Abstract
Mouse embryonic stem (ES) cells differentiate into cells of all three primary germ layers including endodermal cells that produce insulin in vitro. We show that constitutive expression of Pax4 (Pax4+), and to a lesser extent Pdx1 (Pdx1+), affects the differentiation of ES cells and significantly promote the development of insulin-producing cells. In Pax4 overexpressing R1 ES cells, isl-1, ngn3, insulin, islet amyloid polypeptide, and glucose transporter 2 (Glut-2) mRNA levels increase significantly. The number of nestin-expressing (nestin+) cells also increases. Constitutive Pax4 expression combined with selection of nestin+ cells and histotypic culture conditions give rise to spheroids containing insulin-positive granules typical of embryonal and adult β cells. In response to glucose, Pax4+ and wild-type ES-derived cells release insulin. Transplantation of these cells into streptozotocin-treated diabetic mice results in a normalization of blood glucose levels. We conclude that constitutive expression of Pax4 in combination with histotypic cultivation facilitates ES cell differentiation into the pancreatic lineage, which leads to the formation of islet-like spheroid structures that produce increased levels of insulin.
Transplantation of pancreatic islet cells is a promising therapeutic option for the treatment of diabetes. However, the lack of suitable donor tissues remains a major obstacle. Transplantation of adult or fetal islets of Langerhans as a therapy for insulin-dependent diabetes is limited because of the low availability of human donor pancreas (1). Pancreatic stem cells residing within the ductal epithelium have been used to generate mouse and human islet-like clusters (2, 3), which partially reverted insulin-dependent diabetes in animal models (3), but low proliferation rates may limit its wide scale application.
An alternative source for the generation of insulin-producing cells are embryonic stem (ES) cells which have almost unlimited proliferation capabilities while retaining the potential to differentiate in vitro into cells of the three primary germ layers (4, 5). ES cells of mice (6, 7) and human (8) have been shown to differentiate into insulin-secreting cells and to normalize blood glucose levels when transplanted into diabetic mouse models (6). Because this approach may be inefficient and generated in most cases only low amounts of insulin-producing cells, directing ES cells toward the β cell lineage would most likely improve their formation.
During embryogenesis, several growth and transcription factors are involved in β cells differentiation (for review, see ref. 9). Pdx1 and Pax4 are essential for proper β cell development. Pdx1 is expressed throughout the epithelium of early pancreatic buds and becomes restricted to β cells in the adult animal where it plays a role in insulin expression and glucose response (10). Pdx1-mutant mice do not develop any pancreas (11, 12). Pax4 is restricted to the β and δ cell lineages, and mice lacking Pax4 fail to develop any β cells and are diabetic (13).
Pancreatic progenitor cells are at present poorly defined. The four endocrine cell types arise from a common progenitor cell residing within the epithelium of the pancreatic bud. In adult animals, pancreatic stem cells are thought to reside in the exocrine duct system and within the islet itself. Recently, cells expressing the neurofilament protein nestin are proposed to represent multipotent progenitor cells of the pancreas (14, 15), and on special cultivation, insulin-producing cells have been derived in vitro from nestin-positive cells (7, 15).
In the present study, we analyzed the influence of constitutive expression of Pdx1 and Pax4 genes on ES cell differentiation into pancreatic cells and developed protocols for efficient generation of insulin-producing cells. We show that activation of Pax4 expression in ES cells and selective differentiation via nestin+ progenitor cells followed by histotypic culture increases the amount of insulin produced by the cells.
Materials and Methods
Generation of Vectors and Cell Lines.
The mouse Pdx1 or Pax4 gene was placed under the control of the cytomegalovirus (CMV) early promoter/enhancer region by inserting the complete cDNA of each gene into the vector pACCMV.pLpA (16). Mouse ES cells of line R1 [wild type (wt)] (17) were coelectroporated (18) with CMV-Pdx1 or CMV-Pax4 and the neomycin resistance gene under the control of the phosphoglycerate kinase I promoter [pGKneo (19)].
Cell Culture and Differentiation Conditions.
The wt cells and cells constitutively expressing Pdx1 (Pdx1+) and Pax4 (Pax4+) were cultivated on feeder layer of mouse embryonic fibroblasts in the presence of recombinant human leukemia inhibitory factor as described (5). Pdx1+ and Pax4+ cells were additionally supplemented with 300 ng/ml G418.
For differentiation (“basic” protocol), wt, Pdx1+, and Pax4+ ES cells (n = 600) were used to form embryoid bodies (EBs) that were cultivated in hanging drops as described (5). At day 5, EBs were plated in Iscove's modified Dulbecco's medium (IMDM, GIBCO) supplemented with 20% FCS, l-glutamine, nonessential amino acids, and α-monothioglycerol (see ref. 5).
Alternatively, wt and Pax4+ ES cells (n = 200) were cultivated as EBs in hanging drops and plated at day 4, and nestin-positive cells were selected by the “nestin+ selection” protocol (see ref. 20). At day 4 + 8, EB outgrowths were dissociated by 0.1% trypsin (GIBCO)/0.08% EDTA (Sigma) (1:1) in PBS for 1 min, collected by centrifugation, and replated onto poly-l-ornithine/laminin-coated tissue culture dishes in DMEM/F12 (GIBCO) containing 20 nM progesterone, 100 μM putrescine, 1 μg/ml laminin, 10 mM nicotinamide, 25 μg/ml insulin, 30 nM sodium selenite (all from Sigma), 50 μg/ml transferrin, 5 μg/ml fibronectin, B27 media supplement (all from GIBCO), and 20% FCS (7). At day 4 + 9, FCS was removed.
