Abstract
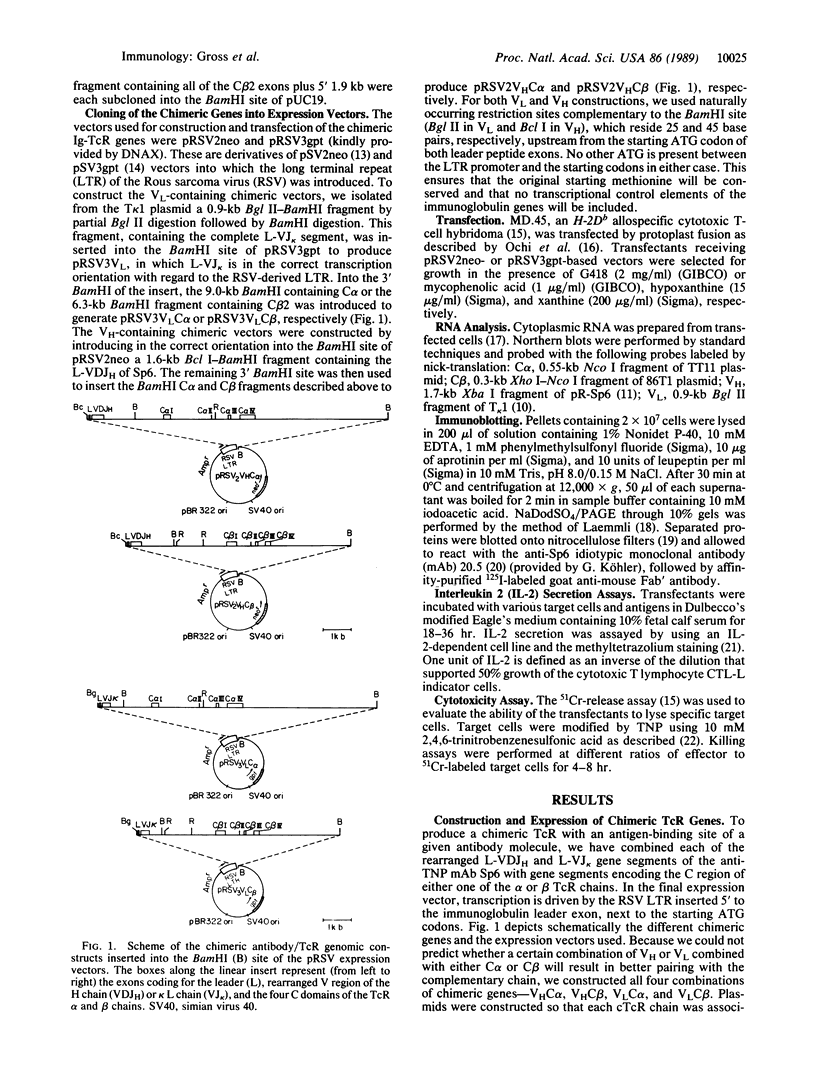
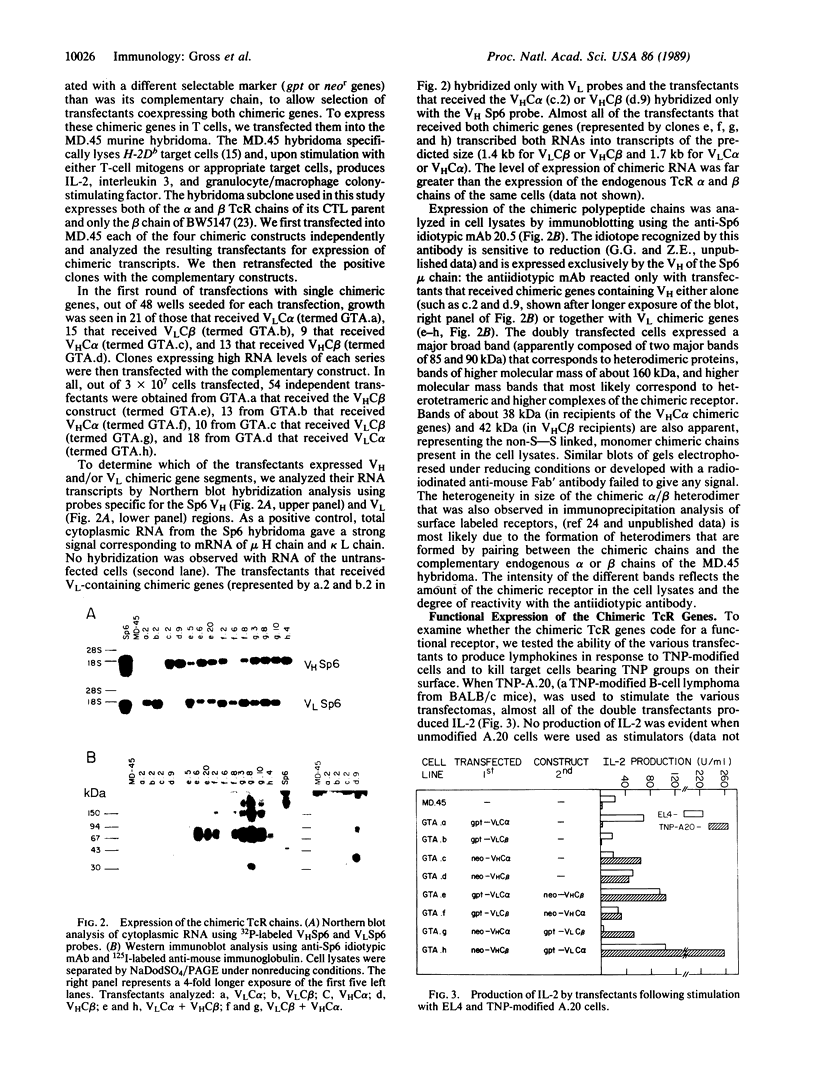
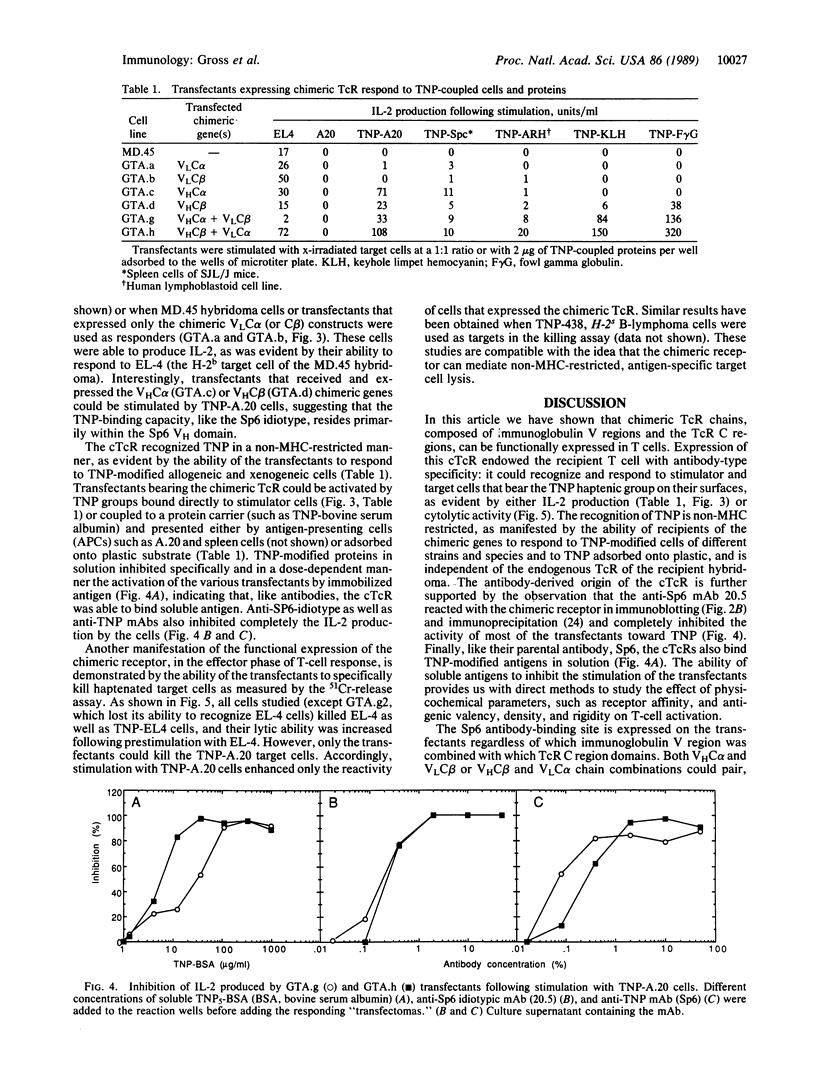
To design and direct at will the specificity of T cells in a non-major histocompatibility complex (MHC)-restricted manner, we have generated and expressed chimeric T-cell receptor (TcR) genes composed of the TcR constant (C) domains fused to the antibody's variable (V) domains. Genomic expression vectors have been constructed containing the rearranged gene segments coding for the V region domains of the heavy (VH) and light (VL) chains of an anti-2,4,6-trinitrophenyl (TNP) antibody (SP6) spliced to either one of the C-region gene segments of the alpha or beta TcR chains. Following transfection into a cytotoxic T-cell hybridoma, expression of a functional TcR was detected. The chimeric TcR exhibited the idiotope of the Sp6 anti-TNP antibody and endowed the T cells with a non-MHC-restricted response to the hapten TNP. The transfectants specifically killed and produced interleukin 2 in response to TNP-bearing target cells across strain and species barriers. Moreover, such transfectants responded to immobilized TNP-protein conjugates, bypassing the need for cellular processing and presentation. In the particular system employed, both the TNP-binding site and the Sp6 idiotope reside almost exclusively in the VH chain region. Hence, introduction into T cells of TcR genes containing only the VHSp6 fused to either the C alpha or C beta was sufficient for the expression of a functional surface receptor. Apparently, the VHC alpha or VHC beta chimeric chains can pair with the endogenous beta or alpha chains of the recipient T cell to form a functional alpha beta heterodimeric receptor. Thus, this chimeric receptor provides the T cell with an antibody-like specificity and is able to effectively transmit the signal for T-cell activation and execution of its effector function.