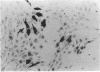
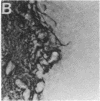
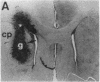
Abstract
Rat fibroblasts were infected with a retroviral vector containing the cDNA for rat tyrosine hydroxylase [TH; tyrosine 3-monooxygenase; L-tyrosine, tetrahydropteridine:oxygen oxidoreductase (3-hydroxylating), EC 1.14.16.2]. A TH-positive clone was identified by biochemical assay and immunohistochemical staining. When supplemented in vitro with pterin cofactors required for TH activity, these cells produced L-dopa and released it into the cell culture medium. Uninfected control cells and fibroblasts infected with the TH vector were grafted separately to the caudate of rats with unilateral 6-hydroxydopamine lesions of the nigrostriatal pathway. Only grafts containing TH-expressing fibroblasts were found to reduce rotational asymmetry. These results have general implications for the application of gene therapy to human neurological disease and specific implications for Parkinson disease.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson W. F. Prospects for human gene therapy. Science. 1984 Oct 26;226(4673):401–409. doi: 10.1126/science.6093246. [DOI] [PubMed] [Google Scholar]
- Becker J. B., Freed W. J. Adrenal medulla grafts enhance functional activity of the striatal dopamine system following substantia nigra lesions. Brain Res. 1988 Oct 18;462(2):401–406. doi: 10.1016/0006-8993(88)90573-2. [DOI] [PubMed] [Google Scholar]
- Brown E. R., Coker G. T., 3rd, O'Malley K. L. Organization and evolution of the rat tyrosine hydroxylase gene. Biochemistry. 1987 Aug 11;26(16):5208–5212. doi: 10.1021/bi00390a046. [DOI] [PubMed] [Google Scholar]
- Brundin P., Strecker R. E., Gage F. H., Lindvall O., Björklund A. Intracerebral transplantation of dopamine neurons: understanding the functional role of the mesolimbocortical dopamine system and developing a therapy for Parkinson's disease. Ann N Y Acad Sci. 1988;537:148–160. doi: 10.1111/j.1749-6632.1988.tb42103.x. [DOI] [PubMed] [Google Scholar]
- Dunnett S. B., Björklund A., Schmidt R. H., Stenevi U., Iversen S. D. Intracerebral grafting of neuronal cell suspensions. IV. Behavioural recovery in rats with unilateral 6-OHDA lesions following implantation of nigral cell suspensions in different forebrain sites. Acta Physiol Scand Suppl. 1983;522:29–37. [PubMed] [Google Scholar]
- Friedmann T. Progress toward human gene therapy. Science. 1989 Jun 16;244(4910):1275–1281. doi: 10.1126/science.2660259. [DOI] [PubMed] [Google Scholar]
- Gage F. H., Wolff J. A., Rosenberg M. B., Xu L., Yee J. K., Shults C., Friedmann T. Grafting genetically modified cells to the brain: possibilities for the future. Neuroscience. 1987 Dec;23(3):795–807. doi: 10.1016/0306-4522(87)90159-x. [DOI] [PubMed] [Google Scholar]
- Hefti F., Melamed E., Wurtman R. J. The site of dopamine formation in rat striatum after L-dopa administration. J Pharmacol Exp Ther. 1981 Apr;217(1):189–197. [PubMed] [Google Scholar]
- Herrera-Marschitz M., Strömberg I., Olsson D., Ungerstedt U., Olson L. Adrenal medullary implants in the dopamine-denervated rat striatum. II. Acute behavior as a function of graft amount and location and its modulation by neuroleptics. Brain Res. 1984 Apr 9;297(1):53–61. doi: 10.1016/0006-8993(84)90542-0. [DOI] [PubMed] [Google Scholar]
- Hornykiewicz O. The mechanisms of action of L-dopa in Parkinson's disease. Life Sci. 1974 Oct 1;15(7):1249–1259. doi: 10.1016/0024-3205(74)90306-3. [DOI] [PubMed] [Google Scholar]
- Iuvone P. M. Calcium, ATP, and magnesium activate soluble tyrosine hydroxylase from rat striatum. J Neurochem. 1984 Nov;43(5):1359–1368. doi: 10.1111/j.1471-4159.1984.tb05395.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewin R. Dramatic results with brain grafts. Science. 1987 Jul 17;237(4812):245–247. doi: 10.1126/science.3603019. [DOI] [PubMed] [Google Scholar]
