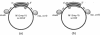Abstract
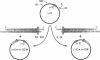
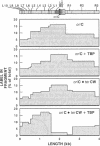
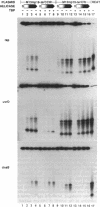
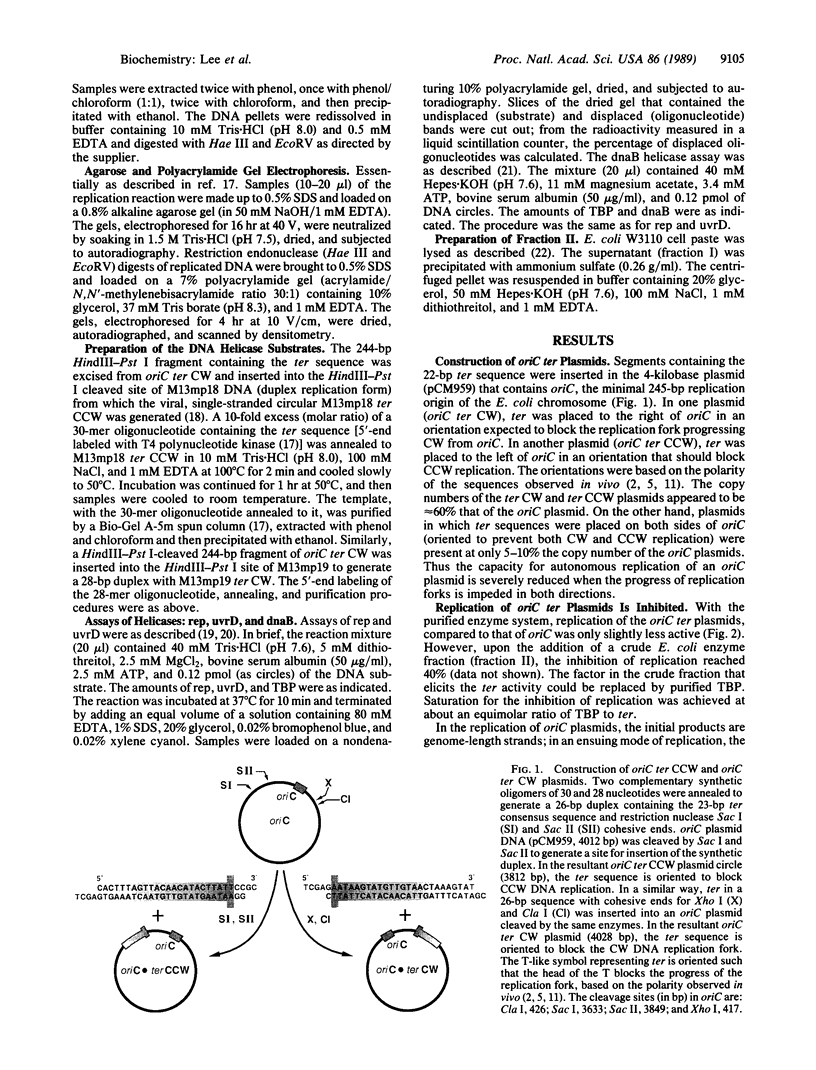
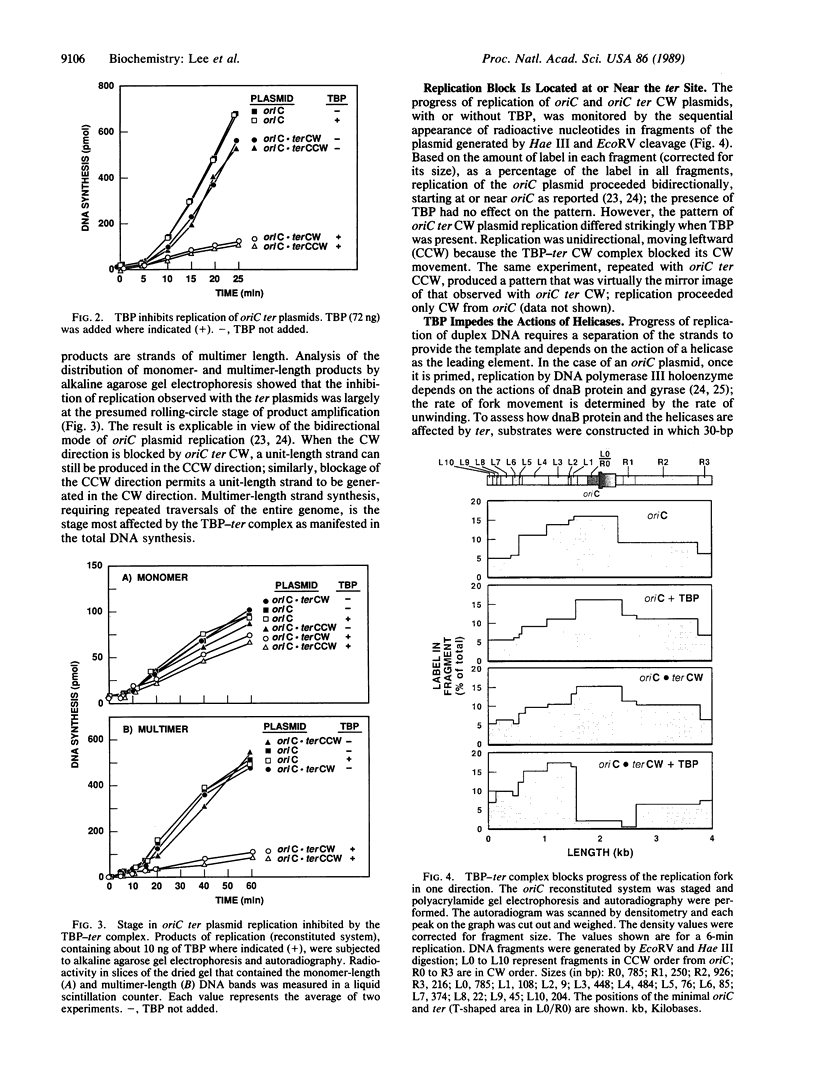
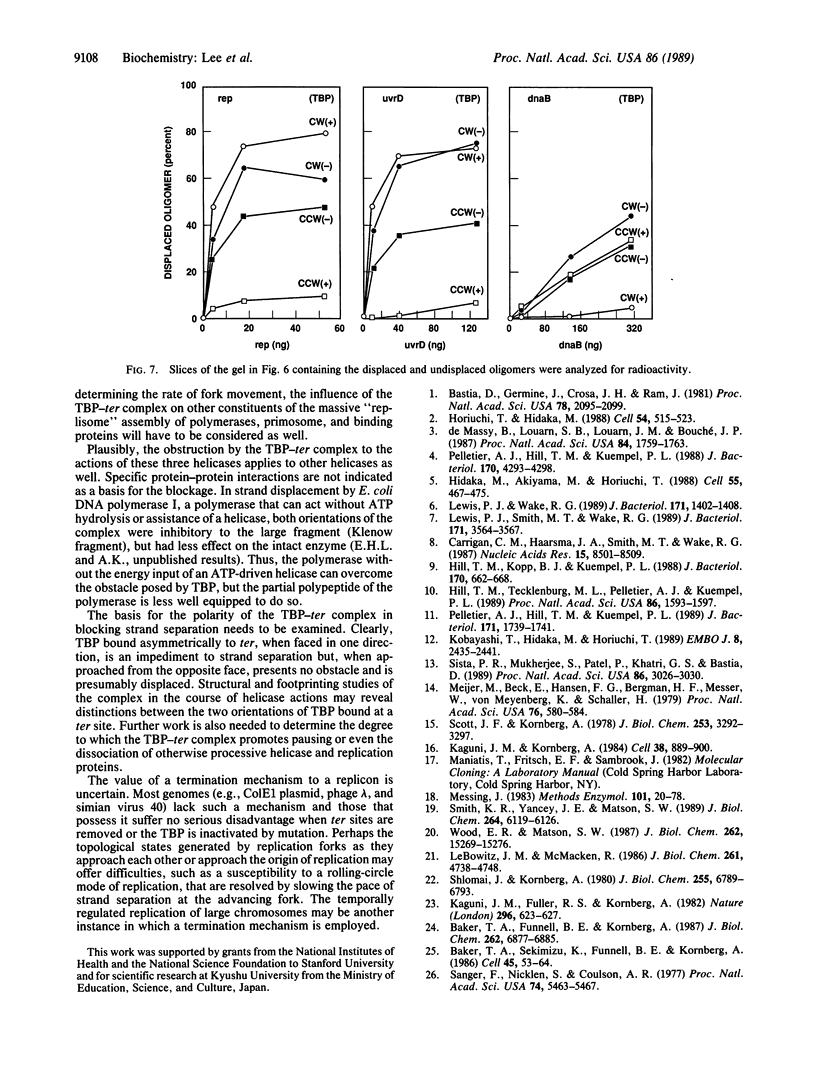
Identification of the consensus sequence for termination of replication (ter) in Escherichia coli and the isolation of the ter-binding protein (TBP) allowed us to test their effects on replication forks initiated at the unique origin of the E. coli chromosome (oriC) in a purified enzyme system. Replication was severely impeded by ter in a unique orientation when purified TBP was supplied to bind it. The target for blockage within the replication complex can now be ascribed to the inability of dnaB helicase to separate the duplex strands when it encounters ter bound by TBP. Other helicases, such as rep and uvrD proteins, that translocate on DNA and displace strands in the direction opposite to that of dnaB protein are also blocked, but only when the TBP-bound ter is oriented in the other direction. From these results, we infer that the orientation of ter confers a particular polarity on the TBP seated on it, such that a helicase is blocked when it confronts TBP from one side, but can act, presumably by displacing TBP, when facing its other side. Thus, the intrinsic nature of the oriented TBP-ter complex is responsible for impeding the helicases, rather than any protein-protein interactions.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baker T. A., Funnell B. E., Kornberg A. Helicase action of dnaB protein during replication from the Escherichia coli chromosomal origin in vitro. J Biol Chem. 1987 May 15;262(14):6877–6885. [PubMed] [Google Scholar]
