Abstract
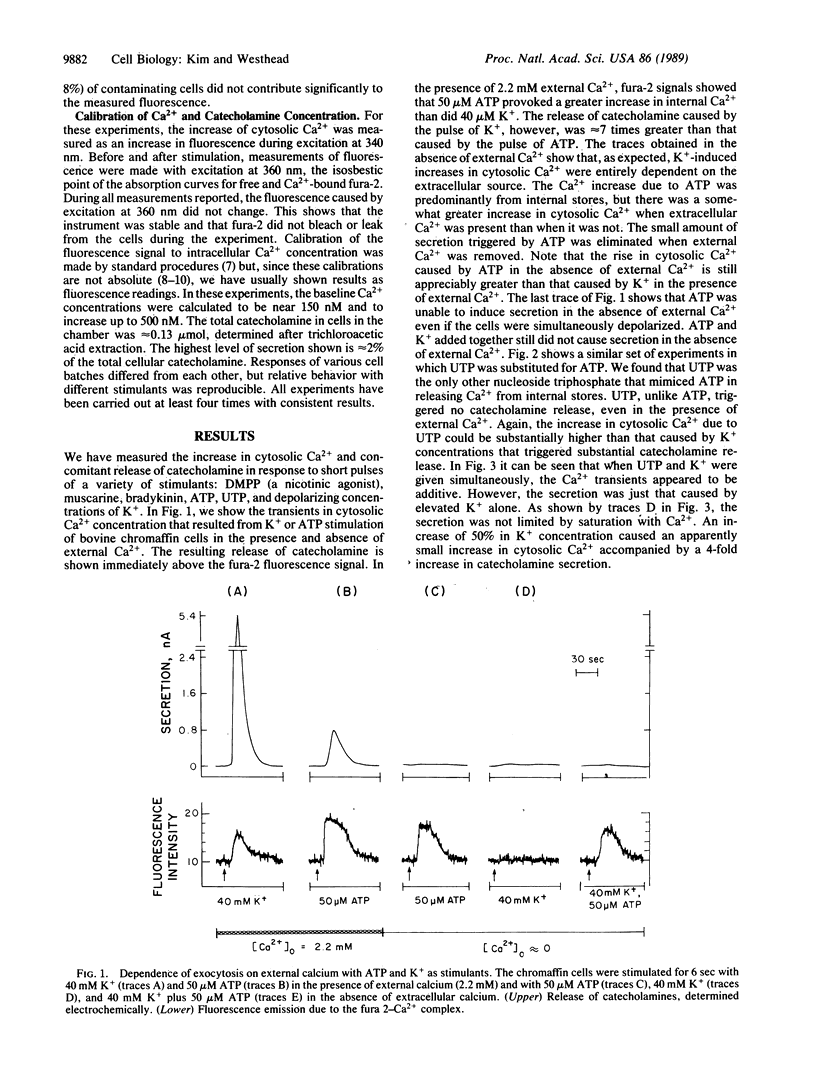
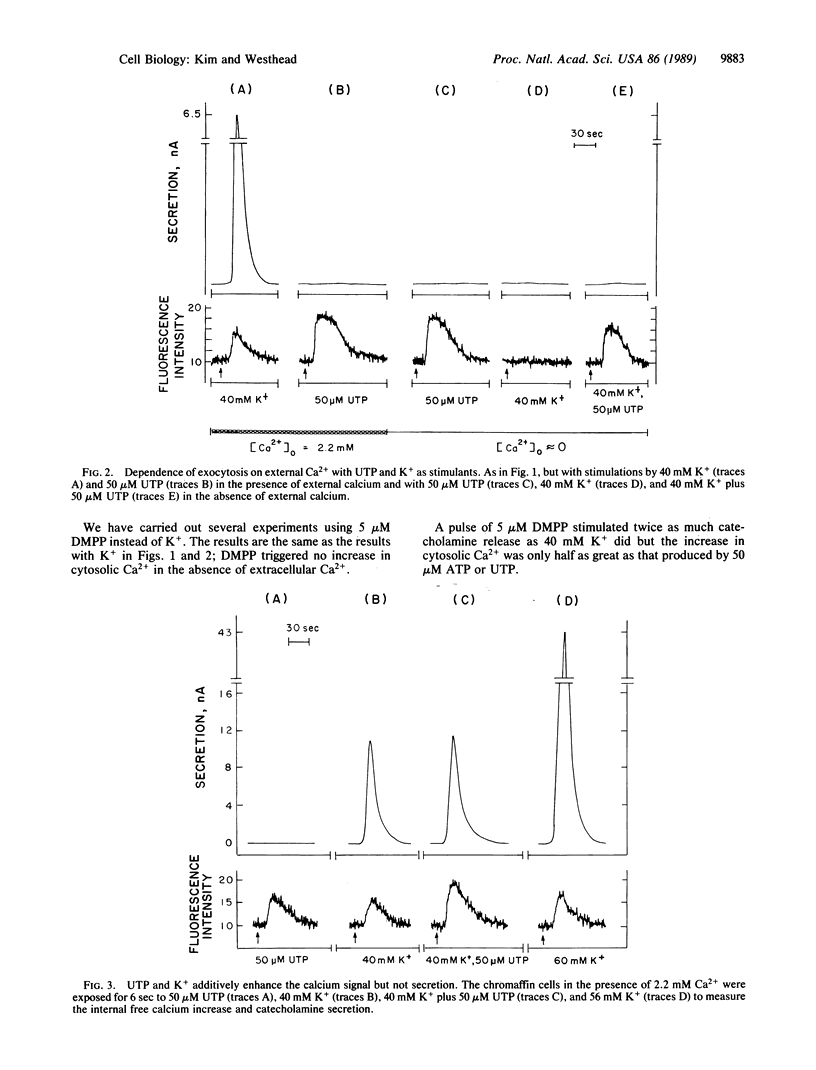
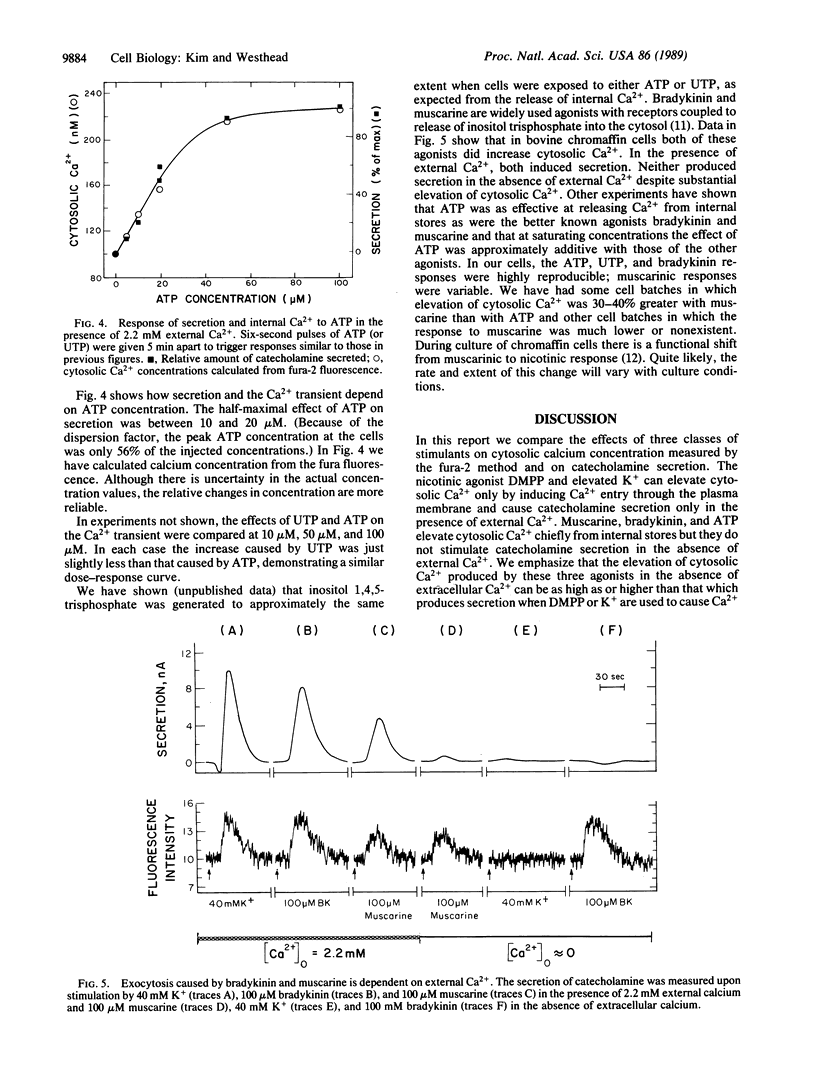
Bovine adrenal medullary cells, cultured on quartz plates, were superfused with buffer to which pulses of stimulant were added. Cytosolic Ca2+ was measured by the fura-2 fluorescence method and the simultaneously released catecholamine was measured electrochemically. When stimulant concentrations were adjusted to given equivalent elevations of cytosolic Ca2+, secretion depended entirely on whether Ca2+ came from internal stores or from the extracellular medium. Calcium from internal stores did not support secretion under these conditions. This nonequivalence of the two sources of cytosolic Ca2+ points to important differences in the physiological roles of the two sources of calcium. Dimethylphenylpiperazinium (a cholinergic agonist) and elevated K+ increased cytosolic Ca2+ and caused secretion only in the presence of external Ca2+. Bradykinin, muscarine, and ATP elevated cytosolic Ca2+ in the presence and absence of extracellular Ca2+ but caused secretion only in the presence of extracellular Ca2+. UTP, which in the absence of extracellular Ca2+ elevated cytosolic Ca2+ as effectively as ATP, did not cause detectable secretion under any circumstance. Because of the high Ca2+-buffering capacity of the cytosol, we expected that Ca2+ gradients, perhaps quite steep, would be produced by a pulse of Ca2+ entering the cytosol. Fura-2 fluorescence measures only the average free cytosolic Ca2+. Our data show that Ca2+ entering across the plasma membrane was much more effective at triggering exocytosis than was Ca2+ released from internal stores, suggesting that the two sources of Ca2+ are effectively compartmentalized, probably by concentration gradients in the cytosol.