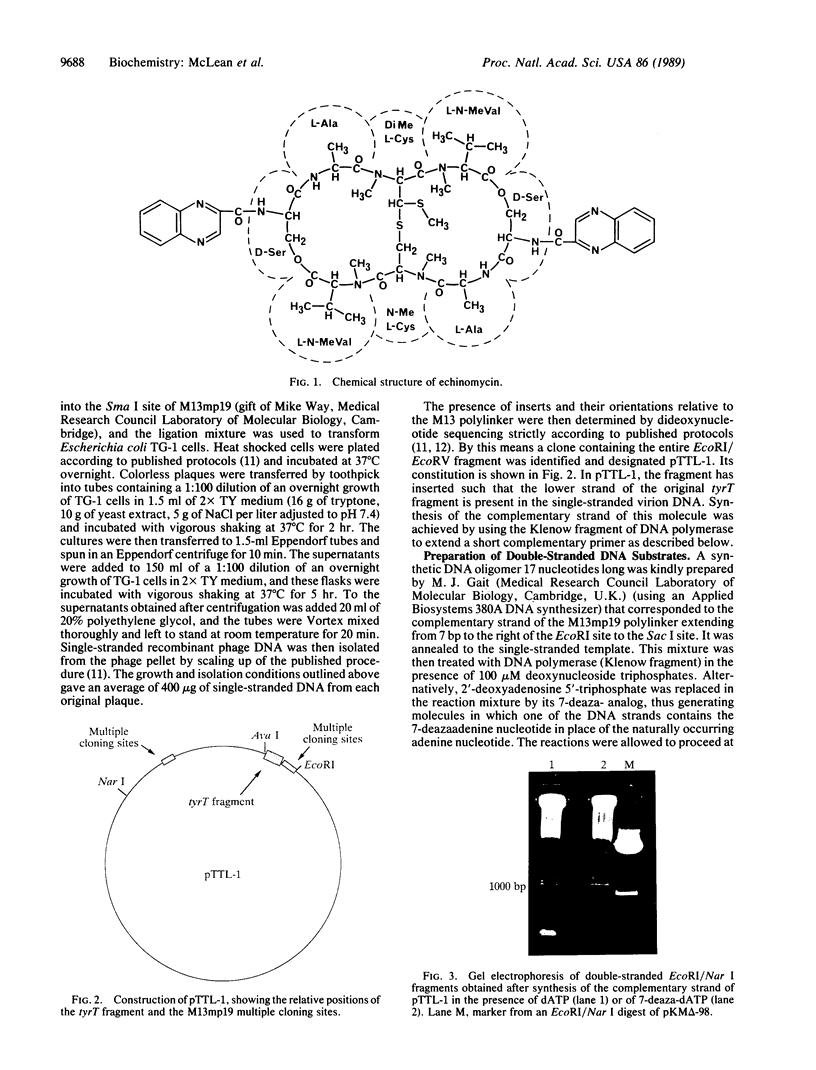
Abstract
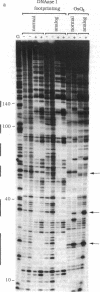
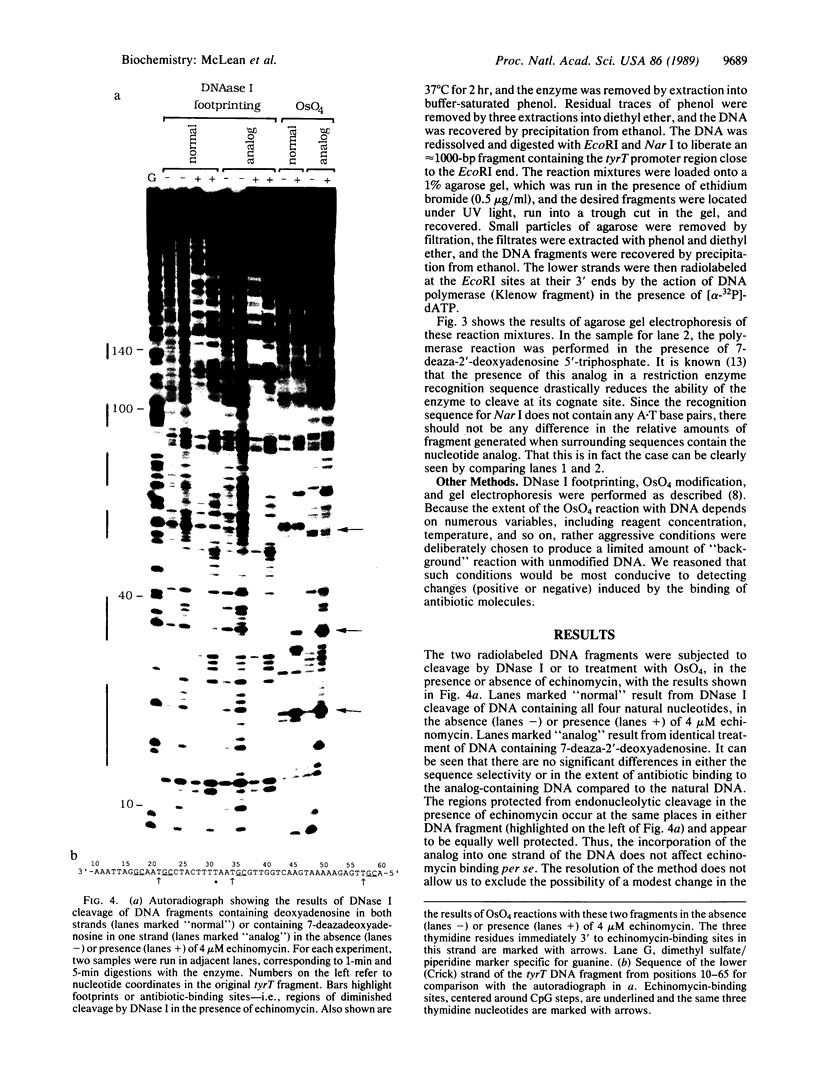
To show conclusively that the critical structural deformation of double-helical DNA that is induced by the binding of quinoxaline antibiotics does not involve the formation of Hoogsteen base pairs, we have prepared a DNA fragment containing the nucleoside analog 7-deaza-2'-deoxyadenosine in one of the two strands. This DNA fragment was subjected to treatment with the thymidine-specific reagent osmium tetroxide and to DNase I "footprinting" in the presence or absence of micromolar concentrations of echinomycin. We report that this anti-tumor antibiotic binds to DNA containing the nucleoside analog as well as to natural DNA and that the previously reported hypersensitivity to osmium tetroxide of certain thymidine residues adjacent to echinomycin binding sites is maintained in analog-containing DNA. Since these thymidines are rendered incapable of participating in Hoogsteen base pairs by the incorporation of 7-deaza-2'-deoxyadenosine, we conclude that this unusual base-pairing scheme is not the cause of the observed hypersensitivity to osmium tetroxide and that it therefore results from a large local unwinding of the DNA in the presence of the antibiotic. Moreover, preventing the possibility of Hoogsteen base pairing does not preclude echinomycin binding.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankier A. T., Weston K. M., Barrell B. G. Random cloning and sequencing by the M13/dideoxynucleotide chain termination method. Methods Enzymol. 1987;155:51–93. doi: 10.1016/0076-6879(87)55009-1. [DOI] [PubMed] [Google Scholar]
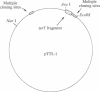
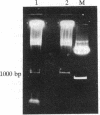
