Abstract
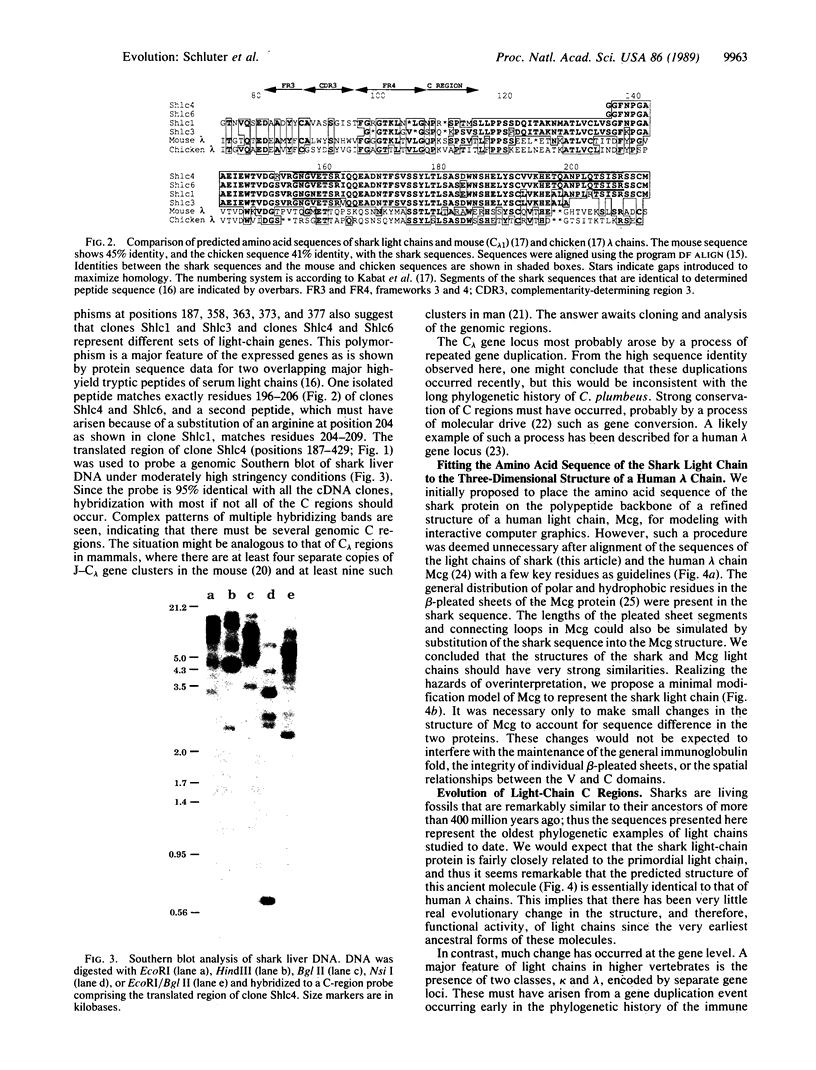
Sharks are living fossils that are indistinguishable morphologically from their Devonian ancestors of approximately equal to 400 million years ago. If parallel conservatism characterizes their biochemical evolution, characterization of their immunoglobulin chains could provide information regarding the primordial features of these essential defense molecules. Shark immunoglobulins are polydisperse like those of mammals, but these species lack homogeneous myeloma proteins. This heterogeneity has precluded direct determination of the sequence of elasmobranch light-chain proteins. We have sequenced four cDNA clones that contain the constant-region sequence as well as varying degrees of variable- or joining-region segments. The sandbar shark (Carcharhinus plumbeus) has at least four distinct light-chain constant regions, and these can be considered homologs of mammalian lambda chains. Approximately 40% identity was found in comparison from sharks to mammals. Certain stretches of sequence were remarkably conserved, whereas others varied in a manner consistent with accepted concepts of speciation. One hexapeptide (Ala-Thr-Leu-Val-Cys-Leu) occurred in lambda constant regions of all vertebrate species. There was a universal conservation of certain cysteines, phenylalanines, tryptophans, and glycines and strong identities in the block of residues from Ser-176 to Trp-186. Comparison of the shark sequence with that of the characterized human lambda myeloma protein Mcg indicates a strong conservation of three-dimensional structure in this light-chain domain representing species whose ancestors diverged early in vertebrate evolution. The shark light-chain sequence contains primordial features shared by mammalian kappa and lambda chains and by T-cell receptor beta chains.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beale D., Coadwell J. Unusual features of the T-cell receptor C domains are revealed by structural comparisons with other members of the immunoglobulin superfamily. Comp Biochem Physiol B. 1986;85(1):205–215. doi: 10.1016/0305-0491(86)90244-0. [DOI] [PubMed] [Google Scholar]
- Bothwell A. L., Paskind M., Reth M., Imanishi-Kari T., Rajewsky K., Baltimore D. Somatic variants of murine immunoglobulin lambda light chains. Nature. 1982 Jul 22;298(5872):380–382. doi: 10.1038/298380a0. [DOI] [PubMed] [Google Scholar]
- Chang H., Dmitrovsky E., Hieter P. A., Mitchell K., Leder P., Turoczi L., Kirsch I. R., Hollis G. F. Identification of three new Ig lambda-like genes in man. J Exp Med. 1986 Feb 1;163(2):425–435. doi: 10.1084/jem.163.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Clem L. W., Small P. A., Jr Phylogeny of immunoglobulin structure and function. I. Immunoglobulins of the lemon shark. J Exp Med. 1967 May 1;125(5):893–920. doi: 10.1084/jem.125.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dover G. A., Flavell R. B. Molecular coevolution: DNA divergence and the maintenance of function. Cell. 1984 Oct;38(3):622–623. doi: 10.1016/0092-8674(84)90255-1. [DOI] [PubMed] [Google Scholar]
- Feng D. F., Doolittle R. F. Progressive sequence alignment as a prerequisite to correct phylogenetic trees. J Mol Evol. 1987;25(4):351–360. doi: 10.1007/BF02603120. [DOI] [PubMed] [Google Scholar]
- Fett J. W., Deutsch H. F. Primary structure of the Mcg lambda chain. Biochemistry. 1974 Sep 24;13(20):4102–4114. doi: 10.1021/bi00717a007. [DOI] [PubMed] [Google Scholar]
- George D. G., Barker W. C., Hunt L. T. The protein identification resource (PIR). Nucleic Acids Res. 1986 Jan 10;14(1):11–15. doi: 10.1093/nar/14.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hood L., Kronenberg M., Hunkapiller T. T cell antigen receptors and the immunoglobulin supergene family. Cell. 1985 Feb;40(2):225–229. doi: 10.1016/0092-8674(85)90133-3. [DOI] [PubMed] [Google Scholar]