- Baker T. A., Sekimizu K., Funnell B. E., Kornberg A. Extensive unwinding of the plasmid template during staged enzymatic initiation of DNA replication from the origin of the Escherichia coli chromosome. Cell. 1986 Apr 11;45(1):53–64. doi: 10.1016/0092-8674(86)90537-4. [DOI] [PubMed] [Google Scholar]
- Bastia D., Germino J., Crosa J. H., Ram J. The nucleotide sequence surrounding the replication terminus of R6K. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2095–2099. doi: 10.1073/pnas.78.4.2095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrigan C. M., Haarsma J. A., Smith M. T., Wake R. G. Sequence features of the replication terminus of the Bacillus subtilis chromosome. Nucleic Acids Res. 1987 Oct 26;15(20):8501–8509. doi: 10.1093/nar/15.20.8501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuller R. S., Funnell B. E., Kornberg A. The dnaA protein complex with the E. coli chromosomal replication origin (oriC) and other DNA sites. Cell. 1984 Oct;38(3):889–900. doi: 10.1016/0092-8674(84)90284-8. [DOI] [PubMed] [Google Scholar]
- Hidaka M., Akiyama M., Horiuchi T. A consensus sequence of three DNA replication terminus sites on the E. coli chromosome is highly homologous to the terR sites of the R6K plasmid. Cell. 1988 Nov 4;55(3):467–475. doi: 10.1016/0092-8674(88)90033-5. [DOI] [PubMed] [Google Scholar]
- Hill T. M., Kopp B. J., Kuempel P. L. Termination of DNA replication in Escherichia coli requires a trans-acting factor. J Bacteriol. 1988 Feb;170(2):662–668. doi: 10.1128/jb.170.2.662-668.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. M., Tecklenburg M. L., Pelletier A. J., Kuempel P. L. tus, the trans-acting gene required for termination of DNA replication in Escherichia coli, encodes a DNA-binding protein. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1593–1597. doi: 10.1073/pnas.86.5.1593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi T., Hidaka M. Core sequence of two separable terminus sites of the R6K plasmid that exhibit polar inhibition of replication is a 20 bp inverted repeat. Cell. 1988 Aug 12;54(4):515–523. doi: 10.1016/0092-8674(88)90073-6. [DOI] [PubMed] [Google Scholar]