Estimation of mRNA Levels by Semiquantitative RT-PCR Analysis.
Total cellular mRNA of wt, Pdx1+, and Pax4+ ES cells, EBs or EB outgrowths were reverse transcribed, amplified by PCR, electrophoretically separated, and analyzed as described (5). For semiquantitative determination of mRNA levels of the candidate genes [shh, isl-1, Pdx1, ngn3, Pax6, insulin, islet amyloid polypeptide, glucose transporter 2 (Glut-2), Pax4, and β-tubulin, primer sequences available on request], transcript levels were standardized to the corresponding β-tubulin level, and for each candidate gene, mRNA levels relative to the highest candidate gene level were estimated in percentage (5).
Immunofluorescence and Quantitative Immunoassay.
Cells were fixed with 4% paraformaldehyde processed for immunofluorescence microscopy as described (5). The following primary Abs were used: mouse anti-insulin (Sigma), rabbit anti-glucagon, rabbit anti-PP, rabbit antisomatostatin (all from Dako), and mouse antinestin (clone rat 401, Developmental Studies Hybridoma Bank, Iowa City). Cy3-conjugated goat anti-mouse IgG and fluorescein (DTAF)-conjugated goat anti rabbit IgG (both from Jackson ImmunoResearch) were used as secondary Abs. Samples were analyzed by the fluorescence microscope ECLIPSE TE300 (Nikon) and the confocal laser scanning microscope LSM-410 (Zeiss). Because insulin- and nestin-positive cells were predominantly found in multilayered, compacted clusters and localized to different structures (cytoplasm or intermediate filament proteins), the percentage values of immunolabeled cells could be estimated only in arbitrary units. Therefore, a semiquantitative immunofluorescence imaging analysis was performed by using the lucia m, version 3.52a software (Nikon). Randomly selected (n = 25) but representative areas of each sample were analyzed for the labeling index (LI) defined by the ratio of the positive signal area to the measured area.
Quantitative Insulin Determination by ELISA.
To estimate total cellular and secreted insulin levels, differentiated wt and Pax4+ cells were preincubated for 90 min at 37°C in Krebs Ringer Bicarbonate Hepes buffer supplemented with 2.5 mM glucose. For induced insulin release, cells were further incubated in Krebs Ringer Bicarbonate Hepes buffer supplemented with 27.7 mM glucose and alternatively with 5.5 mM glucose and 10 μM tolbutamide (Sigma) for 15 min at 37°C. The control was incubated with 5.5 mM glucose. Proteins were extracted from the cells with acid ethanol at 4°C overnight, followed by cell sonification. Determination of cellular and secreted insulin was performed by using an insulin ELISA kit (Mercodia, Uppsala). Protein was determined by the Bradford assay (Bio-Rad).
Histotypic Maturation into Spheroids.
The wt and Pax4+ cell clusters cultivated according to the nestin+ selection protocol at stage 4 + 28 d were dissociated by 0.1% trypsin/0.08% EDTA in PBS (1:1) for 1 min, collected by centrifugation and transferred into 6 cm bacteriological plates in the medium described for nestin+ selection at stage 4 + 9 d. After overnight culture in suspension, spheres were transferred into 100-ml “Spinner” flasks and cultured in the CELLSPIN system (Integra Bioscience, Wallisellen, Switzerland) at 30 rpm agitation at 37°C up to 10 d.
For immunohistochemical detection of insulin and glucagon, spheroids were fixed in Bouin solution, embedded in paraffin, sectioned at 5 μm by conventional techniques, and immunolabeled as described above.
Immunogold Labeling and Electron Microscopy.
Spheroids were fixed in 0.1% glutaraldehyde/4% formaldehyde in 0.1 M cacodylate buffer, transferred to 0.1 M cacodylate buffer, and embedded. Ultrathin sections were sequentially treated with 10% H2O2, washed with 0.9% NaCl, blocked with 3% BSA, incubated overnight with the primary Ab in PBS supplemented with 0.5% BSA (rabbit anti-porcine insulin, 1:50, ICN), washed with PBS, and reblocked with goat serum. Binding of the primary Ab was visualized with gold labeled secondary Ab (15-nm gold particles, goat–anti-rabbit, Amersham Pharmacia), and electronmicroscopy was performed after contrasting the sections. Tissue samples of adult murine pancreas (control) were fixed and processed as described.
Transplantation into Streptozotocin (STZ)-Diabetic Mice.
Nonfasted 8- to 10-wk-old male BALB/c mice were treated with 200 mg/kg body weight STZ (Sigma) freshly dissolved in 0,025 M tri-sodium citrate 2-hydrate (pH 4.0). Mice were anaesthetized by i.p. injection of 15 μl/g body weight avertin (2.5% tribromoethyl alcohol:tertiary amyl alcohol). The left kidney and the spleen were exposed through a lumbar incision and cells were transferred into each tissue by using a blunt 30-gauge needle (Hamilton). Between 1 × 106 and 2 × 106 cells were transplanted per animal 24–48 h after STZ treatment. Blood glucose was measured daily between 9:00 and 11:00 a.m. from snipped tail by using a OneTouch FastTake blood glucose monitoring system (LifeScan, Mountain View, CA). Recipient animals were killed by cervical dislocation 14 d after STZ treatment, and the kidney and spleen were analyzed by immunohistochemistry on paraffin sections for the presence of insulin-producing cells (13).
