Abstract
Lectin-like oxidized low-density lipoprotein receptor-1 (LOX-1) is a major endothelial receptor for oxidized low-density lipoprotein, and is assumed to play a proatherogenic role in atherosclerosis. LOX-1 expression is induced by inflammatory cytokines as well as by proatherogenic stimuli. LOX-1 protein binds aged/apoptotic cells, activated platelets, and bacteria, suggesting that it may have diverse activities in vivo. Here, we reveal a role for LOX-1 in endotoxin-induced inflammation. In a model of endotoxemia, injection of a high dose of endotoxin into rats induced leukopenia within 1 h and death of the animals within 24 h. Preadministration of anti-LOX-1 antibody reduced the degree of leukopenia and completely rescued the animals, whereas control IgG did not. In a model of low-dose endotoxin-induced uveitis, anti-LOX-1 antibody efficiently suppressed leukocyte infiltration and protein exudation. In situ videomicroscopic analyses of leukocyte interactions with retinal veins revealed that anti-LOX-1 antibody reduced the number of rolling leukocytes and increased the velocity of rolling, suggesting that LOX-1 functions as a vascular tethering ligand. The ability of LOX-1 to capture leukocytes under physiologic shear was confirmed in an in vitro flow model. Thus, LOX-1 is an adhesion molecule involved in leukocyte recruitment and may represent an attractive target for modulation of endotoxin-induced inflammation.
Lectin-like oxidized low-density lipoprotein (LDL) receptor-1 (LOX-1) was originally identified as a major receptor for oxidized LDL expressed in endothelial cells (1). LOX-1 has an extracellular C-terminal lectin domain and belongs to the C-type lectin-like protein family with highest homology to natural killer (NK) cell receptors, which are involved in activation of NK cells when NK cells recognize tumor cells (1–4). The genes of LOX-1 and NK cell receptors are colocalized in the short arm of chromosome 12, suggesting that these molecules have been evolved from a common precursor (5, 6). Expression of LOX-1 is induced by inflammatory cytokines and oxidized LDL in vitro (7–9) and proatherogenic conditions in vivo, e.g., hypertension, hyperlipidemia, and diabetes mellitus (10–13). The expression of LOX-1 is induced by stimuli as rapidly as other kinds of cell-adhesion molecules and selectins, suggesting that LOX-1 is in the so-called class of immediate early genes. LOX-1 is a potent mediator of “endothelial dysfunction”: binding of endothelial LOX-1 by ligands induces superoxide generation, inhibits nitric oxide production, enhances endothelial adhesiveness for leukocytes, and induces expression of chemokines (14–16). The versatile activities of LOX-1 include the ability to bind not only oxidized LDL, but also aged red blood cells, apoptotic cells, and activated platelets (17, 18).
Recent progress implicates the inflammatory response in the arterial wall as a fundamental component of atherogenesis (19, 20). Recruitment of leukocytes into the arterial wall, a hallmark of inflammation, is one of the earliest steps in atherosclerosis. Leukocyte recruitment from the blood involves a multistep process of leukocyte–endothelial interaction and diapedesis (21). The process involves leukocyte rolling mediated by microvillous receptors (classically selectins or α4 integrins), activation of leukocyte integrins by chemokines, adhesion to endothelial ligands through activated integrins, and transendothelial migration (21). Recent studies identifying other molecular classes that can mediate leukocyte rolling (22, 23), as well as evidence of novel adhesion pathways that can participate in these events (24–26), indicate that unknown molecular mechanisms may still exist that can play important roles in leukocyte–endothelial interaction and inflammatory cell recruitment.
The studies reported here were initiated to test the hypothesis that LOX-1 plays a role in the inflammatory process, potentially acting as a leukocyte-adhesion molecule at the vascular interface. Our findings confirm this hypothesis, demonstrating an important role for LOX-1 in leukocyte capture and rolling during leukocyte–vascular interactions and in the pathologic consequences of leukocyte recruitment in models of endotoxin-induced inflammation.
Materials and Methods
Animal Model.
Female Lewis rats, each weighing ≈200 g (8 weeks old), were used. Endotoxemia and endotoxin-induced uveitis (EIU) were initiated in each rat by footpad injection of 2 mg/ kg or 0.5 mg/kg lipopolysaccharide (LPS; Salmonella minnesota, Sigma) dissolved in 0.1 ml of sterile saline, respectively.