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Chen K. C., Smith S. D., Rabbitts T. H. Fusion of an immunoglobulin variable gene and a T cell receptor constant gene in the chromosome 14 inversion associated with T cell tumors. Cell. 1985 Dec;43(3 Pt 2):705–713. doi: 10.1016/0092-8674(85)90243-0. [DOI] [PubMed] [Google Scholar]
- Baer R., Forster A., Rabbitts T. H. The mechanism of chromosome 14 inversion in a human T cell lymphoma. Cell. 1987 Jul 3;50(1):97–105. doi: 10.1016/0092-8674(87)90666-0. [DOI] [PubMed] [Google Scholar]
- Becker D. M., Pattern P., Chien Y., Yokota T., Eshhar Z., Giedlin M., Gascoigne N. R., Goodnow C., Wolf R., Arai K. Variability and repertoire size of T-cell receptor V alpha gene segments. Nature. 1985 Oct 3;317(6036):430–434. doi: 10.1038/317430a0. [DOI] [PubMed] [Google Scholar]
- Colman P. M., Deisenhofer J., Huber R. Structure of the human antibody molecule Kol (immunoglobulin G1): an electron density map at 5 A resolution. J Mol Biol. 1976 Jan 25;100(3):257–278. doi: 10.1016/s0022-2836(76)80062-9. [DOI] [PubMed] [Google Scholar]
- Denny C. T., Yoshikai Y., Mak T. W., Smith S. D., Hollis G. F., Kirsch I. R. A chromosome 14 inversion in a T-cell lymphoma is caused by site-specific recombination between immunoglobulin and T-cell receptor loci. Nature. 1986 Apr 10;320(6062):549–551. doi: 10.1038/320549a0. [DOI] [PubMed] [Google Scholar]
- Gascoigne N. R., Goodnow C. C., Dudzik K. I., Oi V. T., Davis M. M. Secretion of a chimeric T-cell receptor-immunoglobulin protein. Proc Natl Acad Sci U S A. 1987 May;84(9):2936–2940. doi: 10.1073/pnas.84.9.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross G., Gorochov G., Waks T., Eshhar Z. Generation of effector T cells expressing chimeric T cell receptor with antibody type-specificity. Transplant Proc. 1989 Feb;21(1 Pt 1):127–130. [PubMed] [Google Scholar]
