Abstract
Previous studies have indicated that stress-activated glucocorticoid hormones induce temporary memory retrieval impairment. The present study examined whether adrenal steroid receptors in the hippocampus mediate such glucocorticoid effects on spatial memory retrieval. The specific glucocorticoid receptor (GR) agonist 11β, 17β-dihydroxy-6,21-dimethyl-17α-pregna-4,6-trien-20yn-3-one (RU 28362; 5 or 15 ng) infused into the hippocampus of male Sprague–Dawley rats 60 min before water-maze retention testing, 24 h after training, dose-dependently impaired probe-trial retention performance, as assessed both by time spent in the training quadrant and initial latency to cross the platform location. The GR agonist did not affect circulating corticosterone levels immediately after the probe trial, indicating that RU 28362 infusions did not influence retention by altering glucocorticoid feedback mechanisms. As infusions of the GR agonist into the hippocampus 60 min before training did not influence water-maze acquisition or immediate recall, the findings indicated that the GR agonist-induced retention impairment was induced selectively by an influence on information retrieval. In contrast, pretest infusions of the GR agonist administered into the basolateral complex of the amygdala (BLA; 2 or 6 ng) did not alter retention performance in the water maze. However, N-methyl-d-aspartate-induced lesions of the BLA, made 1 week before training, blocked the memory retrieval impairment induced by intrahippocampal infusions of RU 28362 given 60 min before the retention test. These findings indicate that the effects of glucocorticoids on retrieval of long-term spatial memory depend on the hippocampus and, additionally, that neuronal input from the BLA is critical in enabling hippocampal glucocorticoid effects on memory retrieval.
Keywords: corticosterone‖glucocorticoid receptor‖stress hormone‖water maze
It is well established that adrenocortical hormones (corticosterone in rats, cortisol in humans) influence many aspects of cognitive performance. Studies of acute glucocorticoid effects on distinct memory phases have revealed that a single administration of glucocorticoids enhances the formation of new memories and, additionally, impairs the recall of previously learned information (1–3). Considerable evidence supports a role for the hippocampus in mediating glucocorticoid enhancement of memory consolidation. For example, either systemic or intracerebroventricular administration of glucocorticoids or specific antagonists influences consolidation of hippocampus-dependent spatial or contextual information (4–9). Furthermore, posttraining intrahippocampal infusions of corticosterone or synthetic glucocorticoid receptor (GR) agonists enhance memory consolidation on a variety of tasks, whereas infusions of a GR antagonist or GR antisense impair memory consolidation (10–16). The basolateral complex of the amygdala (BLA; consisting of the lateral, basal, and accessory basal nuclei) is also a critical component of the neural circuitry mediating glucocorticoid effects on memory consolidation (17, 18). The BLA is particularly important for mediating stress hormone and drug effects on memory consolidation in other brain regions (19–21). Lesions or reversible inactivation of the BLA block the modulation of memory consolidation for inhibitory avoidance and water-maze spatial training initiated by manipulation of GRs in the hippocampus (14, 15). Because the BLA is normally activated by emotionally arousing experiences, these findings are consistent with the emerging view that glucocorticoids may selectively enhance consolidation of emotionally arousing events or material (1, 3).
