Abstract
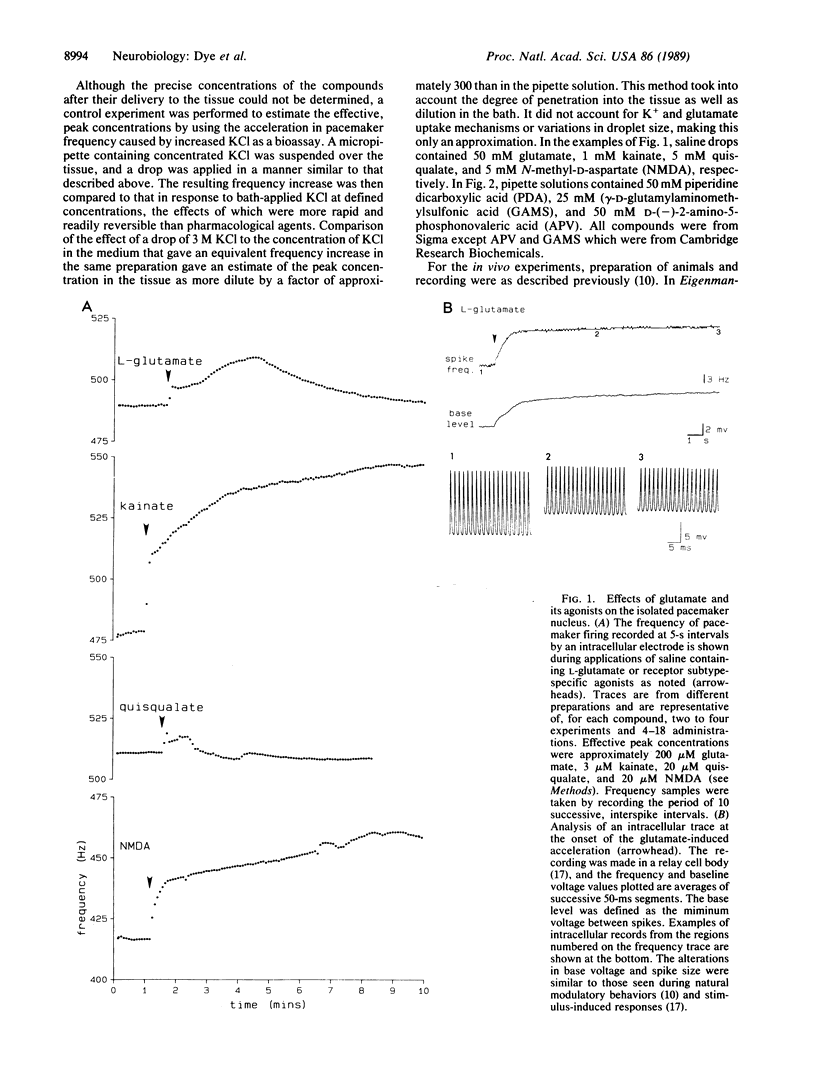
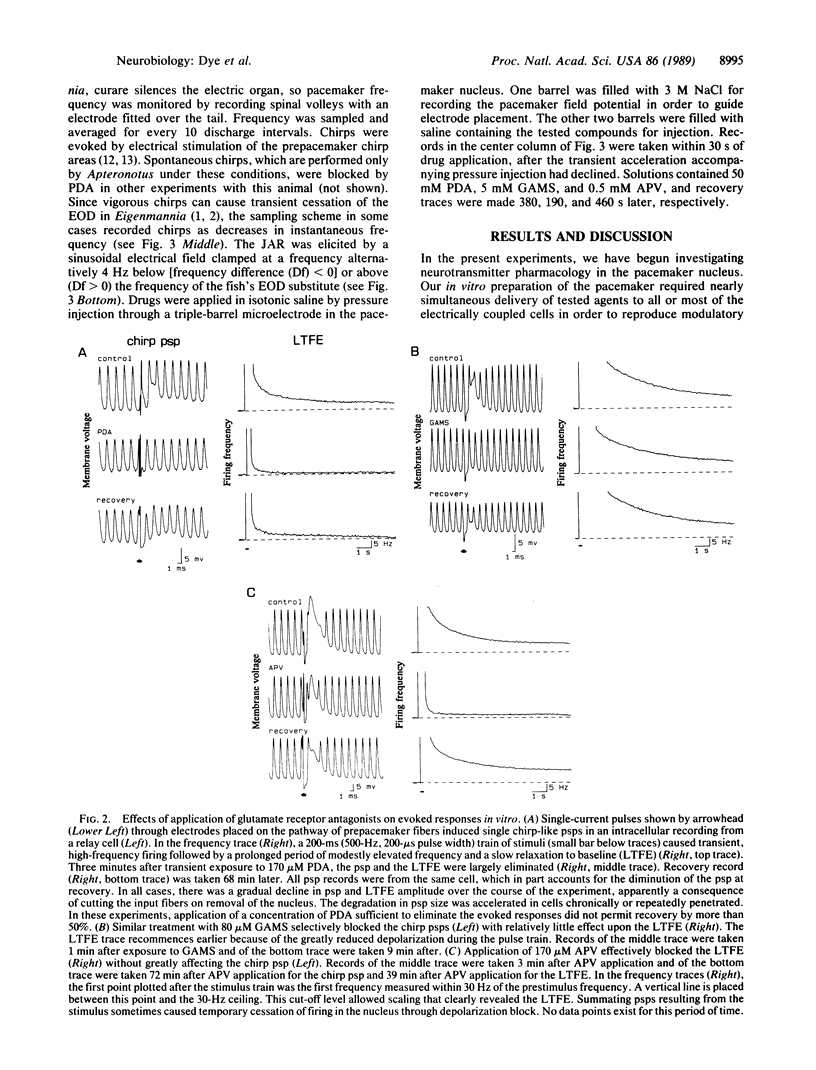
We have taken advantage of the increasing understanding of glutamate neuropharmacology to probe mechanisms of well-defined vertebrate behaviors. Here we report a set of experiments that suggests distinct roles for two major classes of glutamate receptors in a discrete premotor nucleus of the brainstem. The medullary pacemaker nucleus of weakly electric fish is an endogenous oscillator that controls the electric organ discharge (EOD). Its regular frequency of firing is modulated during several distinct behaviors. The pacemaker nucleus continues firing regularly when isolated in vitro, and modulatory behaviors can be reproduced by stimulating the descending input pathway. Glutamate agonists applied to the pacemaker in vitro produced increases in frequency, while glutamate antagonists selectively blocked stimulus-induced modulations. Experiments with glutamate antagonists in the intact animal resulted in specific effects on two well-characterized behaviors. Our data indicate that these behaviors are separately mediated in the pacemaker by receptors displaying characteristics of the kainate/quisqualate and N-methyl-D-aspartate subtypes of glutamate receptor, respectively.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dye J. An in vitro physiological preparation of a vertebrate communicatory behavior: chirping in the weakly electric fish, Apteronotus. J Comp Physiol A. 1988 Aug;163(4):445–458. doi: 10.1007/BF00604899. [DOI] [PubMed] [Google Scholar]
- Dye J. Dynamics and stimulus-dependence of pacemaker control during behavioral modulations in the weakly electric fish, Apteronotus. J Comp Physiol A. 1987 Aug;161(2):175–185. doi: 10.1007/BF00615239. [DOI] [PubMed] [Google Scholar]
- Dye J., Heiligenberg W. Intracellular recording in the medullary pacemaker nucleus of the weakly electric fish, Apteronotus, during modulatory behaviors. J Comp Physiol A. 1987 Aug;161(2):187–200. doi: 10.1007/BF00615240. [DOI] [PubMed] [Google Scholar]
- Elekes K., Szabo T. Synaptology of the medullary command (pacemaker) nucleus of the weakly electric fish (Apteronotus leptorhynchus) with particular reference to comparative aspects. Exp Brain Res. 1985;60(3):509–520. doi: 10.1007/BF00236936. [DOI] [PubMed] [Google Scholar]