- Hawley R. G., Shulman M. J., Murialdo H., Gibson D. M., Hozumi N. Mutant immunoglobulin genes have repetitive DNA elements inserted into their intervening sequences. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7425–7429. doi: 10.1073/pnas.79.23.7425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufmann Y., Berke G., Eshhar Z. Cytotoxic T lymphocyte hybridomas that mediate specific tumor-cell lysis in vitro. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2502–2506. doi: 10.1073/pnas.78.4.2502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kronenberg M., Siu G., Hood L. E., Shastri N. The molecular genetics of the T-cell antigen receptor and T-cell antigen recognition. Annu Rev Immunol. 1986;4:529–591. doi: 10.1146/annurev.iy.04.040186.002525. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Mory Y. Y., Keshet E., Ram D., Kaminchik Y. Analysis of mouse embryonic gene library for the frequency of single and multiple copy genes. Mol Biol Rep. 1980 Dec 31;6(4):203–208. doi: 10.1007/BF00777525. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mulligan R. C., Berg P. Selection for animal cells that express the Escherichia coli gene coding for xanthine-guanine phosphoribosyltransferase. Proc Natl Acad Sci U S A. 1981 Apr;78(4):2072–2076. doi: 10.1073/pnas.78.4.2072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A., Hawley R. G., Hawley T., Shulman M. J., Traunecker A., Köhler G., Hozumi N. Functional immunoglobulin M production after transfection of cloned immunoglobulin heavy and light chain genes into lymphoid cells. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6351–6355. doi: 10.1073/pnas.80.20.6351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A., Hawley R. G., Shulman M. J., Hozumi N. Transfer of a cloned immunoglobulin light-chain gene to mutant hybridoma cells restores specific antibody production. Nature. 1983 Mar 24;302(5906):340–342. doi: 10.1038/302340a0. [DOI] [PubMed] [Google Scholar]