As noted, acute administration of glucocorticoids also impairs memory retrieval. Systemic injections of stress-level doses of corticosterone administered to rats shortly before retention testing impair retrieval in tasks that rely on spatial or contextual information, including water-maze and inhibitory avoidance (ref. 22 and B.R., D.J.-F.d.Q., Gustav Schelling, and J.L.M., unpublished observations). Furthermore, stress-level glucocorticoid administration to human subjects shortly before retention testing impairs hippocampus-dependent recall of previously learned verbal material (23, 24). Numerous studies using lesioning, pharmacological, or imaging techniques implicate the hippocampus in retrieval of spatial/contextual information in rats (25–31) or episodic memory in human subjects (32–34). The evidence that BLA activity is essential for mediating hippocampal stress hormone effects on memory consolidation suggests the possibility that the BLA might also regulate glucocorticoid effects on memory retrieval involving the hippocampus. To address this issue, the present experiments investigated the effects of intrahippocampal or intra-BLA infusions of the specific GR agonist 11β,17β-dihydroxy-6,21-dimethyl-17α-pregna-4,6-trien-20yn-3-one (RU 28362) administered to rats 60 min before water-maze retention testing, assessing memory retrieval of spatial information on a free-swim probe trial. In these studies we used a GR agonist, as our previous findings indicated that only stress-level doses of glucocorticoids, which at high doses primarily bind to GRs (35), impair memory retrieval. It is important to note that previous studies have demonstrated that a selective blockade of mineralocorticoid receptors (MRs) induces performance deficits in a water-maze spatial task (4). However, as MR antagonist infusions impair both acquisition and retention performance, those findings are consistent with the view that MR activation influences behavioral exploration and/or spatial navigation (36, 37). To examine whether GR agonist infusions selectively influenced memory retrieval, other groups of rats received infusions of RU 28362 60 min before water-maze training. We also investigated whether the BLA is required for mediating glucocorticoid effects on memory retrieval involving the hippocampus by determining whether excitotoxic lesions of the BLA, induced 1 week before training, altered the effects of pretest GR agonist infusions administered into the hippocampus on spatial memory retrieval.
Materials and Methods
Animals.
Male adult Sprague–Dawley rats (270–320 g at time of surgery) from Charles River Breeding Laboratories were kept individually in a temperature-controlled (22°C) colony room and maintained on a standard 12-h/12-h light/dark cycle (0700–1900 hours lights on) with ad libitum access to food and water. Training and testing were performed during the light phase of the cycle (1000–1400 hours), at the rat nadir of the circadian cycle for corticosterone. Experimental procedures were performed in compliance with National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
Surgery.
Animals, adapted to the vivarium for at least 1 week, were anaesthetized with sodium pentobarbital (50 mg/kg, i.p.) and given atropine sulfate (0.4 mg/kg, i.p.) to maintain respiration. The skull was positioned in a stereotaxic frame (Kopf Instruments, Tujunga, CA), and two stainless-steel guide cannulae (23 gauge) were implanted bilaterally with the cannula tips either 1.5 mm above the dorsal hippocampus [11 mm long; coordinates: anteroposterior (AP), −3.4 mm from bregma; mediolateral (ML), ±1.7 mm from midline; dorsoventral (DV), −2.7 mm from the skull surface, with the incisor bar −3.3 mm from the interaural line] or 2 mm above the BLA [15 mm long; coordinates: AP, −2.8 mm; ML, ±5.0 mm; DV, −6.5 mm]. The coordinates were based on the atlas of Paxinos and Watson (38). The cannulae were affixed to the skull with two anchoring screws and dental cement. Stylets (11- or 15-mm-long insect dissection pins) were inserted into each cannula to maintain patency and were removed only for the infusion of drugs.
For excitotoxic lesions of the BLA, an N-methyl-d-aspartate (NMDA; Sigma; 12.5 μg per 1.0 μl of phosphate buffer, pH 7.4) solution was back-filled into a 30-gauge microsyringe, driven by a minipump (Sage Instruments, Boston). The needle was placed into the BLA (coordinates: anteroposterior, −2.8 mm; mediolateral, ±5.0 mm; dorsoventral, −8.5 mm), and 0.2 μl of NMDA solution was infused over 25 s, after which the needle was left in place for 3 min to allow for diffusion. For sham BLA lesions, the injection needle tip was lowered only 6.5 mm below the skull surface and left in place for 3 min without an infusion. Immediately after lesioning of the BLA, the animals were placed in another stereotaxic frame for implantation of bilateral cannulae into the hippocampus. After surgery, rats received a 3.0-ml injection of saline (s.c.) to prevent dehydration and were placed into an incubator until recovery from anesthesia. They were allowed to recover a minimum of 7 days before initiation of training and were handled 3 times for 1 min each during this recovery period.
Water Maze and Spatial Learning Procedures.