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol trisphosphate, a novel second messenger in cellular signal transduction. Nature. 1984 Nov 22;312(5992):315–321. doi: 10.1038/312315a0. [DOI] [PubMed] [Google Scholar]
- Chern Y. J., Kim K. T., Slakey L. L., Westhead E. W. Adenosine receptors activate adenylate cyclase and enhance secretion from bovine adrenal chromaffin cells in the presence of forskolin. J Neurochem. 1988 May;50(5):1484–1493. doi: 10.1111/j.1471-4159.1988.tb03034.x. [DOI] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: the concept and clues from chromaffin and other cells. Br J Pharmacol. 1968 Nov;34(3):451–474. doi: 10.1111/j.1476-5381.1968.tb08474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn L. A., Holz R. W. Catecholamine secretion from digitonin-treated adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4989–4993. [PubMed] [Google Scholar]
- Fisher S. K., Holz R. W., Agranoff B. W. Muscarinic receptors in chromaffin cell cultures mediate enhanced phospholipid labeling but not catecholamine secretion. J Neurochem. 1981 Aug;37(2):491–497. doi: 10.1111/j.1471-4159.1981.tb00482.x. [DOI] [PubMed] [Google Scholar]
- Grynkiewicz G., Poenie M., Tsien R. Y. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem. 1985 Mar 25;260(6):3440–3450. [PubMed] [Google Scholar]
