Abstract
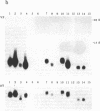
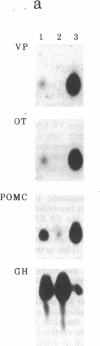
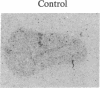
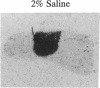
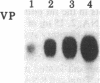
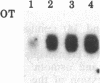
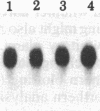
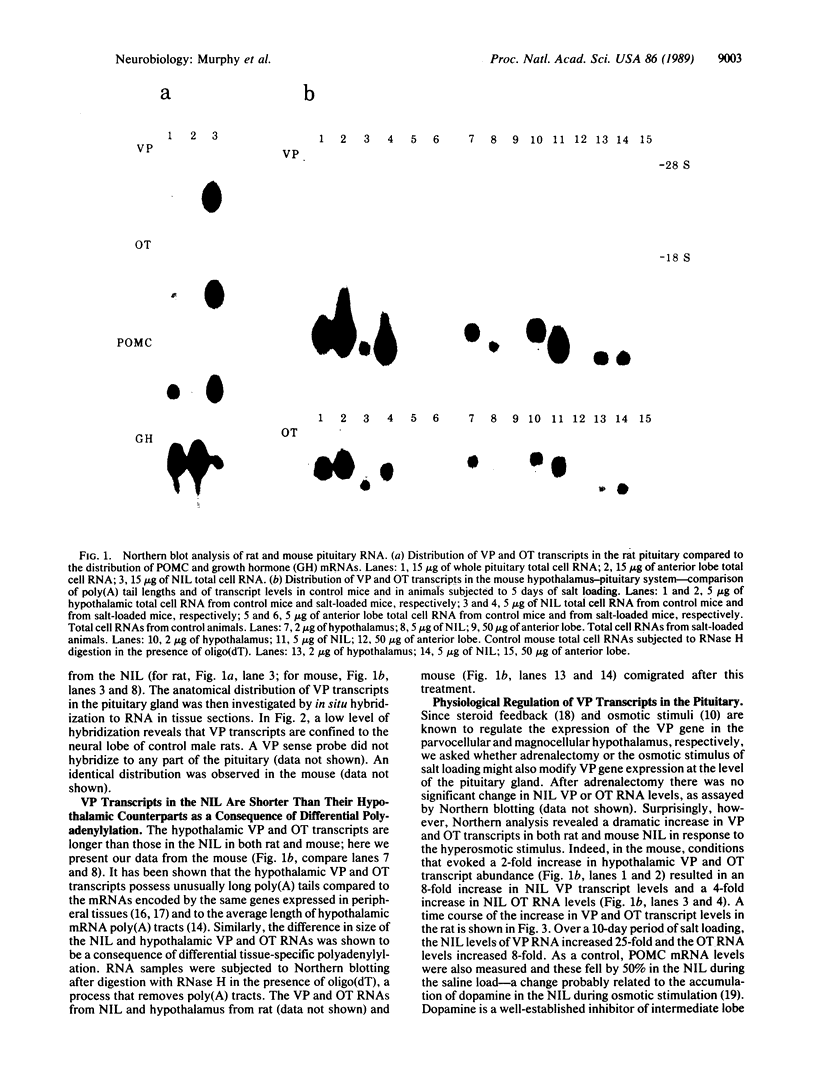
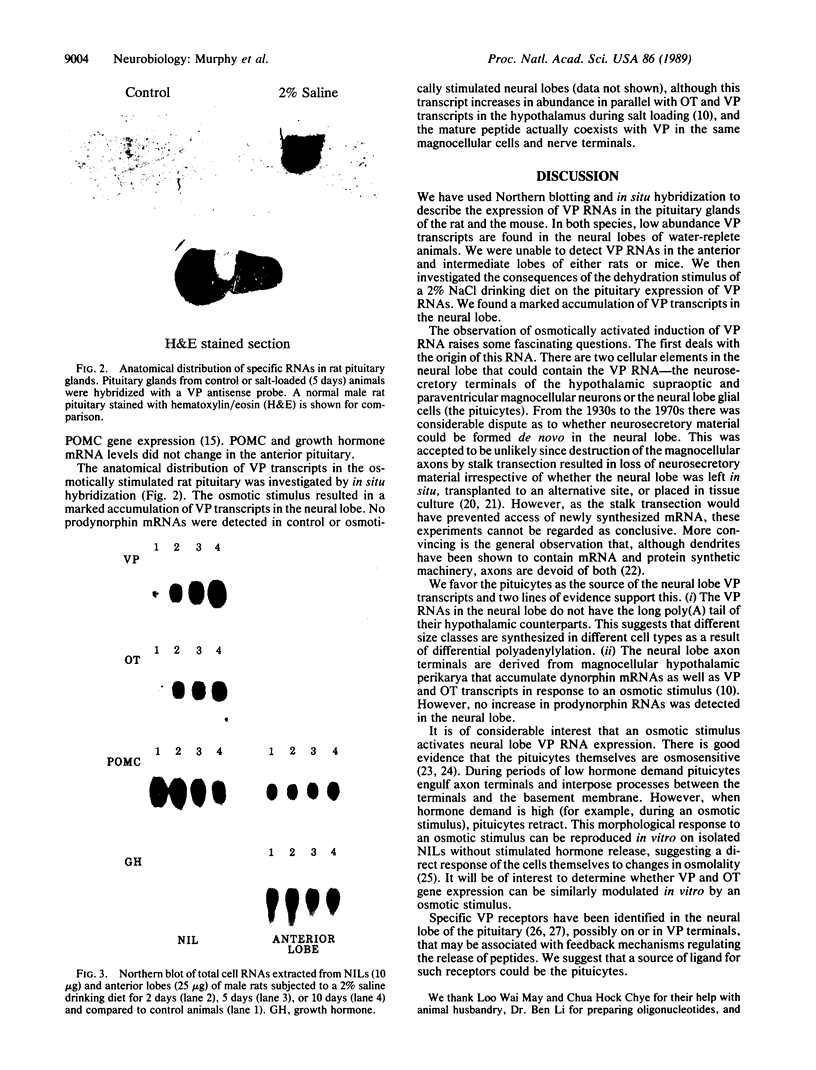
The peptides vasopressin (VP) and oxytocin are derived from preprohormone precursers encoded by highly homologous linked genes that are expressed in discrete groups of hypothalamic neurons. The mature hormones are released into the peripheral circulation from the neural (posterior) lobe of the pituitary and have also been implicated in the regulation of anterior lobe. We have used Northern blotting and in situ hybridization to RNA in tissue sections to describe the presence, anatomical localization, and regulation of VP and oxytocin RNAs in the pituitary gland itself. We were unable to detect VP transcripts in the anterior and intermediate lobes of the pituitary. Rather we found low levels of VP RNA in the neural lobe. Furthermore, the osmotic stimulation of a 2% (wt/vol) NaCl drinking diet resulted in a marked accumulation of VP RNA in the neural lobe. We suggest that VP, locally synthesized in pituicytes, may have paracrine effects on VP receptors in the neural lobe.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bunn S. J., Hanley M. R., Wilkin G. P. Autoradiographic localization of peripheral benzodiazepine, dihydroalprenolol and arginine vasopressin binding sites in the pituitaries of control, stalk transected and Brattleboro rats. Neuroendocrinology. 1986;44(1):76–83. doi: 10.1159/000124625. [DOI] [PubMed] [Google Scholar]
- Carrazana E. J., Pasieka K. B., Majzoub J. A. The vasopressin mRNA poly(A) tract is unusually long and increases during stimulation of vasopressin gene expression in vivo. Mol Cell Biol. 1988 Jun;8(6):2267–2274. doi: 10.1128/mcb.8.6.2267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashwood M. R., Robinson I. C. Specificity of vasopressin binding to the posterior pituitary gland in the rat. An autoradiographic study. Neuroendocrinology. 1988 Aug;48(2):180–187. doi: 10.1159/000125013. [DOI] [PubMed] [Google Scholar]
- Dellmann H. D. Degeneration and regeneration of neurosecretory systems. Int Rev Cytol. 1973;36:215–315. doi: 10.1016/s0074-7696(08)60219-3. [DOI] [PubMed] [Google Scholar]
- Eckland D. J., Todd K., Lightman S. L. Immunoreactive vasopressin and oxytocin in hypothalamo-hypophysial portal blood of the Brattleboro and Long-Evans rat: effect of adrenalectomy and dexamethasone. J Endocrinol. 1988 Apr;117(1):27–34. doi: 10.1677/joe.0.1170027. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Vogelstein B. A technique for radiolabeling DNA restriction endonuclease fragments to high specific activity. Anal Biochem. 1983 Jul 1;132(1):6–13. doi: 10.1016/0003-2697(83)90418-9. [DOI] [PubMed] [Google Scholar]