The water maze was a circular, galvanized-steel tank, 1.83 m in diameter and 0.58 m in height, which was filled with water (25°C) to a depth of 20 cm. A rectangular platform (20 × 25 cm) was placed at a fixed location 25 cm away from the edge of the pool. The platform was submerged 2.5 cm below the water surface and could not be seen by the rats. The maze was located in a room containing several salient, visual, extra-maze cues.
For spatial training, rats were subjected to four trials a session for 3 consecutive days. Before the first training trial, the rat was placed directly on the submerged platform for 30 s. On each of the trials (i.e., swims), the rat was placed into the tank at one of the four designated starting points in a random order. Animals were allowed to find and escape onto the submerged platform. If an animal failed to find the platform within 60 s, it was manually guided to the platform. After mounting the platform, the rat was allowed to remain there for 15 s, and was then placed into a holding cage for 25 s until the start of the next trial. The time each animal spent to reach the platform was recorded as the escape latency.
Retention of the spatial training was assessed 24 h after the last training session with a 60-s free-swim probe trial using a new starting position. The probe trial was videotaped for off-line analysis. The parameters measured from the probe trial were time spent in the quadrant containing the platform during training, initial latency to cross the platform location, and total swim distance. For the acquisition experiment, rats received a single training session consisting of eight trials, followed immediately afterward by a 60-s probe-trial retention test.
Drug and Infusion Procedures.
The specific GR agonist RU 28362 (a gift from Roussel-Uclaf, Romainville, France) was infused into the dorsal hippocampus either 60 min before training or retention testing or into the BLA 60 min before retention testing. Receptor binding studies have shown that this compound has selective and high affinity for GRs (39). RU 28362 was first dissolved in 100% ethanol and subsequently diluted with saline to reach its appropriate concentration. The final concentration of ethanol was 0.25%. The vehicle solution contained 0.25% ethanol in saline only. RU 28362 was kept in a stock solution in 100% ethanol at −20°C. The doses of RU 28362 were based on previous studies indicating memory enhancement after immediate posttraining administration of these concentrations into the hippocampus or the BLA (14, 15, 18). Infusions of RU 28362, or an equivalent volume of control solution, into the hippocampus or the BLA were made by using a 30-gauge injection needle connected to a 10-μl Hamilton microsyringe by polyethylene (PE-20) tubing. For infusions of RU 28362 (5 or 15 ng) into the hippocampus, the injection needle protruded 1.5 mm beyond the tip of the cannula, and a 0.5-μl injection volume per hemisphere was infused over a period of 35 s by an automated syringe pump (Sage Instruments). The injection needle was retained within the cannula for an additional 20 s after drug infusion to maximize diffusion. The experimental procedure for intra-BLA infusions of RU 28362 (2 or 6 ng) was similar to that described for infusions into the hippocampus except that a volume of 0.2 μl was infused over a 25-s period and that the injection needle protruded 2.0 mm beyond the cannula tip. The volume used for the intra-BLA infusions was based on findings that drug infusions of this volume into either the BLA or the adjacent central nucleus of the amygdala induce differential effects on memory consolidation (17, 40).
Corticosterone Assay.
Immediately after the probe trial, the rats were injected with an overdose of pentobarbital. They were decapitated within 90 s of the injection and trunk blood was collected in heparinized (500 units/ml) tubes and stored on ice. After centrifugation at 4,500 × g for 10 min, the supernatant was stored at −50°C until assay. Corticosterone plasma concentrations were determined by a commercially available enzyme immunoassay kit using a 96-well microtiter plate coated with polyclonal antibody raised against corticosterone (Alpco, Windham, NH). The absorbance levels were measured with a photometric microplate reader (Thermo Labsystems, Helsinki) at 450 nm. The sensitivity was 0.023 μg/dl, and coefficients of variation within and between assays were <10%.
Histology.
