI] [PubMed] [Google Scholar]
- Newmeyer D. D., Lucocq J. M., Bürglin T. R., De Robertis E. M. Assembly in vitro of nuclei active in nuclear protein transport: ATP is required for nucleoplasmin accumulation. EMBO J. 1986 Mar;5(3):501–510. doi: 10.1002/j.1460-2075.1986.tb04239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newport J. W., Forbes D. J. The nucleus: structure, function, and dynamics. Annu Rev Biochem. 1987;56:535–565. doi: 10.1146/annurev.bi.56.070187.002535. [DOI] [PubMed] [Google Scholar]
- Paine P. L., Moore L. C., Horowitz S. B. Nuclear envelope permeability. Nature. 1975 Mar 13;254(5496):109–114. doi: 10.1038/254109a0. [DOI] [PubMed] [Google Scholar]
- Richardson W. D., Mills A. D., Dilworth S. M., Laskey R. A., Dingwall C. Nuclear protein migration involves two steps: rapid binding at the nuclear envelope followed by slower translocation through nuclear pores. Cell. 1988 Mar 11;52(5):655–664. doi: 10.1016/0092-8674(88)90403-5. [DOI] [PubMed] [Google Scholar]
- Silver P., Sadler I., Osborne M. A. Yeast proteins that recognize nuclear localization sequences. J Cell Biol. 1989 Sep;109(3):983–989. doi: 10.1083/jcb.109.3.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki L., Kanda P., Lanford R. E. Identification of four nuclear transport signal-binding proteins that interact with diverse transport signals. Mol Cell Biol. 1989 Jul;9(7):3028–3036. doi: 10.1128/mcb.9.7.3028. [DOI] [PMC free article] [PubMed] [Google Scholar]