Abstract
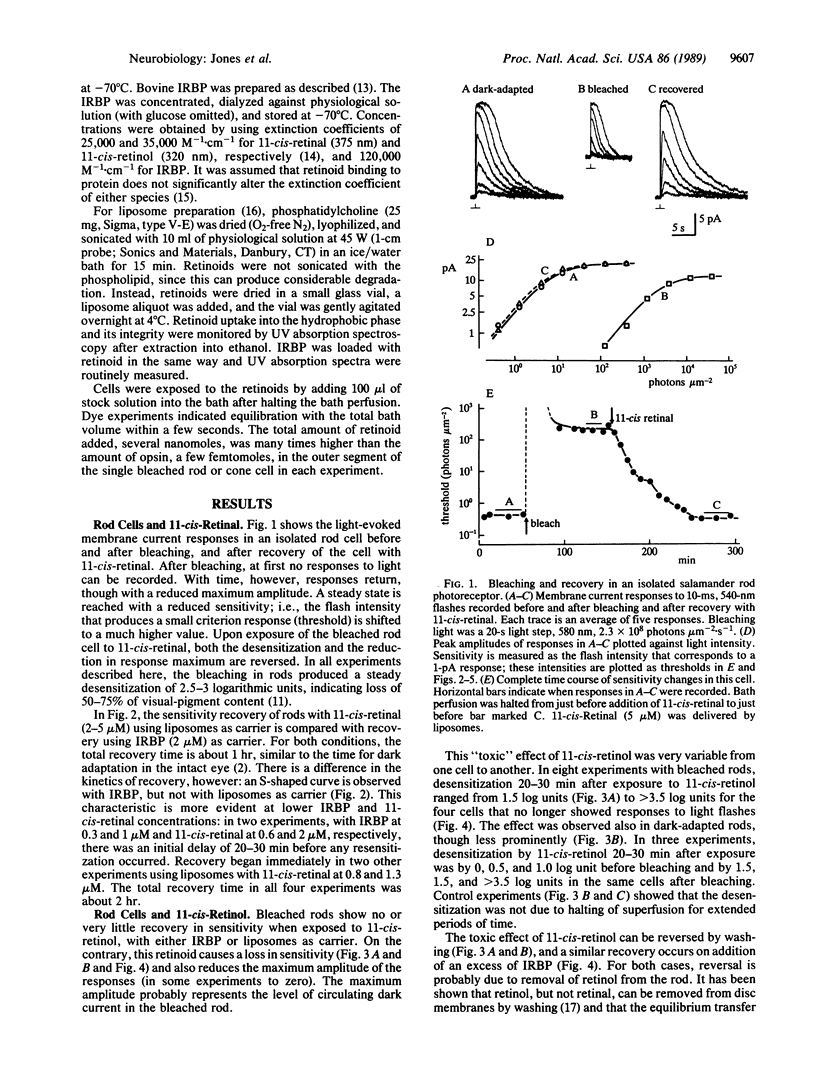
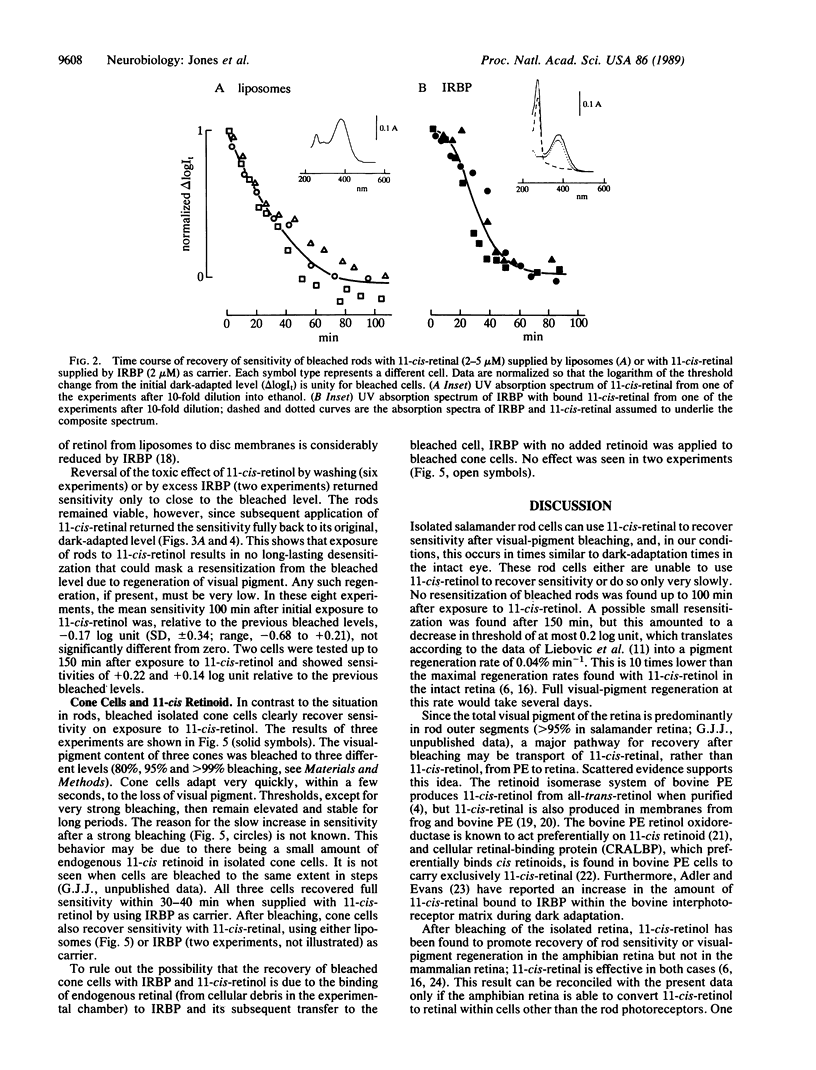
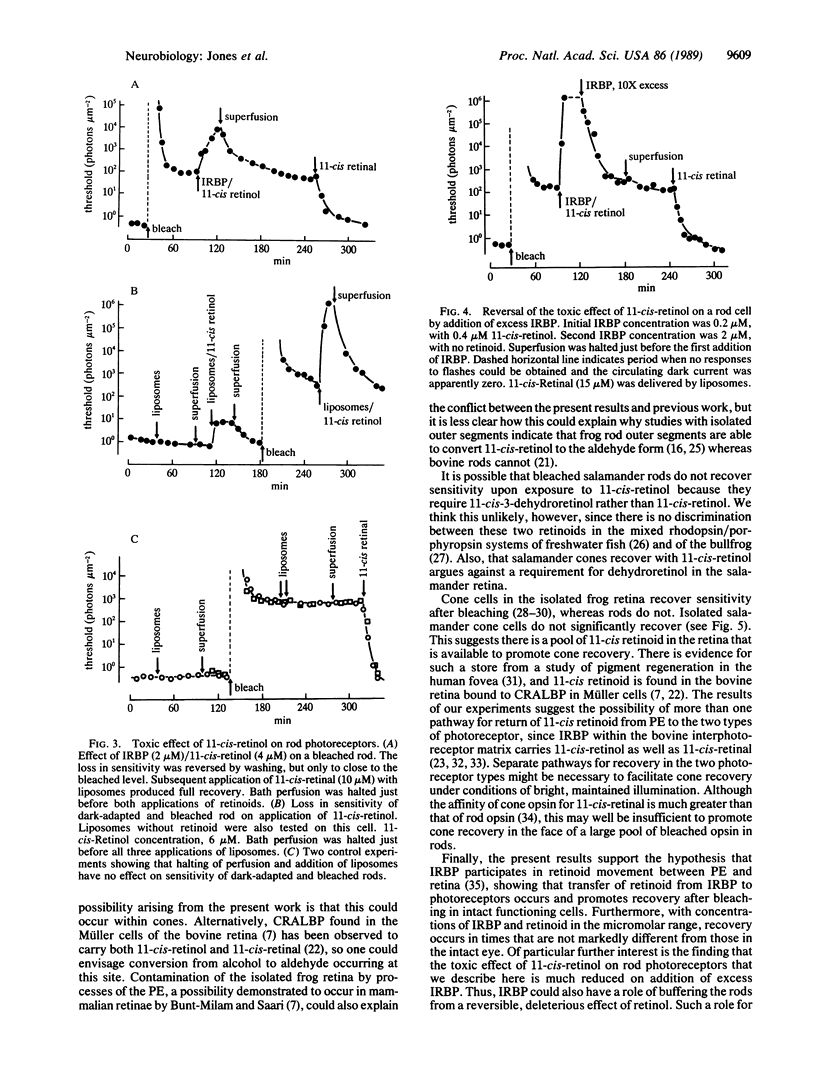
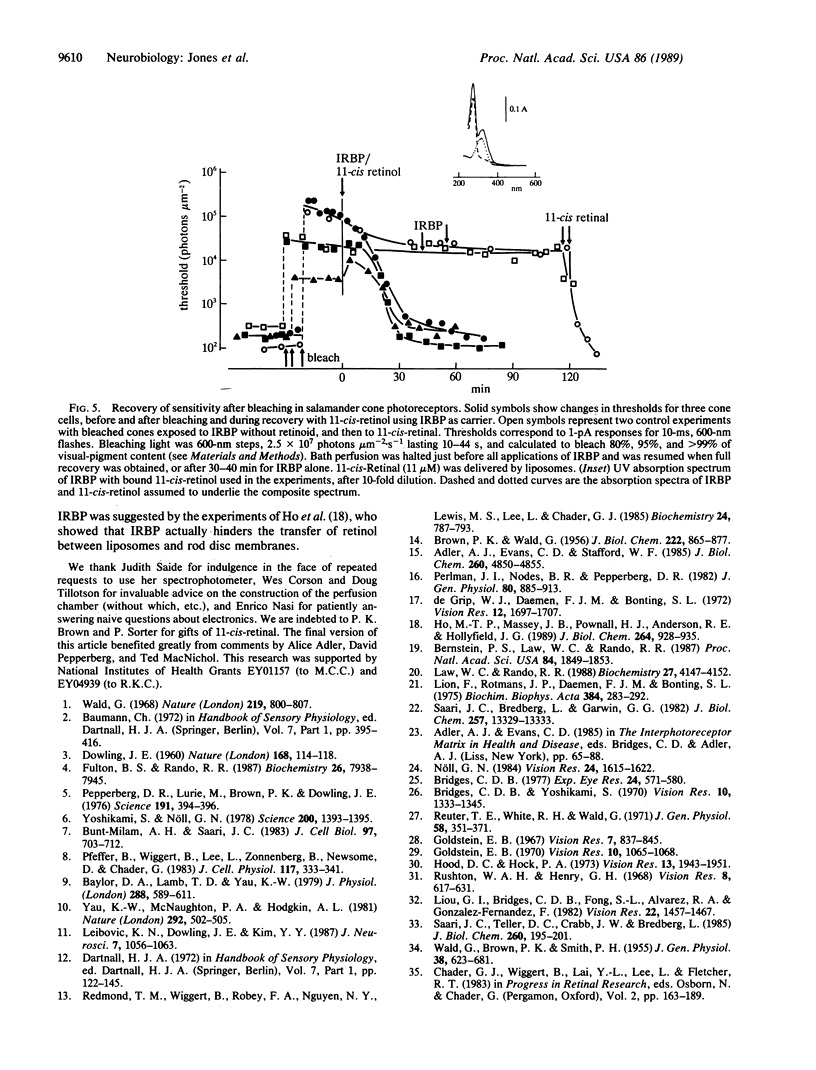
After visual-pigment bleaching, single isolated rod photoreceptors of Ambystoma tigrinum recover their sensitivity to light when supplied with 11-cis-retinal from liposomes or with 11-cis-retinal bound to interphotoreceptor retinoid-binding protein. Bleached rods do not recover sensitivity, or do so only very slowly, after exposure to 11-cis-retinol. The latter retinoid is "toxic" in that rods actually lose sensitivity in its presence. In contrast, bleached isolated cone cells recover sensitivity when either retinoid is supplied. It is suggested that the major pathway for rhodopsin regeneration during dark adaptation in the intact eye is transport of 11-cis-retinal from the pigment epithelium to the retina. The results also suggest that there may be separate pathways for visual-pigment regeneration in rods and cones during dark adaptation.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adler A. J., Evans C. D., Stafford W. F., 3rd Molecular properties of bovine interphotoreceptor retinol-binding protein. J Biol Chem. 1985 Apr 25;260(8):4850–4855. [PubMed] [Google Scholar]
- BROWN P. K., WALD G. The neo-b isomer of vitamin A and retinene. J Biol Chem. 1956 Oct;222(2):865–877. [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Bernstein P. S., Law W. C., Rando R. R. Isomerization of all-trans-retinoids to 11-cis-retinoids in vitro. Proc Natl