Results
Pax4 Activation in ES Cells Modified the Differentiation Pattern and Expression Levels of Pancreatic Genes.
To assess whether developmental control genes expressed during pancreas development can enhance the differentiation of ES cells into insulin-producing cells, we generated ES cell lines expressing the Pdx1 or Pax4 gene under the control of the human CMV early promoter/enhancer region. After EB formation, no differences were observed in the diameter of 5-d-old EBs (between 0.44 and 0.49 mm) derived from wt, Pdx1+, and Pax4+ cells, suggesting that transgene expression did not affect ES cell proliferation within the EB. Constitutive expression of Pdx1 modestly affected the ES cell differentiation pattern; however, significant differences were found in Pax4+-derived cells. Cardiac differentiation was delayed and reduced, and the degree of skeletal and neuronal differentiation was significantly lower in Pax4+ cells in comparison to wt cells (not shown).
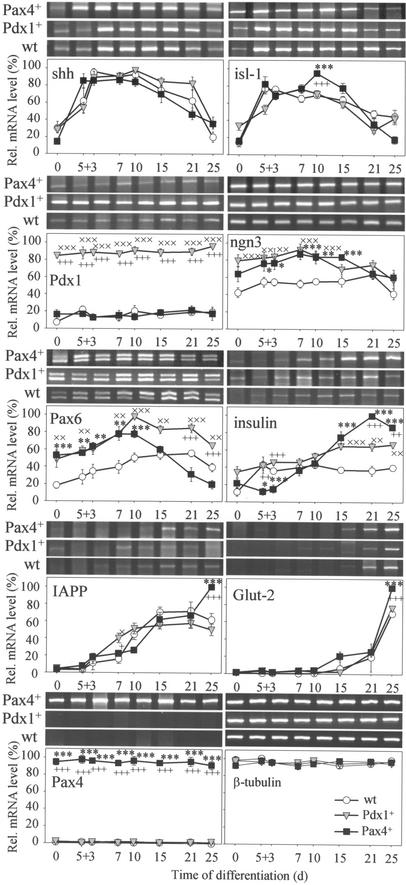
The morphological studies were confirmed by RT-PCR analyses of pancreatic developmental control genes (Fig. 1). Important changes in the expression levels of those genes were detected in Pax4+ and, to a lesser extent, Pdx1+ cells. No differences in shh mRNA levels were found among wt, Pdx1+, and Pax4+ cells. For isl-1, likewise, we detected few differences in expression between wt and Pdx1+ cells, whereas Pax4+-derived cells displayed a maximum mRNA level at differentiation stage 5 + 10 d. As expected, Pdx1+ cells showed 4- to 5-fold higher Pdx1 levels at all stages when compared to wt and Pax4+ cells. Ngn3 mRNA levels were up-regulated at early and intermediate stages in both Pdx1+ and Pax4+ cells. Pax6 levels were significantly higher in Pdx1+-derived cells at all stages, whereas in Pax4+ cells, Pax6 levels were up-regulated during early differentiation stages but significantly down-regulated during later stages. Low insulin mRNA levels were found in undifferentiated wt, Pdx1+, and Pax4+ cells, but after differentiation, insulin expression increased in all cell lines with maximum levels measured in Pax4+ cells. Maximum levels of islet amyloid polypeptide and Glut-2 mRNA were recorded only in Pax4+ cells at a late stage. High Pax4 transcript levels were measured in Pax4+ cells; however, no detectable transcript levels were found in wt and Pdx1+ cells (Fig. 1).
Figure 1.
RT-PCR analysis of wt-, Pdx1+-, and Pax4+-derived cells cultivated according to the basic protocol. Sonic hedgehog (shh), islet-1 (isl-1), Pdx1, neurogenin3 (ngn3), Pax6, insulin, islet amyloid polypeptide (IAPP), Glut-2, Pax4, and β-tubulin mRNA levels were determined. Each value (n = five experiments) represents mean ± SEM. Statistical significance was tested between wt and Pdx1+ (×), wt and Pax4+ (*), and Pdx1+ and Pax4+ (+) cells, respectively, by Student's t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
Pax4 Expression Increased the Number of Insulin- but not Glucagon-Producing Cells.
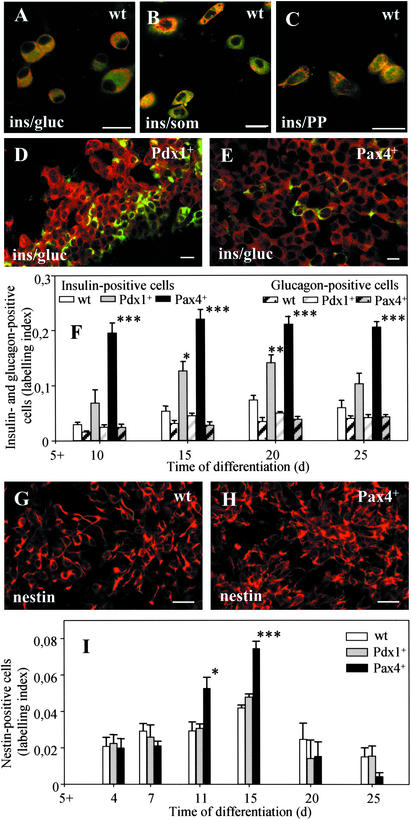
To investigate the expression of the pancreatic hormones insulin, glucagon, somatostatin, and PP in wt, Pdx1+, and Pax4+ cells, immunofluorescence analyses were performed. The wt ES cells spontaneously differentiated into cells predominantly coexpressing all four pancreatic hormones (Fig. 2A–C), whereas few cells were positive for insulin only. The amount of insulin-positive cells was estimated by the LI. The wt cells showed a LI of 0.074 corresponding to ≈10–15% insulin-positive cells (Fig. 2F). No significant changes were detected in the proportion of insulin- vs. glucagon-positive cells during all differentiation stages (Fig. 2F). In Pdx1+ cells, a higher number of insulin-positive cells was observed (LI: 0.12) corresponding to ≈20% of the total cell population (Fig. 2 D and F).