- Gillies G. E., Linton E. A., Lowry P. J. Corticotropin releasing activity of the new CRF is potentiated several times by vasopressin. Nature. 1982 Sep 23;299(5881):355–357. doi: 10.1038/299355a0. [DOI] [PubMed] [Google Scholar]
- Gordon-Weeks P. R. RNA transport in dendrites. Trends Neurosci. 1988 Aug;11(8):342–343. doi: 10.1016/0166-2236(88)90054-9. [DOI] [PubMed] [Google Scholar]
- HILD W. Histological and endocrinological observations in tissue cultures of posterior pituitary of dog and rat. Tex Rep Biol Med. 1954;12(3):474–488. [PubMed] [Google Scholar]
- Hatton G. I., Perlmutter L. S., Salm A. K., Tweedle C. D. Dynamic neuronal-glial interactions in hypothalamus and pituitary: implications for control of hormone synthesis and release. Peptides. 1984;5 (Suppl 1):121–138. doi: 10.1016/0196-9781(84)90271-7. [DOI] [PubMed] [Google Scholar]
- Ivell R., Richter D. The gene for the hypothalamic peptide hormone oxytocin is highly expressed in the bovine corpus luteum: biosynthesis, structure and sequence analysis. EMBO J. 1984 Oct;3(10):2351–2354. doi: 10.1002/j.1460-2075.1984.tb02139.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivell R., Schmale H., Krisch B., Nahke P., Richter D. Expression of a mutant vasopressin gene: differential polyadenylation and read-through of the mRNA 3' end in a frame-shift mutant. EMBO J. 1986 May;5(5):971–977. doi: 10.1002/j.1460-2075.1986.tb04311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy A., Lightman S. L. Quantitative in-situ hybridization histochemistry in the rat pituitary gland: effect of bromocriptine on prolactin and pro-opiomelanocortin gene expression. J Endocrinol. 1988 Aug;118(2):205–210. doi: 10.1677/joe.0.1180205. [DOI] [PubMed] [Google Scholar]
- Lightman S. L., Young W. S., 3rd Vasopressin, oxytocin, dynorphin, enkephalin and corticotrophin-releasing factor mRNA stimulation in the rat. J Physiol. 1987 Dec;394:23–39. doi: 10.1113/jphysiol.1987.sp016858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loh Y. P., Castro M. G., Zeng F. J., Patel-Vaidya U. Presence of pro-vasopressin mRNA, neurophysin and arginine vasopressin in mouse anterior pituitary cells and the AtT-20 corticotrophic tumour cell line. J Mol Endocrinol. 1988 Jul;1(1):39–48. doi: 10.1677/jme.0.0010039. [DOI] [PubMed] [Google Scholar]