Acad Sci U S A. 1987 Apr;84(7):1849–1853. doi: 10.1073/pnas.84.7.1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges C. D. Rhodopsin regeneration in rod outer segments: utilization of 11-cis retinal and retinol. Exp Eye Res. 1977 Jun;24(6):571–580. doi: 10.1016/0014-4835(77)90114-2. [DOI] [PubMed] [Google Scholar]
- Bridges C. D., Yoshikami S. The rhodopsin-porphyropsin system in freshwater fishes. 2. Turnover and interconversion of visual pigment prosthetic groups in light and darkness: role of the pigment epithelium. Vision Res. 1970 Dec;10(12):1333–1345. doi: 10.1016/0042-6989(70)90085-4. [DOI] [PubMed] [Google Scholar]
- Bunt-Milam A. H., Saari J. C. Immunocytochemical localization of two retinoid-binding proteins in vertebrate retina. J Cell Biol. 1983 Sep;97(3):703–712. doi: 10.1083/jcb.97.3.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DOWLING J. E. Chemistry of visual adaptation in the rat. Nature. 1960 Oct 8;188:114–118. doi: 10.1038/188114a0. [DOI] [PubMed] [Google Scholar]
- Fulton B. S., Rando R. R. Biosynthesis of 11-cis-retinoids and retinyl esters by bovine pigment epithelium membranes. Biochemistry. 1987 Dec 1;26(24):7938–7945. doi: 10.1021/bi00398a059. [DOI] [PubMed] [Google Scholar]
- Goldstein E. B. Cone pigment regeneration in the isolated frog retina. Vision Res. 1970 Oct;10(10):1065–1068. doi: 10.1016/0042-6989(70)90082-9. [DOI] [PubMed] [Google Scholar]
- Goldstein E. B. Early receptor potential of the isolated frog (Rana pipiens) retina. Vision Res. 1967 Nov;7(11):837–845. doi: 10.1016/0042-6989(67)90004-1. [DOI] [PubMed] [Google Scholar]
