Abstract
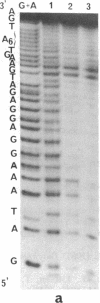
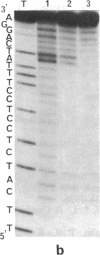
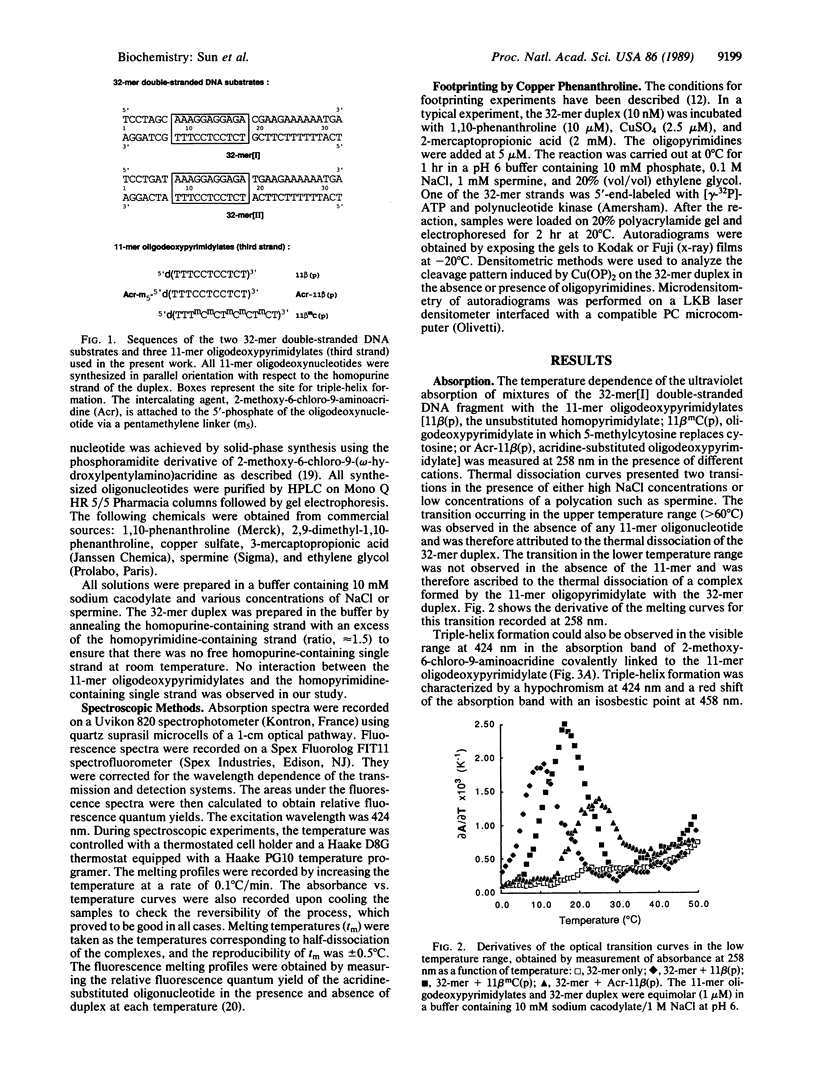
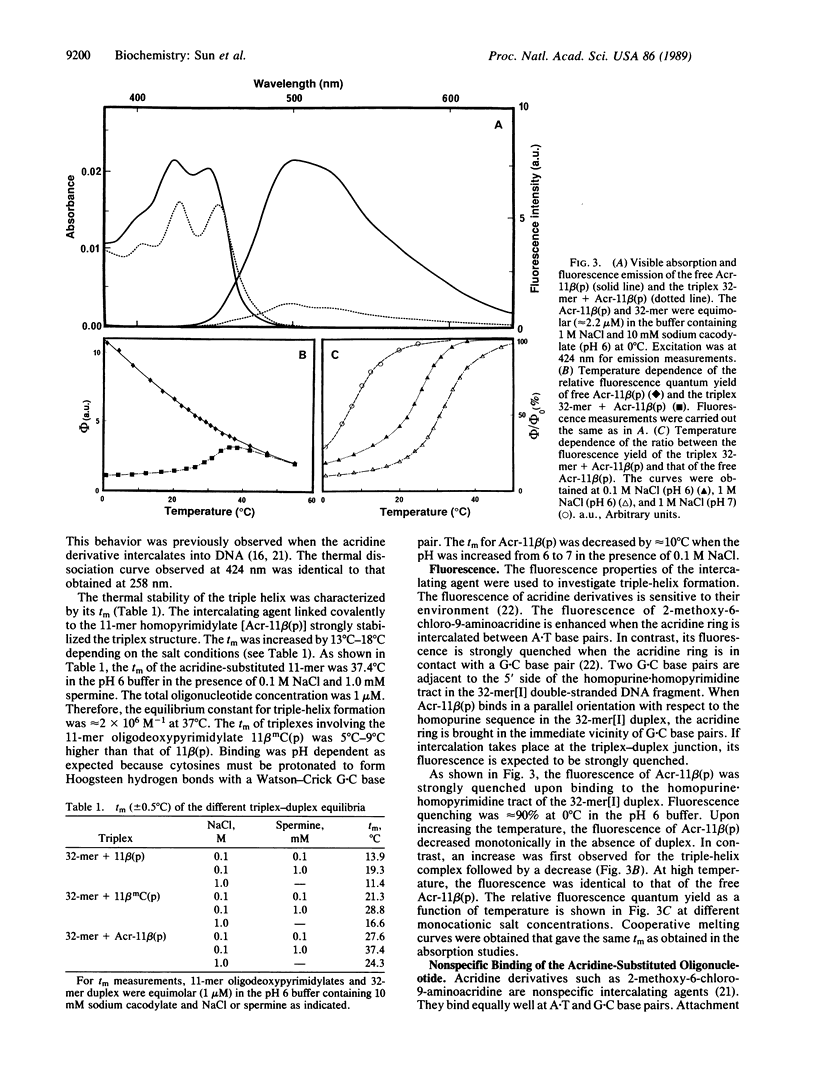
An acridine derivative was covalently linked to the 5' end of a homopyrimidine oligonucleotide. Specific binding to a homopurine-homopyrimidine sequence of duplex DNA was demonstrated by spectroscopic studies (absorption and fluorescence) and by "footprinting" experiments with a copper phenanthroline chelate used as an artificial nuclease. A hypochromism and a red shift of the acridine absorption were observed. Triple-helix formation was also accompanied by a hypochromism in the ultraviolet range. The fluorescence of the acridine ring was quenched by a stacking interaction with a G.C base pair adjacent to the homopurine-homopyrimidine target sequence. The intercalating agent strongly stabilized the complex formed by the oligopyrimidine with its target duplex sequence. Cytosine methylation further increased the stability of the complexes. Footprinting studies revealed that the oligopyrimidine binds in a parallel orientation with respect to the homopurine-containing strand of the duplex. The intercalated acridine extended by 2 base pairs the region of the duplex protected by the oligopyrimidine against degradation by the nuclease activity of the copper phenanthroline chelate. Random intercalation of the acridine ring was lost due to the repulsive effect of the negatively charged oligonucleotide tail. Intercalation occurred only at those double-stranded sequences where the homopyrimidine oligonucleotide recognized the major groove of duplex DNA.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Selsing E. Structures for the polynucleotide complexes poly(dA) with poly (dT) and poly(dT) with poly(dA) with poly (dT). J Mol Biol. 1974 Sep 15;88(2):509–521. doi: 10.1016/0022-2836(74)90498-7. [DOI] [PubMed] [Google Scholar]