- Harish O. E., Kao L. S., Raffaniello R., Wakade A. R., Schneider A. S. Calcium dependence of muscarinic receptor-mediated catecholamine secretion from the perfused rat adrenal medulla. J Neurochem. 1987 Jun;48(6):1730–1735. doi: 10.1111/j.1471-4159.1987.tb05730.x. [DOI] [PubMed] [Google Scholar]
- Kao L. S., Schneider A. S. Calcium mobilization and catecholamine secretion in adrenal chromaffin cells. A Quin-2 fluorescence study. J Biol Chem. 1986 Apr 15;261(11):4881–4888. [PubMed] [Google Scholar]
- Kao L. S., Schneider A. S. Muscarinic receptors on bovine chromaffin cells mediate a rise in cytosolic calcium that is independent of extracellular calcium. J Biol Chem. 1985 Feb 25;260(4):2019–2022. [PubMed] [Google Scholar]
- Knight D. E., Baker P. F. Calcium-dependence of catecholamine release from bovine adrenal medullary cells after exposure to intense electric fields. J Membr Biol. 1982;68(2):107–140. doi: 10.1007/BF01872259. [DOI] [PubMed] [Google Scholar]
- Konishi M., Olson A., Hollingworth S., Baylor S. M. Myoplasmic binding of fura-2 investigated by steady-state fluorescence and absorbance measurements. Biophys J. 1988 Dec;54(6):1089–1104. doi: 10.1016/S0006-3495(88)83045-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kretsinger R. H., Creutz C. E. Cell biology. Consensus in exocytosis. Nature. 1986 Apr 17;320(6063):573–573. doi: 10.1038/320573a0. [DOI] [PubMed] [Google Scholar]