- Ho M. T., Massey J. B., Pownall H. J., Anderson R. E., Hollyfield J. G. Mechanism of vitamin A movement between rod outer segments, interphotoreceptor retinoid-binding protein, and liposomes. J Biol Chem. 1989 Jan 15;264(2):928–935. [PubMed] [Google Scholar]
- Hood D. C., Hock P. A. Recovery of cone receptor activity in the frog's isolated retina. Vision Res. 1973 Oct;13(10):1943–1951. doi: 10.1016/0042-6989(73)90065-5. [DOI] [PubMed] [Google Scholar]
- Law W. C., Rando R. R. Stereochemical inversion at C-15 accompanies the enzymatic isomerization of all-trans- to 11-cis-retinoids. Biochemistry. 1988 May 31;27(11):4147–4152. doi: 10.1021/bi00411a037. [DOI] [PubMed] [Google Scholar]
- Leibovic K. N., Dowling J. E., Kim Y. Y. Background and bleaching equivalence in steady-state adaptation of vertebrate rods. J Neurosci. 1987 Apr;7(4):1056–1063. doi: 10.1523/JNEUROSCI.07-04-01056.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lion F., Rotmans J. P., Daemen F. J., Bonting S. L. Biochemical aspects of the visual process. XXVII. Stereospecificity of ocular retinol dehydrogenases and the visual cycle. Biochim Biophys Acta. 1975 Apr 19;384(2):283–292. doi: 10.1016/0005-2744(75)90030-3. [DOI] [PubMed] [Google Scholar]
- Liou G. I., Bridges C. D., Fong S. L., Alvarez R. A., Gonzalez-Fernandez F. Vitamin A transport between retina and pigment epithelium--an interstitial protein carrying endogenous retinol (interstitial retinol-binding protein). Vision Res. 1982;22(12):1457–1467. doi: 10.1016/0042-6989(82)90210-3. [DOI] [PubMed] [Google Scholar]