Survival Curves and Leukocyte Count in Peripheral Blood After High- Dose LPS Treatment.
LPS (2 mg/kg) was injected at the footpads of 12 rats. Anti-LOX-1 antibody (10 mg/kg) or isotype-matched murine IgG was administered i.v. to six of the rats. Survival of rats was checked every 3 h up to 72 h after LPS injection. Leukocyte number in peripheral blood was counted at 0, 1, 3, and 6 h after LPS treatment. Blood anticoagulated with EDTA was obtained from the tail vein of each rat. The blood sample was analyzed by using a hematology analyzer (Coulter Counter T-890, Beckman Coulter, Tokyo).
Analysis of Protein and Cells in Aqueous Humor.
The lower dose of LPS (0.5 mg/kg) and 10 mg/kg anti-LOX-1 antibody were administered by the same protocol as above (n = 6 for each group). Aqueous humor was collected from the left eye of each rat by anterior chamber puncture by using a 30-gauge needle at 24 h after LPS treatment. The concentration of protein in aqueous humor was determined by the Bradford method (Bio-Rad). The number of cells in 1 μl of aqueous humor was counted under microscope after Wright's staining.
RT-PCR.
The eyes were enucleated 0, 1, 3, 6, 9, 12, 18, 24, and 48 h after LPS treatment (0.5 mg/kg). Three rats were used at each time point. Each enucleated eye was cut into two pieces along the limbus, and then the retina was collected from the posterior segment. Total RNA was isolated from the retina and subjected to RT-PCR analyses as described (10). Data shown represent three reproducible results.
Immunohistochemistry.
The rats were fixed by perfusion of 4% (wt/vol) paraformaldehyde/PBS before enucleation. Subsequently, the retina and iris were isolated and further fixed for 2 h at 4°C in 4% (wt/vol) paraformaldehyde/PBS, then gently shaken overnight at 4°C in 2% (vol/vol) BSA in PBS (“blocking solution”) for 30 min and in a solution of antibodies to LOX-1 or von Willebrand factor for 24 h at 4°C. They were then washed and treated for 12 h with secondary antibodies (Cy3-conjugated anti-mouse IgG or anti-rabbit IgG). Fluorescence was visualized with a confocal laser scanning microscope (Bio-Rad). Data shown represent three reproducible results.
Analyses of Leukocyte Behavior, Rolling Flux, and Rolling Velocities.
Leukocyte behavior in the retina was observed at 12 h after LPS treatment (0.5 mg/kg). Thirty minutes before analyses, 10 mg/kg anti-LOX-1 antibody or isotype-matched murine IgG was administered. Leukocyte behavior in the retina was observed by using acridine orange digital fluorography as described (27). Leukocytes were labeled with fluorescent nuclear dye of acridine orange (Wako Pure Chemical, Osaka), administered i.v., and then imaged with a scanning laser ophthalmoscope (Rodenstock Instruments, Munich). The flux of rolling leukocytes in a vessel was determined from the number of cells crossing a fixed area of the vessel at a distance one disk diameter from the optic disk center per minute. Rolling velocity of leukocyte was calculated as the time required for a leukocyte to travel a given distance along the major retinal vessels. Representative results of three reproducible experiments are shown.
Isolation of Human Polymorphonuclear (PMN) Leukocyte Cells.
Blood was collected from a healthy volunteer with a 21-gauge needle into a syringe containing 1/10 volume of 4% (wt/vol) sodium citrate. After sedimentation of red blood cells with 1% (wt/vol) dextran T500 (Amersham Pharmacia), PMN cells were purified by pelleting the cell suspension on a layer of Ficoll-Histopaque-1077 (Sigma). Contaminated red blood cells were removed by hypoosmolarity shock and centrifugation.
Flow Chamber Assay.
Soluble LOX-1 was purified from conditioned medium of the CHO-K1 cell line that stably expresses the fusion protein (LOX-Fc) of LOX-1 extracellular domain and human IgG1 Fc domain, as described. Culture slides were coated with soluble LOX-1 (50 μg/ml) at 4°C overnight. The unbound LOX-1 was removed, and the slides were exposed to 3% (wt/vol) BSA at 4°C overnight. Immediately before the experiments, BSA was washed out, and the flow chamber with the same specification as described was constructed (28). The rate of flow was controlled by a syringe pump (model 100, KD Scientific, Boston). Experiments were performed at a flow rate of 75 ml/h, which generates a wall shear stress of ≈2 dynes/cm2 (1 dyne = 0.1 N) for a parallel planner layer with a distance of 0.25 mm, as calculated from Poiseuille's Law for Newtonian fluids with a viscosity of 0.01 poise (1 poise = 0.1 Pa⋅sec). The interaction of cells with the coated slides was recorded on videotape, and the behavior of the cells was analyzed frame by frame. Data shown represent four reproducible results.
