Abstract
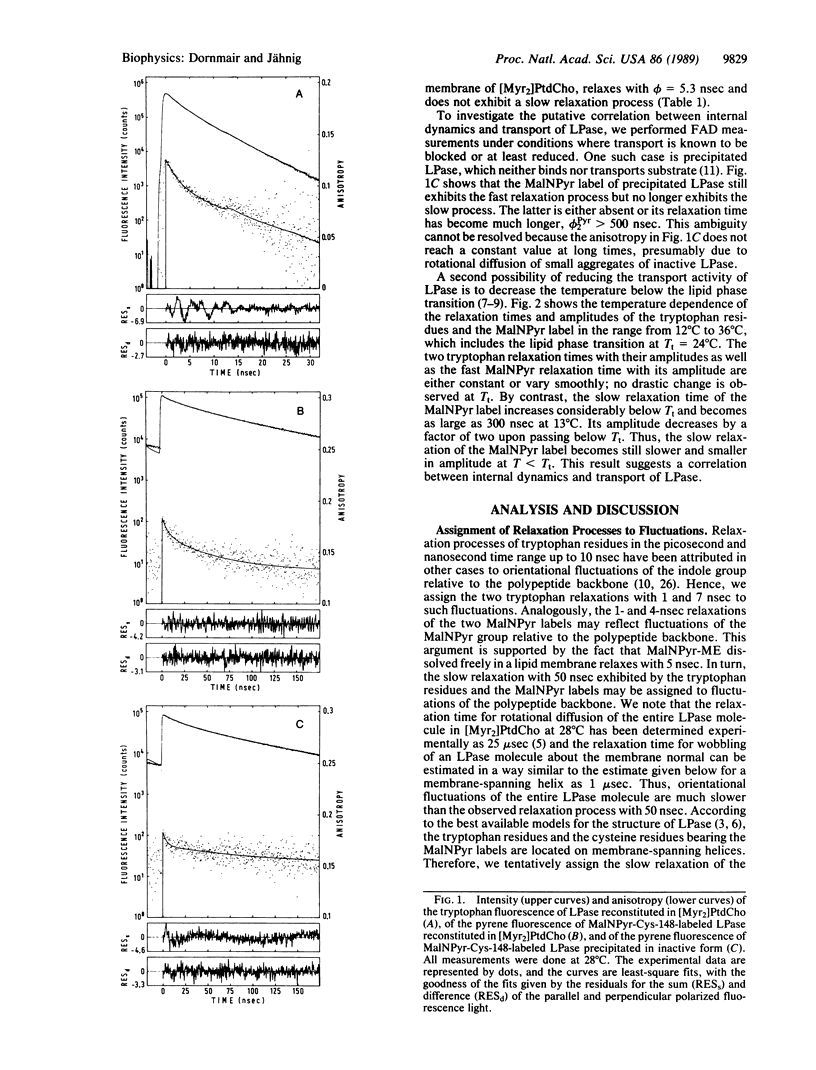
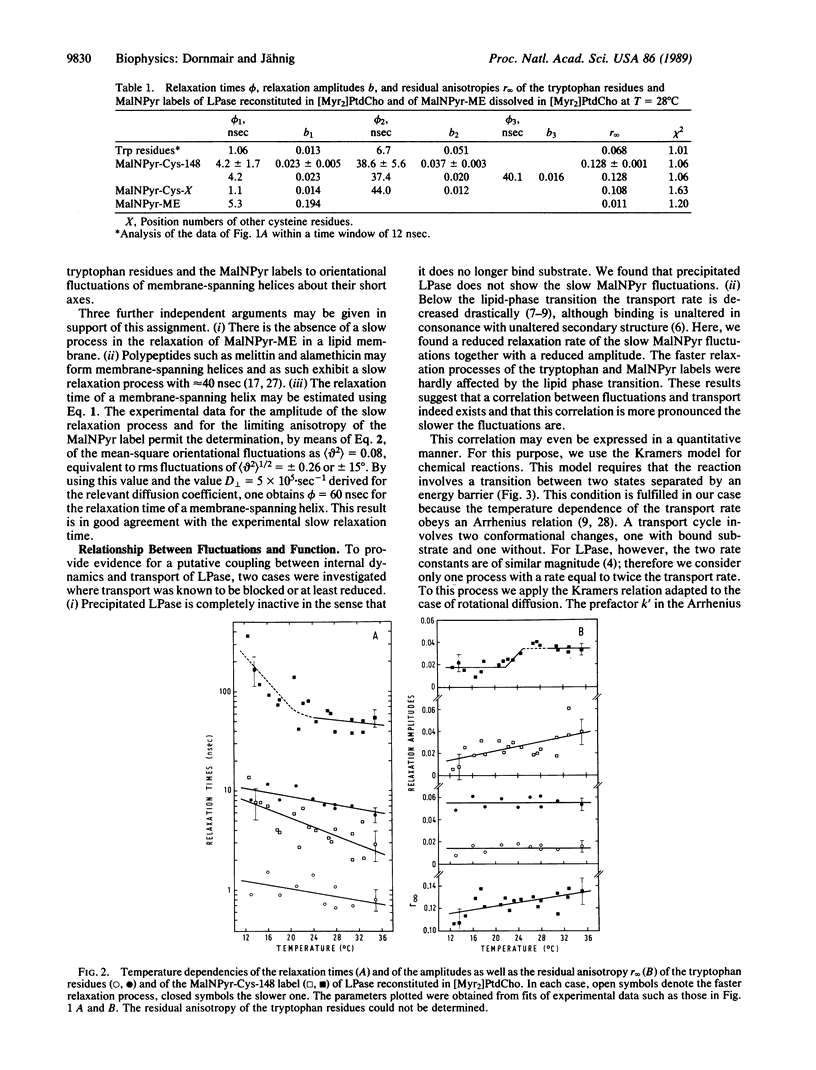
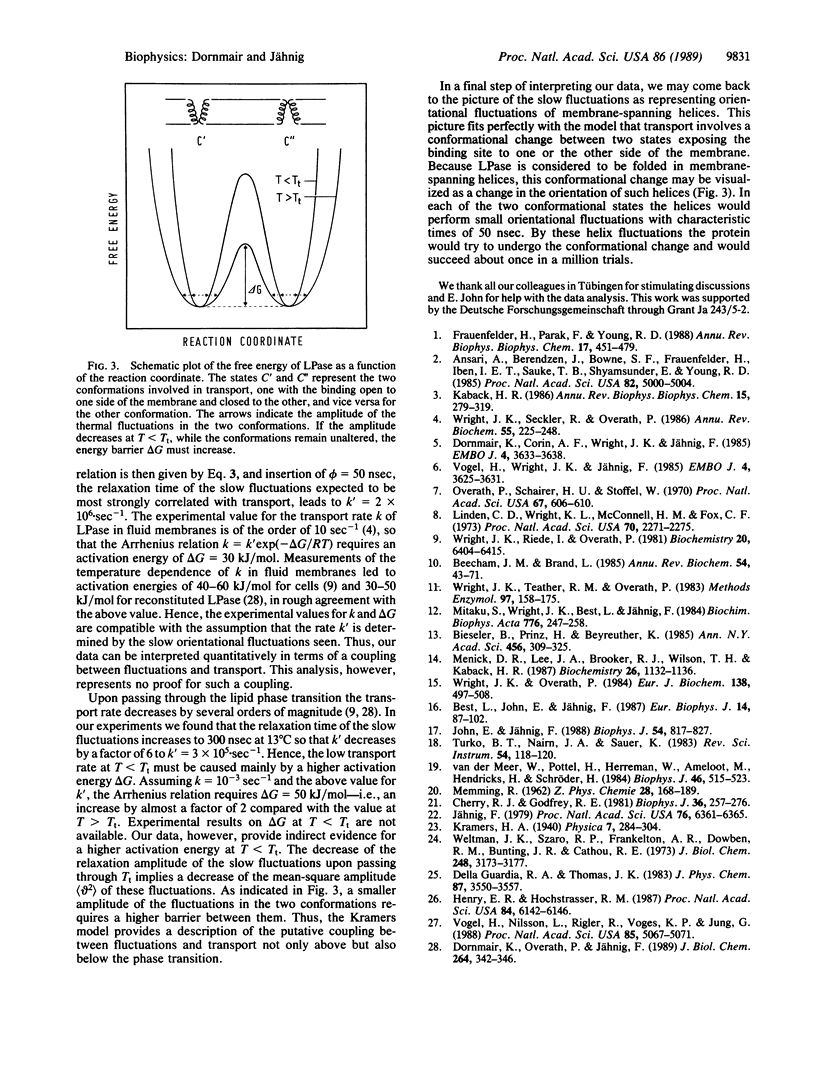
The transport protein lactose permease was reconstituted in vesicles of dimyristoylphosphatidylcholine, and the internal dynamics were studied by measuring the fluorescence anisotropy decay of the tryptophan residues and of a covalently bound pyrene label. For the tryptophans three relaxation processes and for the pyrene two relaxation processes with relaxation times in the nanosecond range were observed. The slowest process, of approximately 50 ns, is assigned to orientational fluctuations of membrane-spanning helices. When the temperature is decreased below the lipid-phase transition, this relaxation process is slowed down and restricted in amplitude. Because the transport rate is known to also decrease below the phase transition, this observation suggests a coupling between internal dynamics and transport. This coupling is analyzed on the basis of the Kramers relation for chemical reactions.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ansari A., Berendzen J., Bowne S. F., Frauenfelder H., Iben I. E., Sauke T. B., Shyamsunder E., Young R. D. Protein states and proteinquakes. Proc Natl Acad Sci U S A. 1985 Aug;82(15):5000–5004. doi: 10.1073/pnas.82.15.5000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beechem J. M., Brand L. Time-resolved fluorescence of proteins. Annu Rev Biochem. 1985;54:43–71. doi: 10.1146/annurev.bi.54.070185.000355. [DOI] [PubMed] [Google Scholar]
- Bieseler B., Prinz H., Beyreuther K. Topological studies of lactose permease of Escherichia coli by protein sequence analysis. Ann N Y Acad Sci. 1985;456:309–325. doi: 10.1111/j.1749-6632.1985.tb14882.x. [DOI] [PubMed] [Google Scholar]
- Cherry R. J., Godfrey R. E. Anisotropic rotation of bacteriorhodopsin in lipid membranes. Comparison of theory with experiment. Biophys J. 1981 Oct;36(1):257–276. doi: 10.1016/S0006-3495(81)84727-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornmair K., Corin A. F., Wright J. K., Jähnig F. The size of the lactose permease derived from rotational diffusion measurements. EMBO J. 1985 Dec 16;4(13A):3633–3638. doi: 10.1002/j.1460-2075.1985.tb04127.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dornmair K., Overath P., Jähnig F. Fast measurement of galactoside transport by lactose permease. J Biol Chem. 1989 Jan 5;264(1):342–346. [PubMed] [Google Scholar]