- Painter R. G., Sage H. J., Tanford C. Contributions of heavy and light chains of rabbit immunoglobulin G to antibody activity. I. Binding studies on isolated heavy and light chains. Biochemistry. 1972 Apr 11;11(8):1327–1337. doi: 10.1021/bi00758a001. [DOI] [PubMed] [Google Scholar]
- Rusconi S., Köhler G. Transmission and expression of a specific pair of rearranged immunoglobulin mu and kappa genes in a transgenic mouse line. 1985 Mar 28-Apr 3Nature. 314(6009):330–334. doi: 10.1038/314330a0. [DOI] [PubMed] [Google Scholar]
- Schmitt-Verhulst A. M., Pettinelli C. B., Henkart P. A., Lunney J. K., Shearer G. M. H-2-restricted cytotoxic effectors generated in vitro by the addition of trinitrophenyl-conjugated soluble proteins. J Exp Med. 1978 Feb 1;147(2):352–368. doi: 10.1084/jem.147.2.352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz R. H. T-lymphocyte recognition of antigen in association with gene products of the major histocompatibility complex. Annu Rev Immunol. 1985;3:237–261. doi: 10.1146/annurev.iy.03.040185.001321. [DOI] [PubMed] [Google Scholar]
- Southern P. J., Berg P. Transformation of mammalian cells to antibiotic resistance with a bacterial gene under control of the SV40 early region promoter. J Mol Appl Genet. 1982;1(4):327–341. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traunecker A., Dolder B., Karjalainen K. A novel approach for preparing anti-T cell receptor constant region antibodies. Eur J Immunol. 1986 Jul;16(7):851–854. doi: 10.1002/eji.1830160722. [DOI] [PubMed] [Google Scholar]
- Traunecker A., Dolder B., Oliveri F., Karjalainen K. Solubilizing the T-cell receptor--problems in solution. Immunol Today. 1989 Jan;10(1):29–32. doi: 10.1016/0167-5699(89)90062-5. [DOI] [PubMed] [Google Scholar]