Statistical Analysis.
All results are expressed as means ± SEM. Unpaired groups of two were compared by using Student's t and Mann–Whitney U tests with correction for multiple comparison when appropriate. Differences were considered significant when P values were <0.05.
Results
Effects of Anti-LOX-1 Antibody on Endotoxin Shock.
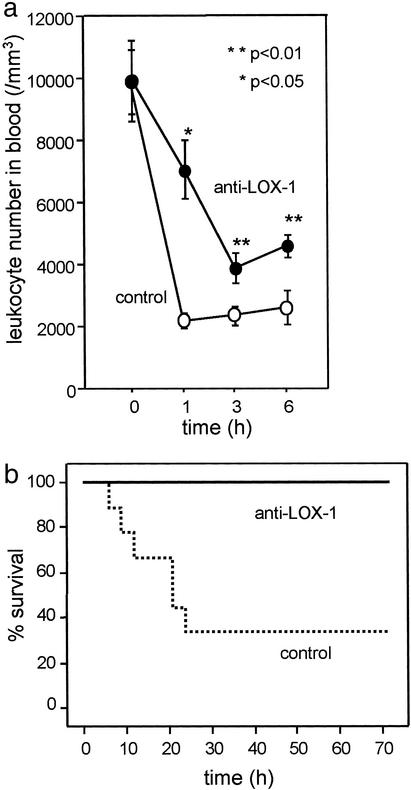
Recent findings on LOX-1, which shows potential relationships to inflammation, prompted us to hypothesize that LOX-1 might work as a component of inflammation. To prove this, we used a typical model of inflammation induced by LPS. Injection of LPS (2 mg/kg) into a footpad of rat together with control IgG1 (10 mg/kg, i.v.) induced transient leukopenia within 1 h. Administration of a neutralizing antibody for LOX-1 (JTX-20, 10 mg/kg, i.v.) at the time of LPS injection significantly ameliorated the leukopenia (Fig. 1a, P < 0.01 at 1 h, P < 0.05 at 3 and 6 h). Neither control IgG1 nor anti-LOX-1 antibody affected leukocyte count in peripheral blood of LPS-untreated rats (data not shown).
Figure 1.
(a) Leukopenia caused by LPS. Anti-LOX-1 antibody administered i.v. at the time of LPS injection ameliorated the leukopenia induced by LPS (P < 0.01 at 1 h, P < 0.05 at 3 and 6 h). (b) Survival of LPS-injected rats that were pretreated with anti-LOX-1 antibody or control IgG. Administration of anti-LOX-1 antibody significantly rescued rats from death.
Furthermore, treatment with anti-LOX-1 antibody completely rescued rats from LPS-induced death, whereas 70% of rats receiving control IgG died within 24 h. Kaplan–Meier's analysis indicated a significant difference in the mortality between control and anti-LOX-1 antibody-treated rats (Fig. 1b).
Induction of LOX-1 Expression in Retina Venules by Endotoxin.
To clarify the mechanism of LOX-1 involvement in the LPS-induced inflammatory response, we studied EIU. In this model, in which retinal inflammation is induced by a lower dose of LPS (0.5 mg/kg), we can analyze leukocyte behavior in retinal blood vessels in vivo with minimal surgical operation (27).
By RT-PCR analysis, the expression of LOX-1 mRNA in the retina was up-regulated over 24 h, with a peak at 9 h after LPS injection (Fig. 2a). By whole mount immunohistochemistry (Fig. 2b), LOX-1-immunopositive vessels were clearly visible across the retina and iris 12 h after LPS injection, while LOX-1 immunoreactivity was not detectable in the retina and the iris–ciliary body in control eyes. Immunostaining with anti-von Willebrand factor confirmed that the cells expressing LOX-1 were endothelial cells (Fig. 2b). Interestingly, LOX-1 was not induced evenly in vessels but was most intense in venules. Because LPS preferentially acts on veins, it is reasonable that LOX-1 induction was more prominent in venules than in arteries and capillaries.