- Lovenberg W., Levine R. A., Robinson D. S., Ebert M., Williams A. C., Calne D. B. Hydroxylase cofactor activity in cerebrospinal fluid of normal subjects and patients with Parkinson's disease. Science. 1979 May 11;204(4393):624–626. doi: 10.1126/science.432666. [DOI] [PubMed] [Google Scholar]
- Mann R., Mulligan R. C., Baltimore D. Construction of a retrovirus packaging mutant and its use to produce helper-free defective retrovirus. Cell. 1983 May;33(1):153–159. doi: 10.1016/0092-8674(83)90344-6. [DOI] [PubMed] [Google Scholar]
- Matson W. R., Gamache P. G., Beal M. F., Bird E. D. EC array sensor concepts and data. Life Sci. 1987 Aug 17;41(7):905–908. doi: 10.1016/0024-3205(87)90192-5. [DOI] [PubMed] [Google Scholar]
- Matson W. R., Langlais P., Volicer L., Gamache P. H., Bird E., Mark K. A. n-Electrode three-dimensional liquid chromatography with electrochemical detection for determination of neurotransmitters. Clin Chem. 1984 Sep;30(9):1477–1488. [PubMed] [Google Scholar]
- Melamed E., Hefti F. Mechanism of action of short- and long-term L-DOPA treatment in parkinsonism: role of the surviving nigrostriatal dopaminergic neurons. Adv Neurol. 1984;40:149–157. [PubMed] [Google Scholar]
- Miller A. D., Buttimore C. Redesign of retrovirus packaging cell lines to avoid recombination leading to helper virus production. Mol Cell Biol. 1986 Aug;6(8):2895–2902. doi: 10.1128/mcb.6.8.2895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Jolly D. J., Friedmann T., Verma I. M. A transmissible retrovirus expressing human hypoxanthine phosphoribosyltransferase (HPRT): gene transfer into cells obtained from humans deficient in HPRT. Proc Natl Acad Sci U S A. 1983 Aug;80(15):4709–4713. doi: 10.1073/pnas.80.15.4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perlow M. J. Brain grafting as a treatment for Parkinson's disease. Neurosurgery. 1987 Feb;20(2):335–342. doi: 10.1227/00006123-198702000-00026. [DOI] [PubMed] [Google Scholar]
- Quade K. Transformation of mammalian cells by avian myelocytomatosis virus and avian erythroblastosis virus. Virology. 1979 Oct 30;98(2):461–465. doi: 10.1016/0042-6822(79)90569-5. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. B., Friedmann T., Robertson R. C., Tuszynski M., Wolff J. A., Breakefield X. O., Gage F. H. Grafting genetically modified cells to the damaged brain: restorative effects of NGF expression. Science. 1988 Dec 16;242(4885):1575–1578. doi: 10.1126/science.3201248. [DOI] [PubMed] [Google Scholar]
- Schmidt R. H., Ingvar M., Lindvall O., Stenevi U., Björklund A. Functional activity of substantia nigra grafts reinnervating the striatum: neurotransmitter metabolism and [14C]2-deoxy-D-glucose autoradiography. J Neurochem. 1982 Mar;38(3):737–748. doi: 10.1111/j.1471-4159.1982.tb08693.x. [DOI] [PubMed] [Google Scholar]
- Sladek J. R., Jr, Gash D. M. Nerve-cell grafting in Parkinson's disease. J Neurosurg. 1988 Mar;68(3):337–351. doi: 10.3171/jns.1988.68.3.0337. [DOI] [PubMed] [Google Scholar]
- Sladek J. R., Jr, Shoulson I. Neural transplantation: a call for patience rather than patients. Science. 1988 Jun 10;240(4858):1386–1388. doi: 10.1126/science.3375820. [DOI] [PubMed] [Google Scholar]
- Smith I., Clayton B. E., Wolff O. H. Letter: A variant of phenylketonuria. Lancet. 1975 Feb 8;1(7902):328–329. doi: 10.1016/s0140-6736(75)91230-1. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Wigler M., Silverstein S., Lee L. S., Pellicer A., Cheng Y. c., Axel R. Transfer of purified herpes virus thymidine kinase gene to cultured mouse cells. Cell. 1977 May;11(1):223–232. doi: 10.1016/0092-8674(77)90333-6. [DOI] [PubMed] [Google Scholar]
- Xu L., Yee J. K., Wolff J. A., Friedmann T. Factors affecting long-term stability of Moloney murine leukemia virus-based vectors. Virology. 1989 Aug;171(2):331–341. doi: 10.1016/0042-6822(89)90600-4. [DOI] [PubMed] [Google Scholar]