After decapitation, the brains were removed and placed in 4% formaldehyde. At least 24 h before sectioning, the brains were submerged in a 20% sucrose solution for cryoprotection. Sections of 40 μm were made by using a freezing microtome and stained with cresyl violet. The sections were examined under a light microscope, and determination of the location of injection needle tips in either the hippocampus or the BLA as well as the size and location of the BLA lesions was made according to the standardized atlas plates of Paxinos and Watson (38) by an observer blind to drug treatment condition. Rats with improper cannula placement or extensive damage to the target tissue were excluded from analyses.
Statistics.
Water-maze training data were analyzed with a one-way ANOVA with trials as repeated measure. For the BLA lesion experiment, training escape latencies were analyzed with a repeated-measures ANOVA with treatment (BLA lesion or sham lesion) as between-subject variable. Probe-trial retention measures were analyzed with one- or two-way ANOVAs for treatment (doses of RU 28362 and BLA lesion). Further analysis used Fisher's post hoc tests to determine the source of the detected significance in the ANOVAs. A probability level of <0.05 was accepted as statistical significance.
Results
GR Agonist Infusions into the Hippocampus Before Retention Testing Impair Water-Maze Spatial Performance.
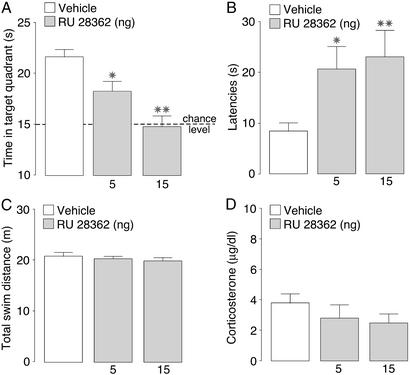
All rats learned to locate the platform during the 3 days of training as indicated by decreasing escape latencies as training progressed (F11,495 = 44.41; P < 0.0001; data not shown). Animals were chosen randomly to receive infusions of either vehicle or the GR agonist RU 28362 (5 or 15 ng) into the dorsal hippocampus 60 min before the 24-h retention test. Fig. 1 shows the effects of intrahippocampal GR agonist infusions on the several performance parameters measured during the probe-trial retention test. One-way ANOVAs for treatment indicated that RU 28362 induced dose-dependent impairment of retention performance, as assessed both by time spent in the quadrant that contained the platform during training (F2,43 = 12.88; P < 0.0001; Fig. 1A) and the initial latency to cross the platform location (F2,43 = 3.46; P < 0.05; Fig. 1B). Post hoc analyses revealed that both doses of RU 28362 significantly decreased the time spent in the training quadrant (5 ng: P < 0.05; 15 ng: P < 0.01) as well as increased initial crossing latencies (5 ng: P < 0.05; 15 ng: P < 0.01). Total swim distance during the probe trial was not affected by intrahippocampal infusions of RU 28362 (F2,43 = 0.44; P = 0.65; Fig. 1C), indicating that the longer crossing latencies were not caused by any gross changes in swimming speed. Furthermore, infusions of RU 28362 did not significantly alter plasma concentrations of corticosterone (F2,43 = 0.88; P = 0.42; Fig. 1D).
Figure 1.
Probe-trial retention performance of rats given infusions of the GR agonist RU 28362 (5 or 15 ng) into the hippocampus 60 min before water-maze retention testing. (A) Time spent in the training quadrant (mean + SEM) in seconds during the 60-s probe trial. (B) Latencies (mean + SEM) in seconds to cross the platform location. (C) Total swim distance (mean + SEM) in meters. (D) Plasma corticosterone levels (mean + SEM) in μg/dl as assessed immediately after the probe trial. *, P < 0.05; **, P < 0.01 compared with the vehicle group (n = 15–16 per group).
GR Agonist Infusions into the Hippocampus Do Not Impair Acquisition or Immediate Retention.
To examine further whether GR agonist infusions selectively affected retention performance or induced general impairments in spatial navigation, other groups of rats received intrahippocampal infusions of RU 28362 60 min before training. ANOVA for treatment with trials as repeated measure indicated that RU 28362 infusions did not alter escape latencies during water-maze acquisition, as assessed with a single training session consisting of eight trials (F2,245 = 0.31; P = 0.74; data not shown). Furthermore, retention testing with a 60-s probe trial conducted immediately after training showed unaltered performance. Neither the time spent in the training quadrant (F2,34 = 0.61; P = 0.55) nor the initial latency to cross the platform location were altered (F2,34 = 0.08; P = 0.93; data not shown).