- Heiligenberg W., Finger T., Matsubara J., Carr C. Input to the medullary pacemaker nucleus in the weakly electric fish, Eigenmannia (sternopygidae, gymnotiformes). Brain Res. 1981 May 4;211(2):418–423. doi: 10.1016/0006-8993(81)90966-5. [DOI] [PubMed] [Google Scholar]
- Hopkins C. D. Neuroethology of electric communication. Annu Rev Neurosci. 1988;11:497–535. doi: 10.1146/annurev.ne.11.030188.002433. [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Heiligenberg W. Individual prepacemaker neurons can modulate the pacemaker cycle of the gymnotiform electric fish, Eigenmannia. J Comp Physiol A. 1988 Jan;162(1):13–21. doi: 10.1007/BF01342699. [DOI] [PubMed] [Google Scholar]
- Kawasaki M., Maler L., Rose G. J., Heiligenberg W. Anatomical and functional organization of the prepacemaker nucleus in gymnotiform electric fish: the accommodation of two behaviors in one nucleus. J Comp Neurol. 1988 Oct 1;276(1):113–131. doi: 10.1002/cne.902760108. [DOI] [PubMed] [Google Scholar]
- Mayer M. L., Westbrook G. L. The physiology of excitatory amino acids in the vertebrate central nervous system. Prog Neurobiol. 1987;28(3):197–276. doi: 10.1016/0301-0082(87)90011-6. [DOI] [PubMed] [Google Scholar]
- Rose G. J., Kawasaki M., Heiligenberg W. 'Recognition units' at the top of a neuronal hierarchy? Prepacemaker neurons in Eigenmannia code the sign of frequency differences unambiguously. J Comp Physiol A. 1988 Apr;162(6):759–772. doi: 10.1007/BF00610965. [DOI] [PubMed] [Google Scholar]
- Tokunaga A., Akert K., Sandri C., Bennett M. V. Cell types and synaptic organization of the medullary electromotor nucleus in a constant frequency weakly electric fish, Sternarchus albifrons. J Comp Neurol. 1980 Aug 1;192(3):407–426. doi: 10.1002/cne.901920304. [DOI] [PubMed] [Google Scholar]
- Watkins J. C., Evans R. H. Excitatory amino acid transmitters. Annu Rev Pharmacol Toxicol. 1981;21:165–204. doi: 10.1146/annurev.pa.21.040181.001121. [DOI] [PubMed] [Google Scholar]
- Zaczek R., Balm M., Arlis S., Drucker H., Coyle J. T. Quisqualate-sensitive, chloride-dependent transport of glutamate into rat brain synaptosomes. J Neurosci Res. 1987;18(3):425–431. doi: 10.1002/jnr.490180307. [DOI] [PubMed] [Google Scholar]