- Frauenfelder H., Parak F., Young R. D. Conformational substates in proteins. Annu Rev Biophys Biophys Chem. 1988;17:451–479. doi: 10.1146/annurev.bb.17.060188.002315. [DOI] [PubMed] [Google Scholar]
- Henry E. R., Hochstrasser R. M. Molecular dynamics simulations of fluorescence polarization of tryptophans in myoglobin. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6142–6146. doi: 10.1073/pnas.84.17.6142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- John E., Jähnig F. Dynamics of melittin in water and membranes as determined by fluorescence anisotropy decay. Biophys J. 1988 Nov;54(5):817–827. doi: 10.1016/S0006-3495(88)83019-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jähnig F. Structural order of lipids and proteins in membranes: evaluation of fluorescence anisotropy data. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6361–6365. doi: 10.1073/pnas.76.12.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Active transport in Escherichia coli: passage to permease. Annu Rev Biophys Biophys Chem. 1986;15:279–319. doi: 10.1146/annurev.bb.15.060186.001431. [DOI] [PubMed] [Google Scholar]
- Linden C. D., Wright K. L., McConnell H. M., Fox C. F. Lateral phase separations in membrane lipids and the mechanism of sugar transport in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2271–2275. doi: 10.1073/pnas.70.8.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menick D. R., Lee J. A., Brooker R. J., Wilson T. H., Kaback H. R. Role of cysteine residues in the lac permease of Escherichia coli. Biochemistry. 1987 Feb 24;26(4):1132–1136. doi: 10.1021/bi00378a022. [DOI] [PubMed] [Google Scholar]
- Mitaku S., Wright J. K., Best L., Jähnig F. Localization of the galactoside binding site in the lactose carrier of Escherichia coli. Biochim Biophys Acta. 1984 Oct 3;776(2):247–258. doi: 10.1016/0005-2736(84)90214-1. [DOI] [PubMed] [Google Scholar]