Figure 2.
LOX-1 is involved in the process of EIU. (a) mRNA level of LOX-1 was increased in retina after LPS treatment. RNA was extracted from retina 0, 1, 3, 6, 9, 12, 18, 24, and 48 h after LPS treatment. cDNA fragments for LOX-1 and β-actin were amplified by PCR after reverse transcription. (b) Immunoreactivity of LOX-1 was increased in iris and retinal vessels. Iris and retinal vessels were stained with anti-von Willebrand factor antiserum or anti-LOX-1 antibody 12 h after the injection of LPS. LOX-1 expression was up-regulated, especially in venules dilated by the effects of LPS. (c and d) Anti-LOX-1 antibody suppressed endotoxin-induced cellular infiltration (c) and protein exudation (d) into anterior chamber of eyes. Anti-LOX-1 antibody was administered i.v. at the time of LPS injection.
Effects of Anti-LOX-1 Antibody on the Inflammation in Uvea.
Next, we analyzed the effects of anti-LOX-1 antibody on the severity of EIU. The aqueous humor in the normal, untreated rats contained 11 ± 5 leukocytes per μl and a protein level of 2.65 mg/ml. At 24 h after LPS treatment, when the number of leukocytes and the protein levels in the aqueous humor reached maximum, the number of leukocytes in the aqueous humor was 472 ± 145 cells per μl in the rats with EIU. The infiltrated leukocytes consisted of 90.2 ± 1.7% neutrophils, 7.9 ± 1.2% lymphocytes, and 1.9 ± 0.5% monocytes. Injection of anti-LOX-1 antibody at the time of LPS administration significantly reduced the aqueous humor cell number to 192 ± 155 cells per μl (P < 0.001). In contrast, control had no significant effect (457 ± 133 cells per μl; Fig. 2c).
The protein concentration in the aqueous humor at 24 h after LPS injection was 27.4 ± 3.0 mg/ml. Anti-LOX-1 antibody significantly reduced the protein levels to 9.3 ± 6.5 mg/ml (P < 0.05), whereas control IgG again had no significant effect (25.6 ± 4.7 mg/ml; Fig. 2d).
Effects of Anti-LOX-1 Antibody on Leukocyte–Vascular Interactions.
Next, we analyzed leukocyte behavior in vivo in the retinal blood vessels by using scanning laser ophthalmoscopy, labeling circulating leukocytes with acridine orange. As previously described, rolling leukocytes were observed along the major retinal veins (Fig. 3a). Twelve hours after LPS treatment, when the flux of rolling leukocytes reached maximum, 10 mg/kg anti-LOX-1 antibody or control IgG were administered i.v. to the rats. The flux of rolling leukocytes was significantly decreased by the treatment with anti-LOX-1 antibody (42% reduction, from 23.2 ± 1.3 to 9.6 ± 1.2 cells per min, P < 0.0001, Fig. 3b). The mean rolling velocity in major retinal veins was significantly higher in the rats treated with anti-LOX-1 antibody than in the IgG-treated rats (111.3 ± 29.1 vs. 24.5 ± 1.9 μm/sec, P < 0.003, Fig. 3 c and d). These results suggest that LOX-1 supports leukocyte–endothelial interaction under inflammatory condition in vivo, playing roles in leukocyte capture and rolling, in this model.
Figure 3.
LOX-1 is involved in leukocyte–endothelium interaction in vivo in retinal blood vessels in endotoxin-induced inflammation. Anti-LOX-1 antibody or control IgG was administered 30 min before analyses. (a) Fundus oculi imaged 12 h after LPS injection. A number of leukocytes associated with endothelium were observed along the wall of major veins (arrowheads), whereas only a few were observed when treated with anti-LOX-1 antibody. (b) Flux of rolling leukocytes at retinal major veins. The flux of rolling leukocytes significantly decreased by anti-LOX-1 antibody compared with control IgG (P < 0.001). (c) Velocities of rolling leukocytes in retinal major veins. The mean velocities of rolling leukocytes significantly decreased by anti-LOX-1 antibody (P < 0.003). (d) Shift of the distribution of rolling velocity in retinal major veins after treatment of anti-LOX-1 antibody (10 mg/kg).
In Vitro Analyses of LOX-1-Mediated Leukocyte Adhesion.