GR Agonist Infusions into the Basolateral Amygdala Before Retention Testing Do Not Impair Water-Maze Spatial Performance.
All rats learned to locate the platform during the 3 days of training (F11,330 = 29.54; P < 0.0001; data not shown). Animals were chosen randomly to receive infusions of either vehicle or the GR agonist RU 28362 (2 or 6 ng) into the BLA 60 min before retention testing. Intra-BLA infusions of the GR agonist did not affect performance parameters measured during the probe-trial retention test. One-way ANOVAs for treatment indicated that RU 28362 did not affect time spent in the training quadrant (F2,30 = 1.05; P = 0.36) or the initial latency to cross the platform location (F2,30 = 0.29; P = 0.75; data not shown). Intra-BLA infusions of RU 28362 also did not affect total swim distance during the probe trial (F2,30 = 0.13; P = 0.88) or plasma levels of corticosterone (F2,30 = 0.38; P = 0.69).
Basolateral Amygdala Lesions Block the Effect of Intrahippocampal GR Agonist Infusions on Water-Maze Retention Impairment.
Experiments investigating glucocorticoid effects on memory consolidation have shown that lesions of the BLA block memory enhancement induced by intrahippocampal infusions of a GR agonist (14). The present experiment examined whether excitotoxic lesions of the BLA, made 1 week before initiation of water-maze training, blocked the effects on retention performance induced by intrahippocampal infusions of RU 28362 given 60 min before retention testing.
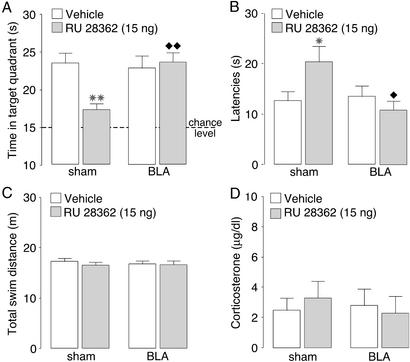
All rats learned to locate the platform position during the 3 days of training, before drug treatment (F11,627 = 68.51; P < 0.0001), and lesions of the BLA did not affect training escape latencies (F1,627 = 1.54; P = 0.22; data not shown). Fig. 2 shows the effects of BLA lesions on retention performance impairment induced by pretest intrahippocampal infusions of RU 28362. A two-way ANOVA for time spent in the training quadrant during the probe trial revealed a significant RU 28362 effect (F1,55 = 6.59; P < 0.05; Fig. 2A), a significant BLA lesion effect (F1,55 = 6.93; P < 0.05), and a significant interaction between both factors (F1,55 = 6.75; P < 0.05). As found in the first experiment, RU 28362 (15 ng) infused into the hippocampus 60 min before retention testing decreased time spent in the training quadrant to a chance level (P < 0.01 as compared with vehicle). Although BLA lesions alone did not affect retention performance, the lesions blocked the retention impairment induced by RU 28362 infused into the hippocampus. BLA-lesioned rats given infusions of RU 28362 into the hippocampus spent significantly more time in the target quadrant than sham-lesioned animals given RU 28362 (P < 0.01). Furthermore, the time spent in the training quadrant by BLA-lesioned rats given RU 28362 did not differ from that of either sham- or BLA-lesioned animals given vehicle infusions (P = 0.97 and P = 0.99, respectively). The pattern of results for initial latency to cross the platform location was similar to that for the time spent in the training quadrant. A two-way ANOVA revealed no RU 28362 effect (F1,55 = 2.61; P = 0.11; Fig. 2B) but did find a significant BLA lesion effect (F1,55 = 5.76; P < 0.05) and a significant interaction between both factors (F1,55 = 4.90; P < 0.05). RU 28362 infusions into the hippocampus increased initial latency to cross the platform location in sham-lesioned rats (P < 0.05), and this drug effect was blocked in animals with BLA lesions. Initial crossing latencies of BLA-lesioned rats given RU 28362 were significantly shorter than those of sham-lesioned animals given RU 28362 (P < 0.05). Furthermore, latencies of BLA-lesioned rats given RU 28362 into the hippocampus did not differ from those of either sham- or BLA-lesioned rats given vehicle (P = 0.49 and P = 0.59, respectively). A two-way ANOVA for total swim distance did not reveal significant RU 28362 (F1,55 = 0.77; P = 0.38), BLA lesion (F1,55 = 0.07; P = 0.79), or interaction effects (F1,55 = 0.10; P = 0.76; Fig. 2C). Two-way ANOVA for plasma corticosterone levels also found no significant RU 28362 (F1,55 = 0.02; P = 0.90), BLA lesion (F1,55 = 0.11; P = 0.75), or interaction effects (F1,55 = 0.42; P = 0.52; Fig. 2D).