- Drew H. R., Travers A. A. DNA structural variations in the E. coli tyrT promoter. Cell. 1984 Jun;37(2):491–502. doi: 10.1016/0092-8674(84)90379-9. [DOI] [PubMed] [Google Scholar]
- Gao X. L., Patel D. J. NMR studies of echinomycin bisintercalation complexes with d(A1-C2-G3-T4) and d(T1-C2-G3-A4) duplexes in aqueous solution: sequence-dependent formation of Hoogsteen A1.T4 and Watson--Crick T1.A4 base pairs flanking the bisintercalation site. Biochemistry. 1988 Mar 8;27(5):1744–1751. doi: 10.1021/bi00405a054. [DOI] [PubMed] [Google Scholar]
- Gilbert D. E., van der Marel G. A., van Boom J. H., Feigon J. Unstable Hoogsteen base pairs adjacent to echinomycin binding sites within a DNA duplex. Proc Natl Acad Sci U S A. 1989 May;86(9):3006–3010. doi: 10.1073/pnas.86.9.3006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeppesen C., Nielsen P. E. Detection of intercalation-induced changes in DNA structure by reaction with diethyl pyrocarbonate or potassium permanganate. Evidence against the induction of Hoogsteen base pairing by echinomycin. FEBS Lett. 1988 Apr 11;231(1):172–176. doi: 10.1016/0014-5793(88)80725-7. [DOI] [PubMed] [Google Scholar]
- Koo H. S., Wu H. M., Crothers D. M. DNA bending at adenine . thymine tracts. Nature. 1986 Apr 10;320(6062):501–506. doi: 10.1038/320501a0. [DOI] [PubMed] [Google Scholar]
- McLean M. J., Waring M. J. Chemical probes reveal no evidence of Hoogsteen base pairing in complexes formed between echinomycin and DNA in solution. J Mol Recognit. 1988 Jun;1(3):138–151. doi: 10.1002/jmr.300010307. [DOI] [PubMed] [Google Scholar]
- Mendel D., Dervan P. B. Hoogsteen base pairs proximal and distal to echinomycin binding sites on DNA. Proc Natl Acad Sci U S A. 1987 Feb;84(4):910–914. doi: 10.1073/pnas.84.4.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson H. C., Finch J. T., Luisi B. F., Klug A. The structure of an oligo(dA).oligo(dT) tract and its biological implications. Nature. 1987 Nov 19;330(6145):221–226. doi: 10.1038/330221a0. [DOI] [PubMed] [Google Scholar]