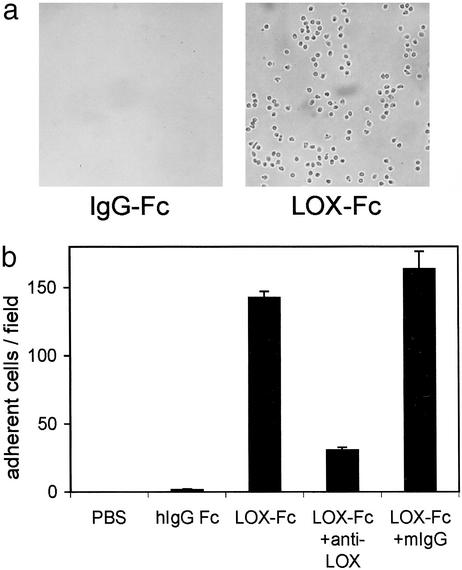
To confirm the ability of LOX-1 to mediate leukocyte adhesion, we analyzed the interaction between LOX-1 and leukocytes in vitro. Because neutrophils were predominant in the leukocytes infiltrated to tissues in the endotoxin-induced inflammation in the present study, we used a fraction of PMN cells that consisted predominantly of neutrophils for the in vitro analyses. A suspension of human PMN cells was infused through a flow chamber constructed with recombinant LOX-1-coated or control protein-coated slides. PMN cells bound only to the LOX-1-coated plates (Fig. 4a). The number of adherent cells was decreased under higher shear stress, but significant numbers of PMN cells still bound up to a shear rate of 5 dynes/cm2 (data not shown). The binding was significantly inhibited by anti-LOX-1 antibody, but not by control IgG, suggesting that the adhesion of PMN cells was directly mediated by binding to recombinant LOX-1 (Fig. 4b).
Figure 4.
(a) Adhesion of PMN cells to the surface coated with recombinant LOX-1 (LOX-Fc) but not to that with control protein (IgG-Fc) under the physiological shear stress at 2 dynes/cm2. (b) Quantitative analyses of adhesion of PMN cells. Anti-LOX-1 antibody blocked the adhesion of PMN cells to LOX-1-coated surface, whereas control human IgG did not.
Discussion
The characteristic lectin-like structure of LOX-1 has homology to the NK cell receptor, which is essential for tumor cell recognition by NK cells (1). The chromosomal localization of the LOX-1 gene is within a cluster of the NK cell receptor gene family (5). This finding prompted us to speculate that LOX-1 might be a molecule working in a context of host defense and cell–cell interaction. In the present study, we demonstrate that LOX-1 is involved in endotoxin-induced inflammatory responses. In vivo, anti-LOX-1 antibody suppressed leukocyte infiltration and protein exudation, leading to survival of endotoxin-treated animals. In vitro, recombinant LOX-1 binds PMN cells under physiological shear force, indicating LOX-1 itself supports leukocyte recruitment.
Families of adhesion molecules have been identified by earlier studies: selectins for rolling, and vascular cell adhesion molecule-1 and intercellular adhesion molecule-1 for integrin-mediated firm adhesion. The importance of these molecules in lymphocyte homing and leukocyte recruitment to inflamed sites has been established (21). Besides these molecules, some candidates for leukocyte adhesion have been reported as antigens for monoclonal antibody, such as vascular monocyte adhesion-associated protein-1 for the adhesion of monocytic cell line, and glucocorticoid receptor antigen for neutrophils (25, 26). Identity of LOX-1 with these candidate molecules is unknown at the present time. However, it is clearly demonstrated that LOX-1 is a hitherto unknown member of the class of cell-adhesion molecules.
Although it is uncertain how LOX-1 shares a role in leukocyte recruitment with the previously identified molecules, LOX-1 blockade showed significant effects on the leukocyte–endothelium interaction and in the consequences upon the inflammatory process. LOX-1 is an inducible molecule. The expression is induced by inflammatory cytokines, such as tumor necrosis factor-α, IFN-γ, and IL-6, and by pathological conditions such as hyperlipidemia, hypertension, and diabetes mellitus (7, 10, 13, 29, 30). This property suggests that the LOX-1-mediated leukocyte recruitment would increase its importance in the inflammation that occurs in aged humans, especially in those suffering from maturity-onset diseases. For example, atherosclerosis and ischemic heart disease are probable candidates for which an inflammatory process is involved and whose risk is predicted well by C-reactive protein, a marker of inflammation (19, 31).