Figure 2.
Probe-trial retention performance of rats with sham or N-methyl-d-aspartate-induced lesions of the BLA given infusions of the GR agonist RU 28362 (15 ng) into the hippocampus 60 min before water-maze retention testing. (A) Time spent in the training quadrant (mean + SEM) in seconds during the 60-s probe trial. (B) Latencies (mean + SEM) in seconds to cross the platform location. (C) Total swim distance (mean + SEM) in meters. (D) Plasma corticosterone levels (mean + SEM) in μg/dl as assessed immediately after the probe trial. *, P < 0.05; **, P < 0.01 compared with the corresponding vehicle group. ⧫, P < 0.05; ⧫⧫, P < 0.01 compared with the sham lesion–RU 28362 group (n = 13–17 per group).
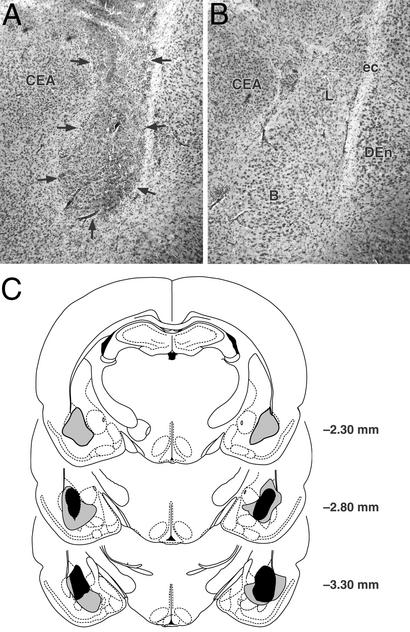
Fig. 3A shows a representative BLA lesion and Fig. 3B shows a sham lesion control. Histological examination of the lesioned BLA indicated that the lesioned area was characterized by pyknosis and loss of neurons, accompanied by extensive gliosis. In most animals, the lesions of the BLA were not complete, leaving the most anterior part intact, but typically 50% or more of the nucleus was damaged bilaterally. In several animals, whose data were included in the analyses, minor damage was seen in the cortex adjacent to the BLA but the central nucleus of the amygdala was intact in all animals. Fig. 3C shows the extent of minimum and maximum BLA lesions of rats included in the experiment.
Figure 3.
Lesions of the BLA. (A) Representative N-methyl-d-aspartate-induced lesion of the BLA. Arrows denote lesion borders. (B) Sham BLA lesion. (C) Smallest (black area) and largest (gray area) lesions from rats used in the experiment. Adapted from ref. 38. B, basal nuclei; CEA, central amygdala; DEn, dorsal endopiriform nucleus; ec, external capsula; L, lateral nuclei.
Discussion
We previously reported that stress exposure or systemic corticosterone injections shortly before water-maze retention testing induced impairment of spatial performance, whereas the same treatments given shortly before training did not affect either acquisition or immediate recall (22). Such a selective influence on retention performance, but not acquisition performance, strongly suggests that glucocorticoid administration induces impairment of spatial memory retrieval. The results of the present experiments extend those of de Quervain et al. (22) and provide evidence indicating that glucocorticoid effects on memory retrieval impairment are mediated by an activation of GRs in the hippocampus. The present findings also indicate that the BLA does not seem to play a direct role in mediating glucocorticoid effects on memory retrieval for spatial information. Importantly, however, an intact BLA is required for enabling GR activation in the hippocampus to impair spatial memory retrieval.