Figure 2.
Immunofluorescence analysis of pancreatic proteins (A–F) and of nestin (G–I) of wt- (A–C and G), Pdx1+- (D), and Pax4+ (E and H)-derived cells differentiated according to the basic protocol. Shown are wt cells expressing insulin and glucagon (ins/gluc, A), insulin and somatostatin (ins/som, B), and insulin and pancreatic polypeptide (ins/PP, C), and shown are Pdx1+ (D), and Pax4+ (E) cells expressing insulin or glucagon (ins/gluc) at days 5 + 15 to 5 + 20. Semiquantitative immunofluorescence analysis was done to estimate the labeling index of insulin and glucagon (F) and of nestin (I) in wt, Pdx1+, and Pax4+ cells. Each value of n = 5 (F) and n = 4 (I) experiments represents mean ± SEM. Statistical significance was tested by Student's t test: *, P < 0.05; **, P < 0.01, ***; P < 0.001. (Bar = 20 μm.)
In Pax4+ cells, the amount of insulin-positive cells was significantly higher with a LI of 0.24 corresponding to ≈60% insulin-positive cells that were mainly organized in compact clusters (Fig. 2E). Pax4+ cells revealed a constant 4.5-fold increase throughout the differentiation period, whereas the level of glucagon-positive cells remained constant at all stages (Fig. 2F), indicating that Pax4+ cells differentiated mainly into cells producing only insulin.
Pax4 Expression Resulted in an Increase in the Number of Nestin-Positive Cells.
Nestin expression has been used to select ES-derived cells capable of differentiation into insulin-producing cells in vitro (7). Consequently, we performed a comparative analysis of nestin expression in wt, Pdx1+, and Pax4+ ES cells after differentiation according to the basic protocol (Fig. 2G–I). Pax4+ cells showed a significant increase in the number of nestin+ cells at stages 5 + 11 d and 5 + 15 d (Fig. 2H) when compared to wt (Fig. 2G) or Pdx1+ (not shown) cells (Fig. 2I).
In subsequent experiments, wt and Pax4+ ES cells were differentiated under culture conditions selective for the development of nestin-expressing cells [nestin+ selection protocol (20)]. An acceleration and increase in the amount of nestin-positive cells (Fig. 3 A and B) was found when compared to the results obtained with the basic protocol (Fig. 2I). Differentiated Pax4+ cells generated a higher number of nestin+ cells at an earlier differentiation stage (compare LI at stages 5 + 4 and 5 + 7 d of Fig. 2I with stages 4 + 4 and 4 + 7 d of Fig. 3F).
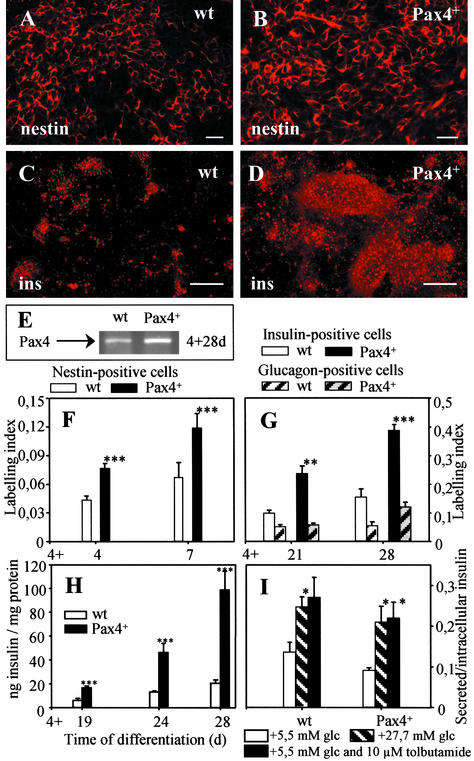
Figure 3.
Immunofluorescence (A–D) and semiquantitative imaging analysis (F and G) of nestin (A, B, and F) and insulin (ins, C, D, and G) labeling of wt- (A and C) and Pax4+ (B and D)-derived cells at days 4 + 7 (A, B, and F; n = 4) and 4 + 28 (C, D, and G; n = 3, with two parallels) differentiated according to the nestin+ selection protocol. (E) Pax4 mRNA level after differentiation via the nestin+ selection protocol at day 4 + 28 in wt and Pax4+ cells. Intracellular insulin levels (H, n = 5) and glucose-induced insulin release (I, n = 3) of wt- and Pax4+-derived cells were determined by ELISA. Insulin release expressed by the ratio of secreted to intracellular insulin levels (I) was measured in response to 27.7 mM glucose (striped), 5.5 mM glucose with 10 μM tolbutamide (black) in wt and Pax4+ cells after 15 min of static incubation; 5.5 mM glucose was used as control (white). Each value represents mean ± SEM. Statistical significance was tested by Student's t test: *, P < 0.05; **, P < 0.01; ***, P < 0.001. [Bar = 20 μm (A and B) and 200 μm (C and D).]