- Nöll G. N. Suitability of retinol, retinal and retinyl palmitate for the regeneration of bleached rhodopsin in the isolated frog retina. Vision Res. 1984;24(11):1615–1622. doi: 10.1016/0042-6989(84)90319-5. [DOI] [PubMed] [Google Scholar]
- Pepperberg D. R., Lurie M., Brown P. K., Dowling J. E. Visual adaptation: effects of externally applied retinal on the light-adapted, isolated skate retina. Science. 1976 Jan 30;191(4225):394–396. doi: 10.1126/science.1246621. [DOI] [PubMed] [Google Scholar]
- Perlman J. I., Nodes B. R., Pepperberg D. R. Utilization of retinoids in the bullfrog retina. J Gen Physiol. 1982 Dec;80(6):885–913. doi: 10.1085/jgp.80.6.885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer B., Wiggert B., Lee L., Zonnenberg B., Newsome D., Chader G. The presence of a soluble interphotoreceptor retinol-binding protein (IRBP) in the retinal interphotoreceptor space. J Cell Physiol. 1983 Dec;117(3):333–341. doi: 10.1002/jcp.1041170308. [DOI] [PubMed] [Google Scholar]
- Redmond T. M., Wiggert B., Robey F. A., Nguyen N. Y., Lewis M. S., Lee L., Chader G. J. Isolation and characterization of monkey interphotoreceptor retinoid-binding protein, a unique extracellular matrix component of the retina. Biochemistry. 1985 Jan 29;24(3):787–793. doi: 10.1021/bi00324a038. [DOI] [PubMed] [Google Scholar]