The GR agonist RU 28362 administered into the hippocampus 60 min before probe-trial retention testing dose-dependently impaired water-maze spatial performance, as assessed both by a decreased time spent in the training quadrant and an increased latency to cross the platform location. As the GR agonist did not affect total swim distance on the probe trial, the retention impairment cannot be attributed to an attenuation of motivation or motor performance. Furthermore, the failure of RU 28362 administered into the hippocampus 60 min before training to affect either water-maze acquisition or immediate retrieval on a probe trial strongly suggests that the RU 28362-induced retention impairment reflects direct effects on retrieval of long-term spatial information. As noted, the findings replicate those of de Quervain et al. (22), indicating that stress exposure or systemic injections of corticosterone induce temporary memory retrieval impairment. Retention performance of those rats was not longer impaired after stress-induced plasma corticosterone levels had returned to baseline. These findings provide strong evidence that after memories are consolidated, the efficacy or accuracy of the information retrieved is vulnerable to glucocorticoid influences at the time of retrieval. Additionally, these results are consistent with the findings that exposure to a stressor immediately after learning impairs performance on hippocampus-dependent tasks when retention is tested while corticosterone levels are still elevated (41, 42), and that glucocorticoids facilitate extinction of avoidance behavior (43). However, all stress hormones do not seem to impair memory retrieval. Other stress hormones, such as epinephrine, adrenocorticotropin, vasopressin, or β-endorphin, administered shortly before retention testing have been reported to enhance memory retrieval, possibly by acting as a retrieval cue (44–47).
Our findings suggest an important and specific role for the hippocampus, which expresses a high density of adrenal steroid receptors (35, 48), in mediating glucocorticoid effects on memory retrieval in a water-maze spatial task. Other evidence, however, indicates that administration of glucocorticoids into the hippocampus can alter glucocorticoid feedback mechanisms (49–51) and suggests that, as a consequence, local GR activation might potentially affect memory retrieval by means of an altered corticosterone influence on other brain regions. The present experiment, however, found no evidence of either an enhancement or impairment of circulating corticosterone levels after GR agonist infusions. Thus, the findings strongly suggest that the GR agonist effects on memory retrieval were due to influences within the hippocampus. Cellular actions of glucocorticoids on hippocampus neurons are consistent with this view. Peripheral injections of stress doses of corticosterone reduce hippocampal firing rate with a delay of ≈30–60 min (52, 53). Additionally, recent findings from an H215O-positron-emission tomography study in human subjects showed that stress doses of cortisone reduced regional blood flow in the right medial temporal lobe, most pronounced in the parahippocampal gyrus, which correlated with memory retrieval impairments on episodic tasks (D.J.-F.d.Q., Katharina Henke, Amanda Aerni, Valerie Treyer, J.L.M., Thomas Berthold, Roger M. Nitsch, Alfred Buck, B.R., and Christoph Hock, unpublished observation). Also, sustained hypercortisolemia in patients with Cushing's syndrome, depression, or aging is associated with a reduced hippocampal volume and impaired cognitive performance (54–56). Although glucocorticoid-induced structural changes in the hippocampus (57, 58) may impair memory encoding and/or consolidation, acute impairing effects of glucocorticoids on memory retrieval may contribute to the memory deficits found with prolonged hypercortisolemia (30).
