Abstract
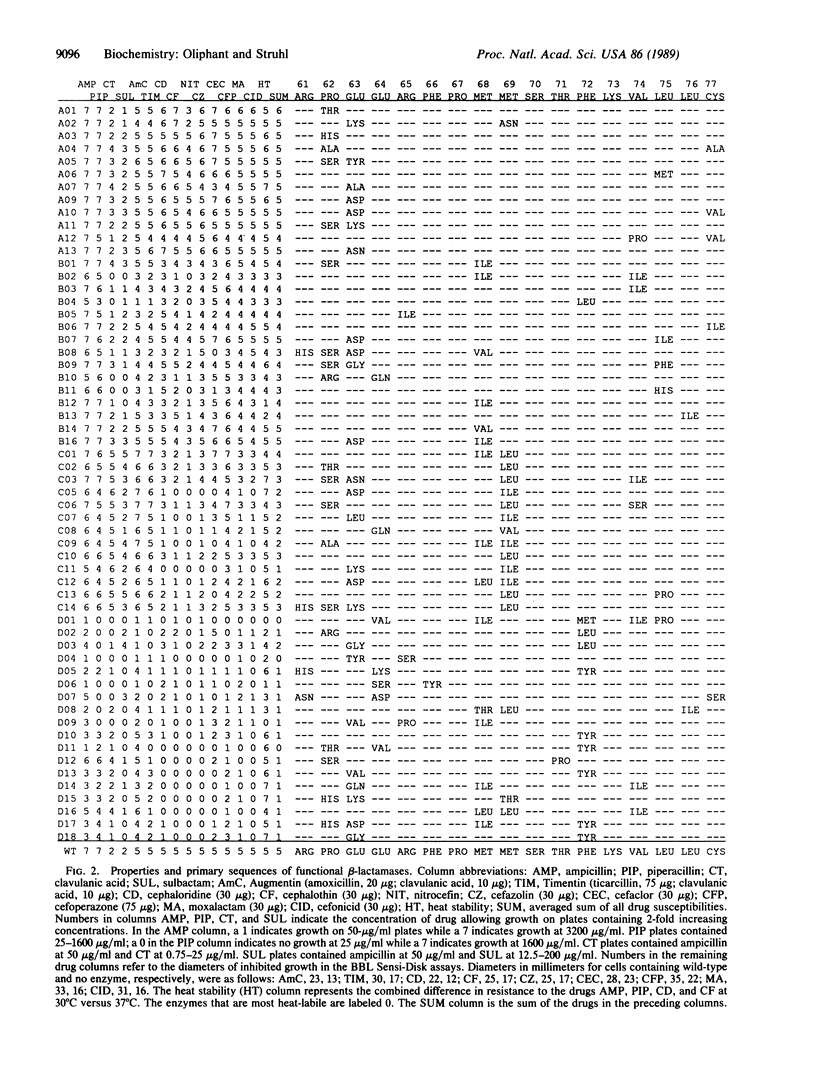
Random-sequence or highly degenerate oligonucleotides have been useful for defining functionally important sequences both in proteins and in nucleic acids. In this approach, such oligonucleotides are used to replace a segment of DNA required for a desired function, and functional sequences are identified by an appropriate genetic or biochemical selection. Here, a collection of 500,000 [corrected] altered beta-lactamase proteins was generated by cloning a mixed-base oligonucleotide in place of the sequences coding for a 17-amino acid portion of the enzyme's active site. Approximately 2000 enzymes from this collection were able to confer ampicillin resistance on Escherichia coli. Fifty-eight of these were chosen for further study after characterization with various beta-lactam substrates. beta-Lactamases having altered specificity against different antibiotics, resistance to the suicide inhibitors clavulanic acid and sulbactam, and temperature-dependent activities were obtained. The amino acid residues responsible for these altered properties as well as for basic enzyme activity are defined. This approach should prove to be an effective and general tool for creating proteins with novel properties, especially in situations in which a high-resolution structure of the protein is not known.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P. The structure of beta-lactamases. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):321–331. doi: 10.1098/rstb.1980.0049. [DOI] [PubMed] [Google Scholar]
- Brenner D. G., Knowles J. R. Penicillanic acid sulfone: nature of irreversible inactivation of RTEM beta-lactamase from Escherichia coli. Biochemistry. 1984 Nov 20;23(24):5833–5839. doi: 10.1021/bi00319a024. [DOI] [PubMed] [Google Scholar]
- Charnas R. L., Fisher J., Knowles J. R. Chemical studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2185–2189. doi: 10.1021/bi00604a025. [DOI] [PubMed] [Google Scholar]
- DeLucia M. L., Kelly J. A., Mangion M. M., Moews P. C., Knox J. R. Tertiary and secondary structure analysis of penicillin-binding proteins. Philos Trans R Soc Lond B Biol Sci. 1980 May 16;289(1036):374–376. [PubMed] [Google Scholar]
- Fisher J., Charnas R. L., Knowles J. R. Kinetic studies on the inactivation of Escherichia coli RTEM beta-lactamase by clavulanic acid. Biochemistry. 1978 May 30;17(11):2180–2184. doi: 10.1021/bi00604a024. [DOI] [PubMed] [Google Scholar]
- Herzberg O., Moult J. Bacterial resistance to beta-lactam antibiotics: crystal structure of beta-lactamase from Staphylococcus aureus PC1 at 2.5 A resolution. Science. 1987 May 8;236(4802):694–701. doi: 10.1126/science.3107125. [DOI] [PubMed] [Google Scholar]