LOX-1 was originally identified as an oxidized LDL receptor that induces functional changes in endothelial cells upon ligation of oxidized LDL; e.g., by generation of superoxide and concomitant reduction of the concentration of nitric oxide, induction of monocyte chemoattractant protein 1, increase of leukocyte adhesiveness, and induction of LOX-1 expression itself (8, 14–16). It is possible that these processes induce inflammatory changes in vessel walls and initiate atherosclerosis. These changes in endothelial cells mediated by LOX-1 also might be involved in endotoxin-induced inflammation. This might explain the beneficial effects of anti-LOX-1 antibody on the endotoxin-induced inflammation. Probably both its property as a cell-adhesion molecule and its property of inducing functional change in endothelial cells work together to facilitate inflammation.
The multiple functions of LOX-1, such as the binding of bacteria, leukocyte adhesion, and superoxide generation, support the idea that LOX-1 might be working as a member of innate immunity (15, 32), though excess activity of LOX-1 might result in the damage of organs. We conclude that manipulation of the activity of LOX-1 at appropriate levels would be beneficial for the control of inflammation-related diseases.
Acknowledgments
This work was supported in part by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan, the Ministry of Health, Labor and Welfare of Japan, the Organization for Pharmaceutical Safety and Research, the Takeda Science Foundation, and the Mitsubishi Foundation.
Abbreviations
- LDL
low-density lipoprotein
- LOX-1
lectin-like oxidized LDL receptor-1
- NK
natural killer
- EIU
endotoxin-induced uveitis
- LPS
lipopolysaccharide
- PMN
polymorphonuclear
References
- 1.Sawamura T, Kume N, Aoyama T, Moriwaki H, Hoshikawa H, Aiba Y, Tanaka T, Miwa S, Katsura Y, Kita T, Masaki T. Nature. 1997;386:73–77. doi: 10.1038/386073a0. [DOI] [PubMed] [Google Scholar]
- 2.Chen M, Narumiya S, Masaki T, Sawamura T. Biochem J. 2001;355:289–296. doi: 10.1042/0264-6021:3550289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M, Inoue K, Narumiya S, Masaki T, Sawamura T. FEBS Lett. 2001;499:215–219. doi: 10.1016/s0014-5793(01)02557-1. [DOI] [PubMed] [Google Scholar]
- 4.Giorda R, Rudert W A, Vavassori C, Chambers W H, Hiserodt J C, Trucco M. Science. 1990;249:1298–1300. doi: 10.1126/science.2399464. [DOI] [PubMed] [Google Scholar]
- 5.Aoyama T, Sawamura T, Furutani Y, Matsuoka R, Yoshida M C, Fujiwara H, Masaki T. Biochem J. 1999;339:177–184. [PMC free article] [PubMed] [Google Scholar]
- 6.Bull C, Sobanov Y, Rohrdanz B, O'Brien J, Lehrach H, Hofer E. Genes Immun. 2000;1:280–287. doi: 10.1038/sj.gene.6363678. [DOI] [PubMed] [Google Scholar]
- 7.Kume N, Murase T, Moriwaki H, Aoyama T, Sawamura T, Masaki T, Kita T. Circ Res. 1998;83:322–327. doi: 10.1161/01.res.83.3.322. [DOI] [PubMed] [Google Scholar]
- 8.Aoyama T, Fujiwara H, Masaki T, Sawamura T. J Mol Cell Cardiol. 1999;31:2101–2114. doi: 10.1006/jmcc.1999.1041. [DOI] [PubMed] [Google Scholar]
- 9.Minami M, Kume N, Kataoka H, Morimoto M, Hayashida K, Sawamura T, Masaki T, Kita T. Biochem Biophys Res Commun. 2000;272:357–361. doi: 10.1006/bbrc.2000.2778. [DOI] [PubMed] [Google Scholar]
- 10.Nagase M, Hirose S, Sawamura T, Masaki T, Fujita T. Biochem Biophys Res Commun. 1997;237:496–498. doi: 10.1006/bbrc.1997.7176. [DOI] [PubMed] [Google Scholar]