- Asseline U., Delarue M., Lancelot G., Toulmé F., Thuong N. T., Montenay-Garestier T., Hélène C. Nucleic acid-binding molecules with high affinity and base sequence specificity: intercalating agents covalently linked to oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1984 Jun;81(11):3297–3301. doi: 10.1073/pnas.81.11.3297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asseline U., Nguyen T. T., Hélène C. Nouvelles substances à forte affinité spécifique pour des séquences d'acides nucléiques: oligodésoxynucléotides liés de façon covalente à un agent intercalant. C R Seances Acad Sci III. 1983;297(7):369–372. [PubMed] [Google Scholar]
- Asseline U., Toulme F., Thuong N. T., Delarue M., Montenay-Garestier T., Hélène C. Oligodeoxynucleotides covalently linked to intercalating dyes as base sequence-specific ligands. Influence of dye attachment site. EMBO J. 1984 Apr;3(4):795–800. doi: 10.1002/j.1460-2075.1984.tb01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bresloff J. L., Crothers D. M. Equilibrium studies of ethidium--polynucleotide interactions. Biochemistry. 1981 Jun 9;20(12):3547–3553. doi: 10.1021/bi00515a038. [DOI] [PubMed] [Google Scholar]
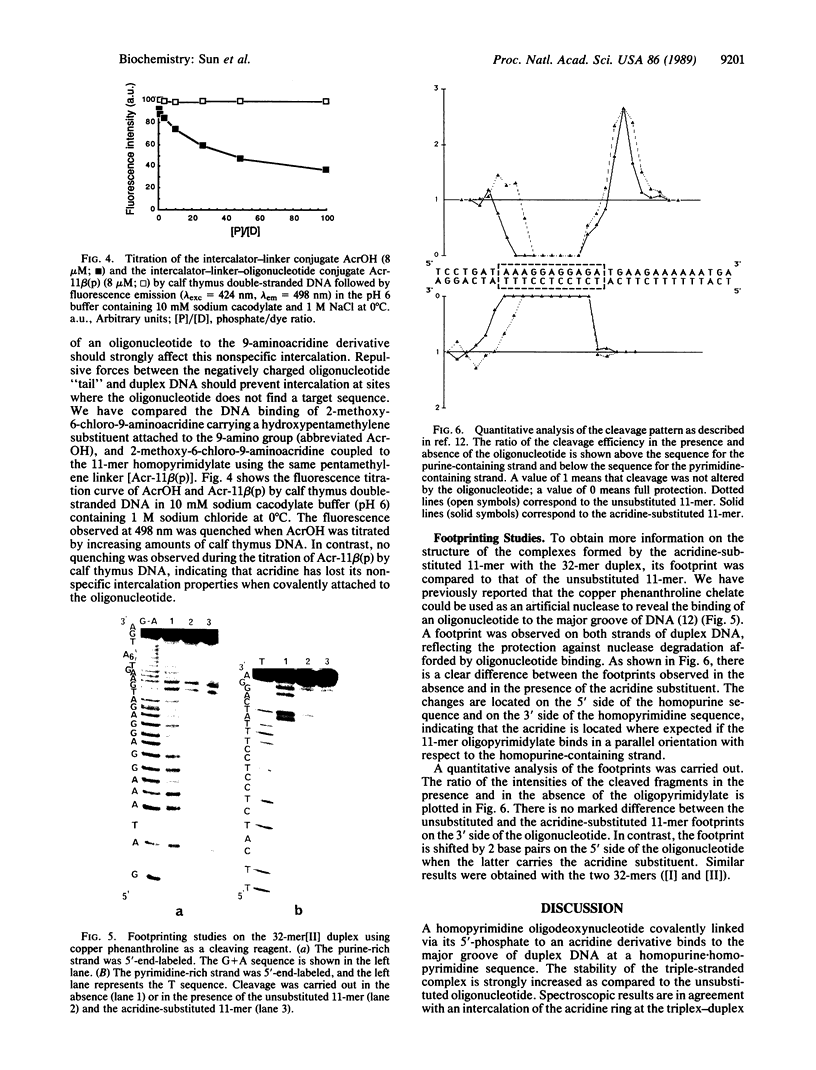
- Cieplak P., Rao S. N., Hélène C., Montenay-Garestier T., Kollman P. A. Conformations of duplex structures formed by oligodeoxynucleotides covalently linked to the intercalator 2-methoxy-6-chloro-9-aminoacridine. J Biomol Struct Dyn. 1987 Oct;5(2):361–382. doi: 10.1080/07391102.1987.10506400. [DOI] [PubMed] [Google Scholar]
- Cooney M., Czernuszewicz G., Postel E. H., Flint S. J., Hogan M. E. Site-specific oligonucleotide binding represses transcription of the human c-myc gene in vitro. Science. 1988 Jul 22;241(4864):456–459. doi: 10.1126/science.3293213. [DOI] [PubMed] [Google Scholar]
- Delbarre A., Delepierre M., Garbay C., Igolen J., Le Pecq J. B., Roques B. P. Geometry of the antitumor drug ditercalinium bisintercalated into d(CpGpCpG)2 by 1H NMR. Proc Natl Acad Sci U S A. 1987 Apr;84(8):2155–2159. doi: 10.1073/pnas.84.8.2155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Chassignol M., Thuong N. T., Hélène C. Nucléases artificielles: coupures spécifiques de la double hélice d'ADN par des oligonucléotides liés au complexe cuivre-phénanthroline. C R Acad Sci III. 1988;307(20):849–854. [PubMed] [Google Scholar]