- Lolait S. J., Markwick A. J., McNally M., Abraham J., Smith A. I., Funder J. W. Anterior pituitary cells from Brattleboro (di/di), Long-Evans and Sprague-Dawley rats contain immunoreactive arginine vasopressin. Neuroendocrinology. 1986;43(5):577–583. doi: 10.1159/000124584. [DOI] [PubMed] [Google Scholar]
- Nagy G., Mulchahey J. J., Smyth D. G., Neill J. D. The glycopeptide moiety of vasopressin-neurophysin precursor is neurohypophysial prolactin releasing factor. Biochem Biophys Res Commun. 1988 Feb 29;151(1):524–529. doi: 10.1016/0006-291x(88)90625-0. [DOI] [PubMed] [Google Scholar]
- Palmiter R. D., Brinster R. L., Hammer R. E., Trumbauer M. E., Rosenfeld M. G., Birnberg N. C., Evans R. M. Dramatic growth of mice that develop from eggs microinjected with metallothionein-growth hormone fusion genes. Nature. 1982 Dec 16;300(5893):611–615. doi: 10.1038/300611a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlmutter L. S., Hatton G. I., Tweedle C. D. Plasticity in the in vitro neurohypophysis: effects of osmotic changes on pituicytes. Neuroscience. 1984 Jun;12(2):503–511. doi: 10.1016/0306-4522(84)90069-1. [DOI] [PubMed] [Google Scholar]
- Racké K., Holzbauer M., Cooper T. R., Sharman D. F. Dehydration increases the electrically evoked dopamine release from the neural and intermediate lobes of the rat hypophysis. Neuroendocrinology. 1986;43(1):6–11. doi: 10.1159/000124501. [DOI] [PubMed] [Google Scholar]
- Richter D. Molecular events in expression of vasopressin and oxytocin and their cognate receptors. Am J Physiol. 1988 Aug;255(2 Pt 2):F207–F219. doi: 10.1152/ajprenal.1988.255.2.F207. [DOI] [PubMed] [Google Scholar]
- Rivier C., Vale W. Interaction of corticotropin-releasing factor and arginine vasopressin on adrenocorticotropin secretion in vivo. Endocrinology. 1983 Sep;113(3):939–942. doi: 10.1210/endo-113-3-939. [DOI] [PubMed] [Google Scholar]
- Tweedle C. D., Hatton G. I. Morphological adaptability at neurosecretory axonal endings on the neurovascular contact zone of the rat neurohypophysis. Neuroscience. 1987 Jan;20(1):241–246. doi: 10.1016/0306-4522(87)90016-9. [DOI] [PubMed] [Google Scholar]
- Watkins W. B., Moore L. G., Spiess J. Characterization of neurophysin in the anterior pituitary gland of sheep. Neuroendocrinology. 1988 Feb;47(2):142–148. doi: 10.1159/000124904. [DOI] [PubMed] [Google Scholar]
- Whitfeld P. L., Seeburg P. H., Shine J. The human pro-opiomelanocortin gene: organization, sequence, and interspersion with repetitive DNA. DNA. 1982;1(2):133–143. doi: 10.1089/dna.1.1982.1.133. [DOI] [PubMed] [Google Scholar]
- Wolfson B., Manning R. W., Davis L. G., Arentzen R., Baldino F., Jr Co-localization of corticotropin releasing factor and vasopressin mRNA in neurones after adrenalectomy. Nature. 1985 May 2;315(6014):59–61. doi: 10.1038/315059a0. [DOI] [PubMed] [Google Scholar]