- Kaguni J. M., Fuller R. S., Kornberg A. Enzymatic replication of E. coli chromosomal origin is bidirectional. Nature. 1982 Apr 15;296(5858):623–627. doi: 10.1038/296623a0. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Hidaka M., Horiuchi T. Evidence of a ter specific binding protein essential for the termination reaction of DNA replication in Escherichia coli. EMBO J. 1989 Aug;8(8):2435–2441. doi: 10.1002/j.1460-2075.1989.tb08374.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LeBowitz J. H., McMacken R. The Escherichia coli dnaB replication protein is a DNA helicase. J Biol Chem. 1986 Apr 5;261(10):4738–4748. [PubMed] [Google Scholar]
- Lewis P. J., Smith M. T., Wake R. G. A protein involved in termination of chromosome replication in Bacillus subtilis binds specifically to the terC site. J Bacteriol. 1989 Jun;171(6):3564–3567. doi: 10.1128/jb.171.6.3564-3567.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis P. J., Wake R. G. DNA and protein sequence conservation at the replication terminus in Bacillus subtilis 168 and W23. J Bacteriol. 1989 Mar;171(3):1402–1408. doi: 10.1128/jb.171.3.1402-1408.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer M., Beck E., Hansen F. G., Bergmans H. E., Messer W., von Meyenburg K., Schaller H. Nucleotide sequence of the origin of replication of the Escherichia coli K-12 chromosome. Proc Natl Acad Sci U S A. 1979 Feb;76(2):580–584. doi: 10.1073/pnas.76.2.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Pelletier A. J., Hill T. M., Kuempel P. L. Location of sites that inhibit progression of replication forks in the terminus region of Escherichia coli. J Bacteriol. 1988 Sep;170(9):4293–4298. doi: 10.1128/jb.170.9.4293-4298.