- François J. C., Saison-Behmoaras T., Hélène C. Sequence-specific recognition of the major groove of DNA by oligodeoxynucleotides via triple helix formation. Footprinting studies. Nucleic Acids Res. 1988 Dec 23;16(24):11431–11440. doi: 10.1093/nar/16.24.11431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hélène C., Lancelot G. Interactions between functional groups in protein-nucleic acid associations. Prog Biophys Mol Biol. 1982;39(1):1–68. doi: 10.1016/0079-6107(83)90013-5. [DOI] [PubMed] [Google Scholar]
- Le Doan T., Perrouault L., Praseuth D., Habhoub N., Decout J. L., Thuong N. T., Lhomme J., Hélène C. Sequence-specific recognition, photocrosslinking and cleavage of the DNA double helix by an oligo-[alpha]-thymidylate covalently linked to an azidoproflavine derivative. Nucleic Acids Res. 1987 Oct 12;15(19):7749–7760. doi: 10.1093/nar/15.19.7749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Pecq J. B., Paoletti C. Interaction du bromhydrate d'éthidium (BET) avec les polyribonucléotides. Applications à l'étude des réactions d'hybridation. C R Acad Sci Hebd Seances Acad Sci D. 1965 Jun 28;260(26):7033–7036. [PubMed] [Google Scholar]
- Lee J. S., Woodsworth M. L., Latimer L. J., Morgan A. R. Poly(pyrimidine) . poly(purine) synthetic DNAs containing 5-methylcytosine form stable triplexes at neutral pH. Nucleic Acids Res. 1984 Aug 24;12(16):6603–6614. doi: 10.1093/nar/12.16.6603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letai A. G., Palladino M. A., Fromm E., Rizzo V., Fresco J. R. Specificity in formation of triple-stranded nucleic acid helical complexes: studies with agarose-linked polyribonucleotide affinity columns. Biochemistry. 1988 Dec 27;27(26):9108–9112. doi: 10.1021/bi00426a007. [DOI] [PubMed] [Google Scholar]
- Morgan A. R., Wells R. D. Specificity of the three-stranded complex formation between double-stranded DNA and single-stranded RNA containing repeating nucleotide sequences. J Mol Biol. 1968 Oct 14;37(1):63–80. doi: 10.1016/0022-2836(68)90073-9. [DOI] [PubMed] [Google Scholar]
- Moser H. E., Dervan P. B. Sequence-specific cleavage of double helical DNA by triple helix formation. Science. 1987 Oct 30;238(4827):645–650. doi: 10.1126/science.3118463. [DOI] [PubMed] [Google Scholar]
- Neidle S., Abraham Z. Structural and sequence-dependent aspects of drug intercalation into nucleic acids. CRC Crit Rev Biochem. 1984;17(1):73–121. doi: 10.3109/10409238409110270. [DOI] [PubMed] [Google Scholar]