- Kokubu F., Hinds K., Litman R., Shamblott M. J., Litman G. W. Complete structure and organization of immunoglobulin heavy chain constant region genes in a phylogenetically primitive vertebrate. EMBO J. 1988 Jul;7(7):1979–1988. doi: 10.1002/j.1460-2075.1988.tb03036.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litman G. W., Berger L., Murphy K., Litman R., Hinds K., Erickson B. W. Immunoglobulin VH gene structure and diversity in Heterodontus, a phylogenetically primitive shark. Proc Natl Acad Sci U S A. 1985 Apr;82(7):2082–2086. doi: 10.1073/pnas.82.7.2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchalonis J. J., Schluter S. F., Hubbard R. A., Diamanduros A., Barker W. C., Pumphrey R. S. Conservation of immunoglobulin variable and joining region structure and the design of universal anti-immunoglobulin antibodies reactive with antigen-binding T cell receptors. Int Rev Immunol. 1988 Apr;3(3):241–273. doi: 10.3109/08830188809051191. [DOI] [PubMed] [Google Scholar]
- Marchalonis J. J., Schluter S. F., Hubbard R. A., McCabe C., Allen R. C. Immunoglobulin epitopes defined by synthetic peptides corresponding to joining region sequence: conservation of determinants and dependence upon the presence of an arginyl or lysyl residue for cross-reaction between light chains and T-cell receptor chains. Mol Immunol. 1988 Aug;25(8):771–784. doi: 10.1016/0161-5890(88)90113-7. [DOI] [PubMed] [Google Scholar]
- Marchalonis J., Edelman G. M. Phylogenetic origins of antibody structure. I. Multichain structure of immunoglobulins in the smooth dogfish (Mustelus canis). J Exp Med. 1965 Sep 1;122(3):601–618. doi: 10.1084/jem.122.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikoryak C. A., Steiner L. A. Amino acid sequence of the constant region of immunoglobulin light chains from Rana catesbeiana. Mol Immunol. 1988 Aug;25(8):695–703. doi: 10.1016/0161-5890(88)90105-8. [DOI] [PubMed] [Google Scholar]
- Novotný J., Tonegawa S., Saito H., Kranz D. M., Eisen H. N. Secondary, tertiary, and quaternary structure of T-cell-specific immunoglobulin-like polypeptide chains. Proc Natl Acad Sci U S A. 1986 Feb;83(3):742–746. doi: 10.1073/pnas.83.3.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenshein I. L., Marchalonis J. J. The immunoglobulins of carcharhine sharks: a comparison of serological and biochemical properties. Comp Biochem Physiol B. 1987;86(4):737–747. doi: 10.1016/0305-0491(87)90221-5. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selsing E., Miller J., Wilson R., Storb U. Evolution of mouse immunoglobulin lambda genes. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4681–4685. doi: 10.1073/pnas.79.15.4681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamblott M. J., Litman G. W. Complete nucleotide sequence of primitive vertebrate immunoglobulin light chain genes. Proc Natl Acad Sci U S A. 1989 Jun;86(12):4684–4688. doi: 10.1073/pnas.86.12.4684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udey J. A., Blomberg B. B. Intergenic exchange maintains identity between two human lambda light chain immunoglobulin gene intron sequences. Nucleic Acids Res. 1988 Apr 11;16(7):2959–2969. doi: 10.1093/nar/16.7.2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagi Y., Yoshikai Y., Leggett K., Clark S. P., Aleksander I., Mak T. W. A human T cell-specific cDNA clone encodes a protein having extensive homology to immunoglobulin chains. Nature. 1984 Mar 8;308(5955):145–149. doi: 10.1038/308145a0. [DOI] [PubMed] [Google Scholar]