- Portugal J., Fox K. R., McLean M. J., Richenberg J. L., Waring M. J. Diethyl pyrocarbonate can detect a modified DNA structure induced by the binding of quinoxaline antibiotics. Nucleic Acids Res. 1988 May 11;16(9):3655–3670. doi: 10.1093/nar/16.9.3655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quigley G. J., Ughetto G., van der Marel G. A., van Boom J. H., Wang A. H., Rich A. Non-Watson-Crick G.C and A.T base pairs in a DNA-antibiotic complex. Science. 1986 Jun 6;232(4755):1255–1258. doi: 10.1126/science.3704650. [DOI] [PubMed] [Google Scholar]
- Rosenthal A., Billwitz H., Kehne A., Seela F. Solid-phase methods for sequencing nucleic acids. VIII. CCS paper-supported degradation of oligodeoxyribonucleotides containing 2'-deoxytubercidin. Nucleic Acids Res. 1988 Feb 25;16(4):1631–1632. doi: 10.1093/nar/16.4.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seela F., Kehne A. Palindromic octa- and dodecanucleotides containing 2'-deoxytubercidin: synthesis, hairpin formation, and recognition by the endodeoxyribonuclease EcoRI. Biochemistry. 1987 Apr 21;26(8):2232–2238. doi: 10.1021/bi00382a024. [DOI] [PubMed] [Google Scholar]
- Ulanovsky L. E., Trifonov E. N. Estimation of wedge components in curved DNA. Nature. 1987 Apr 16;326(6114):720–722. doi: 10.1038/326720a0. [DOI] [PubMed] [Google Scholar]
- Wang A. H., Ughetto G., Quigley G. J., Hakoshima T., van der Marel G. A., van Boom J. H., Rich A. The molecular structure of a DNA-triostin A complex. Science. 1984 Sep 14;225(4667):1115–1121. doi: 10.1126/science.6474168. [DOI] [PubMed] [Google Scholar]
- Waring M. J., Wakelin L. P. Echinomycin: a bifunctional intercalating antibiotic. Nature. 1974 Dec 20;252(5485):653–657. doi: 10.1038/252653a0. [DOI] [PubMed] [Google Scholar]