- Praseuth D., Perrouault L., Le Doan T., Chassignol M., Thuong N., Hélène C. Sequence-specific binding and photocrosslinking of alpha and beta oligodeoxynucleotides to the major groove of DNA via triple-helix formation. Proc Natl Acad Sci U S A. 1988 Mar;85(5):1349–1353. doi: 10.1073/pnas.85.5.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reuben J., Baker B. M., Kallenbach N. R. Structure of mutagen nucleic acid complexes in solution. Proton chemical shifts in 9-aminoacridine complexes with dG-dC, dC-dG, and dA-dT-dG-dC-dA-dT. Biochemistry. 1978 Jul 11;17(14):2915–2919. doi: 10.1021/bi00607a033. [DOI] [PubMed] [Google Scholar]
- Riley M., Maling B. Physical and chemical characterization of two- and three-stranded adenine-thymine and adenine-uracil homopolymer complexes. J Mol Biol. 1966 Sep;20(2):359–389. doi: 10.1016/0022-2836(66)90069-6. [DOI] [PubMed] [Google Scholar]
- Sun J. S., Asseline U., Rouzaud D., Montenay-Garestier T., Nguyen T. T., Hélène C. Oligo-[alpha]-deoxynucleotides covalently linked to an intercalating agent. Double helices with parallel strands are formed with complementary oligo-[beta]-deoxynucleotides. Nucleic Acids Res. 1987 Aug 11;15(15):6149–6158. doi: 10.1093/nar/15.15.6149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veal J. M., Rill R. L. Sequence specificity of DNA cleavage by bis(1,10-phenanthroline)copper(I): effects of single base pair transitions on the cleavage of preferred pyrimidine-purine-pyrimidine triplets. Biochemistry. 1989 Apr 18;28(8):3243–3250. doi: 10.1021/bi00434a019. [DOI] [PubMed] [Google Scholar]
- Williams L. D., Thivierge J., Goldberg I. H. Specific binding of o-phenanthroline at a DNA structural lesion. Nucleic Acids Res. 1988 Dec 23;16(24):11607–11615. doi: 10.1093/nar/16.24.11607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer C., Wähnert U. Nonintercalating DNA-binding ligands: specificity of the interaction and their use as tools in biophysical, biochemical and biological investigations of the genetic material. Prog Biophys Mol Biol. 1986;47(1):31–112. doi: 10.1016/0079-6107(86)90005-2. [DOI] [PubMed] [Google Scholar]