- Reuter T. E., White R. H., Wald G. Rhodopsin and porphyropsin fields in the adult bullfrog retina. J Gen Physiol. 1971 Oct;58(4):351–371. doi: 10.1085/jgp.58.4.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushton W. A., Henry G. H. Bleaching and regeneration of cone pigments in man. Vision Res. 1968 Jun;8(6):617–631. doi: 10.1016/0042-6989(68)90040-0. [DOI] [PubMed] [Google Scholar]
- Saari J. C., Bredberg L., Garwin G. G. Identification of the endogenous retinoids associated with three cellular retinoid-binding proteins from bovine retina and retinal pigment epithelium. J Biol Chem. 1982 Nov 25;257(22):13329–13333. [PubMed] [Google Scholar]
- Saari J. C., Teller D. C., Crabb J. W., Bredberg L. Properties of an interphotoreceptor retinoid-binding protein from bovine retina. J Biol Chem. 1985 Jan 10;260(1):195–201. [PubMed] [Google Scholar]
- Uoshikami S., Nöll G. N. Isolated retinas synthesize visual pigments from tetinol congeners delivered by liposomes. Science. 1978 Jun 23;200(4348):1393–1395. doi: 10.1126/science.307275. [DOI] [PubMed] [Google Scholar]
- WALD G., BROWN P. K., SMITH P. H. Iodopsin. J Gen Physiol. 1955 May 20;38(5):623–681. doi: 10.1085/jgp.38.5.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wald G. The molecular basis of visual excitation. Nature. 1968 Aug 24;219(5156):800–807. doi: 10.1038/219800a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- de Grip W. J., Daemen F. J., Bonting S. L. Enrichment of rhodopsin in rod outer segment membrane preparations. Biochemical aspects of the visual process. 18. Vision Res. 1972 Oct;12(10):1697–1707. doi: 10.1016/0042-6989(72)90040-5. [DOI] [PubMed] [Google Scholar]