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletier A. J., Hill T. M., Kuempel P. L. Termination sites T1 and T2 from the Escherichia coli chromosome inhibit DNA replication in ColE1-derived plasmids. J Bacteriol. 1989 Mar;171(3):1739–1741. doi: 10.1128/jb.171.3.1739-1741.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott J. F., Kornberg A. Purification of the rep protein of Escherichia coli. An ATPase which separates duplex DNA strands in advance of replication. J Biol Chem. 1978 May 10;253(9):3292–3297. [PubMed] [Google Scholar]
- Shlomai J., Kornberg A. A prepriming DNA replication enzyme of Escherichia coli. I. Purification of protein n': a sequence-specific, DNA-dependent ATPase. J Biol Chem. 1980 Jul 25;255(14):6789–6793. [PubMed] [Google Scholar]
- Sista P. R., Mukherjee S., Patel P., Khatri G. S., Bastia D. A host-encoded DNA-binding protein promotes termination of plasmid replication at a sequence-specific replication terminus. Proc Natl Acad Sci U S A. 1989 May;86(9):3026–3030. doi: 10.1073/pnas.86.9.3026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. R., Yancey J. E., Matson S. W. Identification and purification of a protein that stimulates the helicase activity of the Escherichia coli Rep protein. J Biol Chem. 1989 Apr 15;264(11):6119–6126. [PubMed] [Google Scholar]
- Wood E. R., Matson S. W. Purification and characterization of a new DNA-dependent ATPase with helicase activity from Escherichia coli. J Biol Chem. 1987 Nov 5;262(31):15269–15276. [PubMed] [Google Scholar]
- de Massy B., Béjar S., Louarn J., Louarn J. M., Bouché J. P. Inhibition of replication forks exiting the terminus region of the Escherichia coli chromosome occurs at two loci separated by 5 min. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1759–1763. doi: 10.1073/pnas.84.7.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]