Differentiation of Pax4+ Cells According to the Nestin+ Selection Protocol Resulted in an Increase of Intracellular Insulin Levels.
By applying culture conditions selective for the development of nestin+ cells (20) followed by induction of pancreatic differentiation (7), Pax4+ ES cells resulted in a considerable increase in the number of insulin-producing cells (Fig. 3D) in comparison to wt cells (Fig. 3C). Differentiation of wt cells according to the nestin+ selection protocol resulted in significant Pax4 transcript levels at stage 4 + 28 d (compare Fig. 3E to Fig. 1).
Imaging analysis of immunofluorescence signals confirmed that Pax4 expression significantly increased the LI (0.39 corresponding to 80% insulin-positive cells), whereas wt cells resulted in a lower LI of 0.15 equivalent to ≈20–25% insulin-positive cells (Fig. 3 C, D, and G). No significant differences in glucagon-labeling were observed between wt and Pax4+ cells (Fig. 3G).
Intracellular insulin levels analyzed by ELISA were significantly increased in Pax4+ cells (98.7 ng insulin/mg protein), whereas wt cells showed only a moderate increase (20.7 ng insulin/mg protein) at stage 4 + 28 d (Fig. 3H). To determine, whether wt and Pax4+-derived cells were glucose-responsive, we analyzed insulin release in the presence of low (5 mM) and high glucose (27.7 mM) concentration. Both, wt and Pax4+ cells secreted insulin in response to glucose at an advanced stage of 4 + 32 d (but not at 4 + 28 d), suggesting a maturation effect during differentiation. wt and Pax4+ cells also responded to tolbutamide, a sulfonylurea known to stimulate insulin secretion (Fig. 3I).
Histotypic Generation of Spheroids and Ultrastructural Analysis of Insulin-Producing Cells.
Cells generated according to the nestin+ selection protocol for 4 + 28 d followed by 10-d histotypic “Spinner” cultivation showed significant accumulation of insulin and glucagon in spheroids (Fig. 4 A and B). The intracellular insulin levels amounted to 297.4 ± 18.0 (wt) and 455.5.6 ± 64.8 (Pax4+) ng/mg protein.
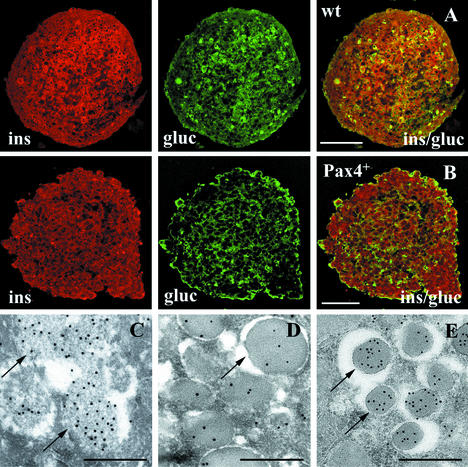
Figure 4.
Immunofluorescence (A and B) and electronmicroscopical (C–E) analysis of wt- (A) and Pax4+ (B–D)-derived spheroids generated by nestin+ selection and 10-d differentiation in histotypic culture. Shown are insulin- (red) and glucagon (green)-labeled sections of Pax4+ spheroids with faint globular structures (C, arrow) filled with floccular low-density material labeled with gold grains indicating insulin. More differentiated Pax4+ cells showed cytoplasmic granules with an electron dense core (D) in comparison to insulin-labeled secretory granules of β cells of adult mouse pancreas (E). [Bar = 50 μm (A and B) and 0.5 μm (C-–E).]
Immune electronmicroscopy of Pax4+ cells showed numerous insulin-positive cells (Fig. 4 C and D). Immunogold-labeling detected faint globular structures of different size filled with floccular low density material (Fig. 4C) and some granules with an electron dense core at the apical pole of the cells (Fig. 4D). The insulin-positive granules were of comparable size to secretory granules of β cells of the adult murine pancreas (Fig. 4E); however the labeling density was lower in Pax4+ derived spheroids (Fig. 4D) as compared to adult β cells (Fig. 4E). Differentiated wt cells did not show any secretory granules and only occasionally cytoplasmic insulin labeling (not shown).
Transplantation of ES Cell-Derived Insulin-Producing Cells Maintained Glucose Homeostasis in Diabetic Animals.