- Overath P., Schairer H. U., Stoffel W. Correlation of in vivo and in vitro phase transitions of membrane lipids in Escherichia coli. Proc Natl Acad Sci U S A. 1970 Oct;67(2):606–612. doi: 10.1073/pnas.67.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H., Nilsson L., Rigler R., Voges K. P., Jung G. Structural fluctuations of a helical polypeptide traversing a lipid bilayer. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5067–5071. doi: 10.1073/pnas.85.14.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel H., Wright J. K., Jähnig F. The structure of the lactose permease derived from Raman spectroscopy and prediction methods. EMBO J. 1985 Dec 16;4(13A):3625–3631. doi: 10.1002/j.1460-2075.1985.tb04126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weltman J. K., Szaro R. P., Frackelton A. R., Jr, Dowben R. M., Bunting J. R., Cathou B. E. N-(3-pyrene)maleimide: a long lifetime fluorescent sulfhydryl reagent. J Biol Chem. 1973 May 10;248(9):3173–3177. [PubMed] [Google Scholar]
- Wright J. K., Overath P. Purification of the lactose:H+ carrier of Escherichia coli and characterization of galactoside binding and transport. Eur J Biochem. 1984 Feb 1;138(3):497–508. doi: 10.1111/j.1432-1033.1984.tb07944.x. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Riede I., Overath P. Lactose carrier protein of Escherichia coli: interaction with galactosides and protons. Biochemistry. 1981 Oct 27;20(22):6404–6415. doi: 10.1021/bi00525a019. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Seckler R., Overath P. Molecular aspects of sugar:ion cotransport. Annu Rev Biochem. 1986;55:225–248. doi: 10.1146/annurev.bi.55.070186.001301. [DOI] [PubMed] [Google Scholar]
- Wright J. K., Teather R. M., Overath P. Lactose permease of Escherichia coli. Methods Enzymol. 1983;97:158–175. doi: 10.1016/0076-6879(83)97130-6. [DOI] [PubMed] [Google Scholar]
- van der Meer W., Pottel H., Herreman W., Ameloot M., Hendrickx H., Schröder H. Effect of orientational order on the decay of the fluorescence anisotropy in membrane suspensions. A new approximate solution of the rotational diffusion equation. Biophys J. 1984 Oct;46(4):515–523. doi: 10.1016/S0006-3495(84)84049-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