- 11.Chen H, Li D, Sawamura T, Inoue K, Mehta J L. Biochem Biophys Res Commun. 2000;276:1100–1104. doi: 10.1006/bbrc.2000.3532. [DOI] [PubMed] [Google Scholar]
- 12.Kataoka H, Kume N, Miyamoto S, Minami M, Moriwaki H, Murase T, Sawamura T, Masaki T, Hashimoto N, Kita T. Circulation. 1999;99:3110–3117. doi: 10.1161/01.cir.99.24.3110. [DOI] [PubMed] [Google Scholar]
- 13.Chen M, Nagase M, Fujita T, Narumiya S, Masaki T, Sawamura T. Biochem Biophys Res Commun. 2001;287:962–968. doi: 10.1006/bbrc.2001.5674. [DOI] [PubMed] [Google Scholar]
- 14.Li D, Mehta J L. Circulation. 2000;101:2889–2895. doi: 10.1161/01.cir.101.25.2889. [DOI] [PubMed] [Google Scholar]
- 15.Cominacini L, Pasini A F, Garbin U, Davoli A, Tosetti M L, Campagnola M, Rigoni A, Pastorino A M, Lo Cascio V, Sawamura T. J Biol Chem. 2000;275:12633–12638. doi: 10.1074/jbc.275.17.12633. [DOI] [PubMed] [Google Scholar]
- 16.Cominacini L, Rigoni A, Pasini A F, Garbin U, Davoli A, Campagnola M, Pastorino A M, Lo Cascio V, Sawamura T. J Biol Chem. 2001;276:13750–13755. doi: 10.1074/jbc.M010612200. [DOI] [PubMed] [Google Scholar]
- 17.Oka K, Sawamura T, Kikuta K, Itokawa S, Kume N, Kita T, Masaki T. Proc Natl Acad Sci USA. 1998;95:9535–9540. doi: 10.1073/pnas.95.16.9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kakutani M, Masaki T, Sawamura T. Proc Natl Acad Sci USA. 2000;97:360–364. doi: 10.1073/pnas.97.1.360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ross R. N Engl J Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- 20.Lusis A J. Nature. 2000;407:233–241. doi: 10.1038/35025203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Butcher E C, Williams M, Youngman K, Rott L, Briskin M. Adv Immunol. 1999;72:209–253. doi: 10.1016/s0065-2776(08)60022-x. [DOI] [PubMed] [Google Scholar]
- 22.Smith D J, Salmi M, Bono P, Hellman J, Leu T, Jalkanen S. J Exp Med. 1998;188:17–27. doi: 10.1084/jem.188.1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.DeGrendele H C, Estess P, Picker L J, Siegelman M H. J Exp Med. 1996;183:1119–1130. doi: 10.1084/jem.183.3.1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Forlow S B, Ley K. Am J Physiol. 2001;280:H634–H641. doi: 10.1152/ajpheart.2001.280.2.H634. [DOI] [PubMed] [Google Scholar]
- 25.McEvoy L M, Sun H, Tsao P S, Cooke J P, Berliner J A, Butcher E C. J Exp Med. 1997;185:2069–2077. doi: 10.1084/jem.185.12.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jutila M A, Wilson E, Kurk S. J Exp Med. 1997;186:1701–1711. doi: 10.1084/jem.186.10.1701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishiwaki H, Ogura Y, Kimura H, Kiryu J, Miyamoto K, Matsuda N. Invest Ophthalmol Visual Sci. 1996;37:1341–1347. [PubMed] [Google Scholar]
- 28.Lawrence M B, Springer T A. Cell. 1991;65:859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- 29.Nagase M, Abe J, Takahashi K, Ando J, Hirose S, Fujita T. J Biol Chem. 1998;273:33702–33707. doi: 10.1074/jbc.273.50.33702. [DOI] [PubMed] [Google Scholar]
- 30.Chen M, Kakutani M, Minami M, Kataoka H, Kume N, Narumiya S, Kita T, Masaki T, Sawamura T. Arterioscler Thromb Vasc Biol. 2000;20:1107–1115. doi: 10.1161/01.atv.20.4.1107. [DOI] [PubMed] [Google Scholar]
- 31.Plutzky J. Am J Cardiol. 2001;88:10K–15K. doi: 10.1016/s0002-9149(01)01924-5. [DOI] [PubMed] [Google Scholar]
- 32.Shimaoka T, Kume N, Minami M, Hayashida K, Sawamura T, Kita T, Yonehara S. J Immunol. 2001;166:5108–5114. doi: 10.4049/jimmunol.166.8.5108. [DOI] [PubMed] [Google Scholar]