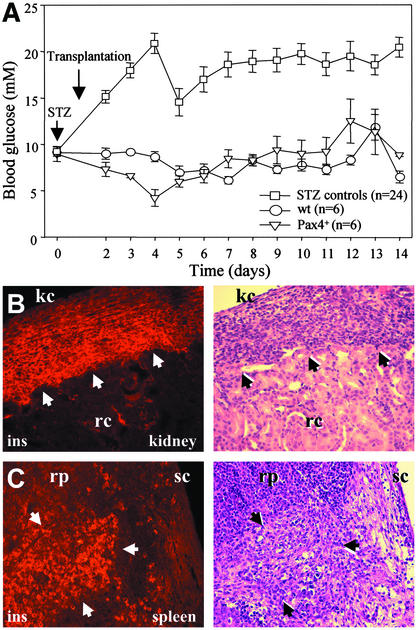
The potential of wt- and Pax4+-derived insulin-producing cells to maintain normal glucose homeostasis was investigated by transplanting the cells into STZ-induced diabetic mice. Between 1 and 2 million cells were transplanted under the kidney capsule and into the spleen. Mice transplanted with wt or Pax4+ cells (differentiated according to the nestin+ selection protocol) 24 h after STZ treatment retained normal blood glucose levels (i.e., below 10 mmol/liter) over a period of 14 d (Fig. 5A). Nontransplanted control animals became hyperglycemic 48 h after STZ treatment, with blood glucose levels >10 mmol/liter. Recipient animals were then killed and the transplanted cells were analyzed by immunohistology and conventional hematoxylin/eosin staining. Numerous insulin-positive cells were observed under the kidney capsule (Fig. 5B) and in the spleen (Fig. 5C) of animals transplanted with Pax4+ (Fig. 5 B and C) and wt (not shown) cells.
Figure 5.
Transplantation of wt and Pax4+ insulin-producing cells into STZ-treated diabetic mice. Shown are blood glucose levels in control STZ mice that did not receive any cells (control) and mice transplanted with wt or Pax4+ cells (A), and the immunofluorescence analyses by using an insulin-specific Ab of kidney (B) and spleen (C) of mice transplanted with Pax4+ cells. Arrowheads in hematoxylin/eosin stainings define the area of transplanted insulin-positive cells in kidney and spleen. kc, kidney capsule; rc, renal cortex; rp, red pulp; and sc, splenic capsule.
Discussion
Whereas recent studies have shown the generation of insulin-producing cells from progenitor cells of the pancreas (2, 3), liver (21), and pluripotent ES cells of mouse (6, 7) and human (8) origin, the efficiency of in vitro generated insulin-producing cells is low and presently insufficient for large scale therapeutic applications.
To overcome this limitation, we devised a strategy to induce the formation of insulin-producing cells by overexpressing the Pax4 gene in ES cells. We show here that constitutive expression of Pax4 revealed a significant up-regulation of genes involved in β-cell development and function resulting in an increased number of insulin-positive cells and in the amount of insulin being produced. This increase in the efficiency of Pax4+ cells to direct undifferentiated ES cells into insulin-producing cells was seen in three different culture systems: the basic and nestin+ selection protocol, and a histotypic culture model. Pax4 overexpression also resulted in an increase in the differentiation status of the cells, as shown by ultrastructural analyses and by a higher proportion of cells producing insulin only, whereas wt cells differentiated mainly into cells coexpressing both insulin and glucagon.
The RT-PCR data showed no effects of Pax4 and Pdx1 expression on shh, isl-1, and Pdx1 transcript levels, genes affecting early pancreatic differentiation. However, expression levels of genes controlling endocrine precursor specification, such as ngn3 and Pax6 (22, 23), were up-regulated in Pax4+ (and Pdx1+) cells. The early activation of Pax6 transcript levels, but down-regulation at later stages in Pax4+ cells could be explained by specific regulatory functions of Pax4 on α- vs. β-cell specification. However, Pax6, as well as ngn3 and isl-1, are also significantly expressed during neuronal differentiation and specification (24–26) indicating that the expression of these genes may be associated with other endodermal or neuroectodermal cell types present in differentiating EBs.
The results suggested that Pax4 may play a significant role in directing undifferentiated ES cells into endocrine insulin-producing cells, but low insulin levels were obtained in all cell lines differentiated according to the basic protocol. However, when a differentiation procedure selecting for potential nestin+ precursor cells was applied (7), Pax4+ cells showed an increase in the formation of insulin-producing cells from ≈60% (basic protocol) to 80% (nestin+ selection) compared to 10–15% (basic protocol) and 20–25% (nestin+ selection) insulin-producing cells generated from wt cells. As a consequence, a fivefold increase of the intracellular insulin level was detected in Pax4+ cells.
This finding of increased differentiation efficiency of insulin-producing cells under conditions that selected for a nestin+ cell population (7) raised several questions. Although, nestin+ cells may participate in the neogenesis of endocrine islet cells (14, 15), recent results (27) showed that, in vivo, nestin is expressed in mesenchymal and not in epithelial cells, where endocrine progenitor cells reside.
The role of nestin expression and the significance of nestin+ cells as a potential progenitor cell type is not yet known. Nestin is transiently expressed in different cell types of embryonic and adult tissues and is suggested to play a transient role in proliferation and migration processes of progenitor cells (28). One might speculate that nestin+ cells are characterized by a high developmental plasticity and represent a common progenitor cell population which in vitro under the influence of genetic and/or epigenetic factors can be programmed either into a neural (29), hepatic (21), or pancreatic endocrine (7, 15) fate. Previous findings would support this hypothesis: The expression of transcription factors ngn3, isl-1, Pax6, and Pax4, and of neuropeptide-processing enzymes were reported both, for cultured neuronal (29) and pancreatic endocrine cells (30).
In parallel to the selective differentiation via nestin+ cells, insulin levels could be further enhanced when cells were additionally differentiated in a histotypic culture system as 3D spheroids. Obviously, communication via cell–cell interactions is necessary to support cell-type-specific differentiation in vitro as demonstrated for liver, retinal, and pancreatic cells (31, 32). Spheroids generated from wt and Pax4+ ES cells showed insulin- and glucagon-producing cells in a histotypic organization. Similarly, reaggregation of human fetal islet cells increased the differentiation state (33).