- Kaiser C. A., Preuss D., Grisafi P., Botstein D. Many random sequences functionally replace the secretion signal sequence of yeast invertase. Science. 1987 Jan 16;235(4786):312–317. doi: 10.1126/science.3541205. [DOI] [PubMed] [Google Scholar]
- Ma J., Ptashne M. A new class of yeast transcriptional activators. Cell. 1987 Oct 9;51(1):113–119. doi: 10.1016/0092-8674(87)90015-8. [DOI] [PubMed] [Google Scholar]
- Min K. T., Kim M. H., Lee D. S. Search for the optimal sequence of the ribosome binding site by random oligonucleotide-directed mutagenesis. Nucleic Acids Res. 1988 Jun 10;16(11):5075–5088. doi: 10.1093/nar/16.11.5075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Napper A. D., Benkovic S. J., Tramontano A., Lerner R. A. A stereospecific cyclization catalyzed by an antibody. Science. 1987 Aug 28;237(4818):1041–1043. doi: 10.1126/science.3616626. [DOI] [PubMed] [Google Scholar]
- Neu H. C. Contribution of beta-lactamases to bacterial resistance and mechanisms to inhibit beta-lactamases. Am J Med. 1985 Nov 29;79(5B):2–12. doi: 10.1016/0002-9343(85)90123-8. [DOI] [PubMed] [Google Scholar]
- Oliphant A. R., Brandl C. J., Struhl K. Defining the sequence specificity of DNA-binding proteins by selecting binding sites from random-sequence oligonucleotides: analysis of yeast GCN4 protein. Mol Cell Biol. 1989 Jul;9(7):2944–2949. doi: 10.1128/mcb.9.7.2944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant A. R., Nussbaum A. L., Struhl K. Cloning of random-sequence oligodeoxynucleotides. Gene. 1986;44(2-3):177–183. doi: 10.1016/0378-1119(86)90180-0. [DOI] [PubMed] [Google Scholar]
- Oliphant A. R., Struhl K. Defining the consensus sequences of E.coli promoter elements by random selection. Nucleic Acids Res. 1988 Aug 11;16(15):7673–7683. doi: 10.1093/nar/16.15.7673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliphant A. R., Struhl K. The use of random-sequence oligonucleotides for determining consensus sequences. Methods Enzymol. 1987;155:568–582. doi: 10.1016/0076-6879(87)55037-6. [DOI] [PubMed] [Google Scholar]
- Pollack S. J., Jacobs J. W., Schultz P. G. Selective chemical catalysis by an antibody. Science. 1986 Dec 19;234(4783):1570–1573. doi: 10.1126/science.3787262. [DOI] [PubMed] [Google Scholar]
- Reidhaar-Olson J. F., Sauer R. T. Combinatorial cassette mutagenesis as a probe of the informational content of protein sequences. Science. 1988 Jul 1;241(4861):53–57. doi: 10.1126/science.3388019. [DOI] [PubMed] [Google Scholar]