- Nakaki T., Sasakawa N., Yamamoto S., Kato R. Functional shift from muscarinic to nicotinic cholinergic receptors involved in inositol trisphosphate and cyclic GMP accumulation during the primary culture of adrenal chromaffin cells. Biochem J. 1988 Apr 15;251(2):397–403. doi: 10.1042/bj2510397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poenie M., Alderton J., Steinhardt R., Tsien R. Calcium rises abruptly and briefly throughout the cell at the onset of anaphase. Science. 1986 Aug 22;233(4766):886–889. doi: 10.1126/science.3755550. [DOI] [PubMed] [Google Scholar]
- Sasakawa N., Nakaki T., Yamamoto S., Kato R. Stimulation by ATP of inositol trisphosphate accumulation and calcium mobilization in cultured adrenal chromaffin cells. J Neurochem. 1989 Feb;52(2):441–447. doi: 10.1111/j.1471-4159.1989.tb09140.x. [DOI] [PubMed] [Google Scholar]
- Schneider A. S., Cline H. T., Lemaire S. Rapid rise in cyclic GMP accompanies catecholamine secretion in suspensions of isolated adrenal chromaffin cells. Life Sci. 1979 Apr 9;24(15):1389–1394. doi: 10.1016/0024-3205(79)90009-2. [DOI] [PubMed] [Google Scholar]
- Tsien R. Y. Fluorescent probes of cell signaling. Annu Rev Neurosci. 1989;12:227–253. doi: 10.1146/annurev.ne.12.030189.001303. [DOI] [PubMed] [Google Scholar]
- Volpe P., Krause K. H., Hashimoto S., Zorzato F., Pozzan T., Meldolesi J., Lew D. P. "Calciosome," a cytoplasmic organelle: the inositol 1,4,5-trisphosphate-sensitive Ca2+ store of nonmuscle cells? Proc Natl Acad Sci U S A. 1988 Feb;85(4):1091–1095. doi: 10.1073/pnas.85.4.1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson S. P., Kirshner N. Calcium-evoked secretion from digitonin-permeabilized adrenal medullary chromaffin cells. J Biol Chem. 1983 Apr 25;258(8):4994–5000. [PubMed] [Google Scholar]