The role of histotypic differentiation of ES-derived pancreatic cells with respect to cellular specification was further supported by ultrastructural analysis. Spheroids showed an intracellular accumulation of insulin, and Pax4+ clusters revealed similar granular structures as described for embryonic β cells (34). Although, the density of insulin labeling in the secretory granules of Pax4+ cells was lower as compared to insulin granules in adult β cells (reflecting differences in intracellular insulin content) histotypic differentiation was capable to increase the maturation stage of insulin-positive cells in vitro. This would support the idea that a characteristic “biosociology” of pancreatic endocrine cells is necessary for tissue-specific functions (35).
Transplantation of wt and Pax4+ insulin-producing cells was sufficient to preserve normal blood glucose levels in diabetic mice. In contrast to Lumelsky et al. (7), we found that wt insulin-producing cells generated by the nestin+ selection protocol also maintained blood glucose. An explanation could be the different location of the implanted cells (kidney capsule and spleen vs. subcutaneous). Indeed, our results are consistent with the results of Soria et al. (6) showing blood glucose normalization after intrasplenic transplantation of ES-derived insulin producing cells. Moreover, successful transplantation of purified islets has been shown to be dependent on islet number and implantation site (36).
Tumor formation is an important safety concern when considering transplantation therapies based on ES cells. We observed tumors in the kidney and spleen of some animals transplanted with wt and Pax4+ cells. It is well known that undifferentiated ES cells can form teratomas or teratocarcinomas (see ref. 37). Although Pax4 expression in ES cells resulted in up to 80% insulin-producing cells, remaining undifferentiated cells may still possess oncogenic properties. Lineage selection by using cell-trapping systems (6) and/or fluorescence-activated cell sorting methods similar to those developed for the isolation of β cells (35) have to be further adapted to prevent tumor formation.
In conclusion, our findings present evidence that constitutive Pax4 expression in ES cells, selective differentiation via nestin+ progenitor cells, followed by histotypic maturation, efficiently increases the development of insulin-producing cells.
Acknowledgments
We thank Mrs. S. Sommerfeld, O. Weiss, K. Meier, and S. Kappler for excellent technical assistance; Dr. A. Navarrete-Santos, University of Halle, for paraffin sections of spheroids; and Drs. C. Wollheim and B. Gauthier, University Medical Center, Geneva, Switzerland, for critical reading of the manuscript. This work was supported by the European Commission (Grant QLG1-CT-2001-02233 to L.S.-O.), the Deutsche Forschungsgemeinschaft (WO 503/3-1 to A.M.W.), and Fonds der Chemischen Industrie, Germany (to A.M.W.).
Abbreviations
- EB
embryoid body
- ES
embryonic stem
- Glut-2
glucose transporter 2
- LI
labeling index
- STZ
streptozotocin
- CMV
cytomegalovirus
- wt
wild type
References
- 1.Shapiro A M, Lakey J R, Ryan E A, Korbutt G S, Toth E, Warnock G L, Kneteman N M, Rajotte R V. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 2.Bonner-Weir S, Taneja M, Weir G C, Tatarkiewicz K, Song K H, Sharma A, O'Neil J J. Proc Natl Acad Sci USA. 2000;97:7999–8004. doi: 10.1073/pnas.97.14.7999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramiya V K, Maraist M, Arfors K E, Schatz D A, Peck A B, Cornelius J G. Nat Med. 2000;6:278–282. doi: 10.1038/73128. [DOI] [PubMed] [Google Scholar]
- 4.Wobus A M. Mol Aspects Med. 2001;22:149–164. doi: 10.1016/s0098-2997(01)00006-1. [DOI] [PubMed] [Google Scholar]
- 5.Wobus A M, Guan K, Yang H-T, Boheler K. In: Embryonic Stem Cells: Methods and Protocols, Methods in Molecular Biology. Turksen K, editor. Vol. 185. Totowa, NJ: Humana; 2002. pp. 127–156. [DOI] [PubMed] [Google Scholar]
- 6.Soria B, Roche E, Berna G, Leon-Quinto T, Reig J A, Martin F. Diabetes. 2000;49:157–162. doi: 10.2337/diabetes.49.2.157. [DOI] [PubMed] [Google Scholar]
- 7.Lumelsky N, Blondel O, Laeng P, Velasco I, Ravin R, McKay R. Science. 2001;292:1389–1394. doi: 10.1126/science.1058866. [DOI] [PubMed] [Google Scholar]
- 8.Assady S, Maor G, Amit M, Itskovitz-Eldor J, Skorecki K L, Tzukerman M. Diabetes. 2001;50:1691–1697. doi: 10.2337/diabetes.50.8.1691. [DOI] [PubMed] [Google Scholar]
- 9.Soria B. Differentiation. 2001;68:205–219. doi: 10.1046/j.1432-0436.2001.680408.x. [DOI] [PubMed] [Google Scholar]
- 10.Ahlgren U, Jonsson J, Jonsson L, Simu K, Edlund H. Genes Dev. 1998;12:1763–1768. doi: 10.1101/gad.12.12.1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jonsson J, Carlsson L, Edlund T, Edlund H. Nature. 1994;371:606–609. doi: 10.1038/371606a0. [DOI] [PubMed] [Google Scholar]
- 12.Ahlgren U, Jonsson J, Edlund H. Development (Cambridge, UK) 1996;122:1409–1416. doi: 10.1242/dev.122.5.1409. [DOI] [PubMed] [Google Scholar]
- 13.Sosa-Pineda B, Chowdhury K, Torres M, Oliver G, Gruss P. Nature. 1997;386:399–402. doi: 10.1038/386399a0. [DOI] [PubMed] [Google Scholar]
- 14.Hunziker E, Stein M. Biochem Biophys Res Commun. 2000;271:116–119. doi: 10.1006/bbrc.2000.2611. [DOI] [PubMed] [Google Scholar]
- 15.Zulewski H, Abraham E J, Gerlach M J, Daniel P B, Moritz W, Muller B, Vallejo M, Thomas M K, Habener J F. Diabetes. 2001;50:521–533. doi: 10.2337/diabetes.50.3.521. [DOI] [PubMed] [Google Scholar]
- 16.Perry J R, Basrai M A, Steiner H Y, Naider F, Becker J M. Mol Cell Biol. 1994;14:104–115. doi: 10.1128/mcb.14.1.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagy A, Rossant J, Nagy R, Abramow-Newerly W, Roder J C. Proc Natl Acad Sci USA. 1993;90:8424–8428. doi: 10.1073/pnas.90.18.8424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansouri A. In: Cell Biology: A Laboratory Handbook. 2nd Ed. Celis J E, editor. Vol. 3. San Diego: Academic; 1998. pp. 478–486. [Google Scholar]
- 19.Soriano P, Montgomery C, Geske R, Bradley A. Cell. 1991;64:693–702. doi: 10.1016/0092-8674(91)90499-o. [DOI] [PubMed] [Google Scholar]
- 20.Rolletschek A, Chang H, Guan K, Czyz J, Meyer M, Wobus A M. Mech Dev. 2001;105:93–104. doi: 10.1016/s0925-4773(01)00385-9. [DOI] [PubMed] [Google Scholar]
- 21.Yang L, Li S, Hatch H, Ahrens K, Cornelius J G, Petersen B E, Peck A B. Proc Natl Acad Sci USA. 2002;99:8078–8083. doi: 10.1073/pnas.122210699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mansouri A, St-Onge L, Gruss P. Trends Endocrinol Metab. 1999;10:164–167. doi: 10.1016/s1043-2760(98)00133-7. [DOI] [PubMed] [Google Scholar]
- 23.Schwitzgebel V M, Scheel D W, Conners J R, Kalamaras J, Lee J E, Anderson D J, Sussel L, Johnson J D, German M S. Development (Cambridge, UK) 2000;127:3533–3542. doi: 10.1242/dev.127.16.3533. [DOI] [PubMed] [Google Scholar]
- 24.Walther C, Gruss P. Development (Cambridge, UK) 1991;113:1435–1449. doi: 10.1242/dev.113.4.1435. [DOI] [PubMed] [Google Scholar]
- 25.Ericson J, Thor S, Edlund T, Jessell T M, Yamada T. Science. 1992;256:1555–1560. doi: 10.1126/science.1350865. [DOI] [PubMed] [Google Scholar]
- 26.Sommer L, Ma Q, Anderson D J. Mol Cell Neurosci. 1996;8:221–241. doi: 10.1006/mcne.1996.0060. [DOI] [PubMed] [Google Scholar]
- 27.Selander L, Edlund H. Mech Dev. 2002;113:189–192. doi: 10.1016/s0925-4773(02)00023-0. [DOI] [PubMed] [Google Scholar]
- 28.Mokry J, Nemecek S. Acta Medica (Hradec Králové) 1998;41:73–80. [PubMed] [Google Scholar]
- 29.Cattaneo E, McKay R. Nature. 1990;347:762–765. doi: 10.1038/347762a0. [DOI] [PubMed] [Google Scholar]
- 30.Edlund H. Diabetes. 2001;50 Suppl. 1:S5–S9. doi: 10.2337/diabetes.50.2007.s5. [DOI] [PubMed] [Google Scholar]
- 31.Layer P G, Robitzki A, Rothermel A, Willbold E. Trends Neurosci. 2002;25:131–134. doi: 10.1016/s0166-2236(00)02036-1. [DOI] [PubMed] [Google Scholar]
- 32.Pipeleers D G, in't Veld P A, Van de Winkel M, Maes E, Schuit F C, Gepts W. Endocrinology. 1985;117:806–816. doi: 10.1210/endo-117-3-806. [DOI] [PubMed] [Google Scholar]
- 33.Beattie G M, Rubin J S, Mally M I, Otonkoski T, Hayek A. Diabetes. 1996;45:1223–1228. doi: 10.2337/diab.45.9.1223. [DOI] [PubMed] [Google Scholar]
- 34.Like A A. Lab Invest. 1967;16:937–951. [PubMed] [Google Scholar]
- 35.Pipeleers D. Diabetologia. 1987;30:277–291. doi: 10.1007/BF00299019. [DOI] [PubMed] [Google Scholar]
- 36.Montana E, Bonner-Weir S, Weir G C. J Clin Invest. 1993;91:780–787. doi: 10.1172/JCI116297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wobus A M, Holzhausen H, Jakel P, Schöneich J. Exp Cell Res. 1984;152:212–219. doi: 10.1016/0014-4827(84)90246-5. [DOI] [PubMed] [Google Scholar]