Considerable evidence indicates that the hippocampus is involved in memory retrieval. Partial lesions of the hippocampus disrupt retrieval of spatial memory in a water maze but fail to affect new learning or retrieval of a task that is acquired postoperatively (25). Reversible neural inactivation of the hippocampus with an α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)/kainate receptor antagonist shortly before retention testing also impairs memory retrieval in a water-maze spatial task (27). Furthermore, lesions or pharmacological inhibition of the hippocampus impair contextual memory retrieval of fear memories (26, 29). More broadly, findings of studies of amnesic patients and neuroimaging studies indicate that successful retrieval of episodic memory depends on hippocampal function (32–34). Other studies, investigating the molecular substrate of memory retrieval, have suggested a role for hippocampal metabotropic glutamate receptors, protein kinase A, and mitogen-activated protein kinases in memory retrieval in an inhibitory avoidance task (28, 59). Cellular imaging studies examining the expression of the plasticity-associated immediate early gene zif268 during recall of fear memories have shown selective increases of zif268 levels in the hippocampus during retrieval of contextual but not cued fear conditioning, supporting a role of this immediate early gene during recall of contextual information (60).
The finding that infusions of the GR agonist administered into the BLA shortly before retention testing did not impair memory retention performance indicates that GR activation in the BLA is not crucially involved in memory retrieval. These findings are consistent with the evidence that BLA lesions do not impair performance on this task (5, 61) as well as with the findings that infusions of a metabotropic glutamate receptor antagonist into the amygdala before testing do not impair inhibitory avoidance retention (62). Other studies have reported that amygdala lesions or reversible inactivation impair retention in fear-based tasks (63–65) but as the amygdala is known to be critically involved in the expression of both conditioned and unconditioned fear behaviors (66, 67), it is unlikely that these effects specifically reflect memory retrieval deficits.
The important finding of the present study is that lesions of the BLA blocked memory retrieval impairment induced by intrahippocampal infusions of RU 28362, suggesting that neuronal input from the BLA is critical for enabling hippocampal glucocorticoid effects on memory retrieval. Such a role of the BLA in enabling glucocorticoids effects on memory retrieval involving the hippocampus is highly comparable to that suggested by previous findings investigating its function in mediating drug effects on memory consolidation. BLA lesions do not block acquisition or retention performance alone but block memory enhancement induced by either posttraining systemic or intrahippocampal administration of glucocorticoids (1, 14). Anatomical evidence is consistent with a role for the BLA in regulating hippocampal processes, as the BLA is known to project extensively to discrete hippocampal subfields (68). Studies have indicated that BLA effects on encoding and consolidation reflect the emotional or motivational significance of experiences (20, 69). The present findings indicate that the role of the BLA in regulating stress hormone effects on cognition in other brain regions is more general and extends to processes of memory retrieval. In a broader theoretical framework, our findings suggest that the BLA may play a promiscuous role in mediating stress hormone effects on two independently regulated memory processes, consolidation and retrieval. Alternatively, it is possible that glucocorticoid effects on memory retrieval impairment are linked to those on memory consolidation enhancement in terms of function and neurobiological substrate, and that BLA activation influences these related processes simultaneously. According to the view, exposure to emotionally arousing events or glucocorticoids may facilitate the consolidation and/or storage of new information, and simultaneously impair the recall of previously learned information. Hasselmo et al. (70) have suggested that changes in proactive interference, induced by functional changes in local hippocampal circuitry, can explain opposing drug effects on memory encoding and retrieval. Our findings suggest that there is a dynamic interplay between the BLA and the hippocampus in regulating stress hormone effects on different memory phases (3). However, it should be recognized that this system is only part of a larger interconnected network of cortical and subcortical structures important for mediating stress hormone effects on cognition (21).
Acknowledgments
We thank Lukasz Gorski for assistance with the corticosterone assay; Gabriel Hui for preparation of the figures; and Christina Buranday, Jayme McReynolds, and Sheila Nathan for performing the histology. This research was supported by Grant 32-58420.99 from the Swiss National Science Foundation (to D.J.-F.d.Q.) and U.S. Public Health Service Grant MH12526 (to J.L.M.). Q.K.G. was supported by National Institutes of Health Undergraduate Training Grant GM55246-03.
Abbreviations
- BLA
basolateral complex of the amygdala
- GR
glucocorticoid receptor
- RU 28362
11β,17β-dihydroxy-6,21-dimethyl-17α-pregna-4,6-trien-20yn-3-one
References
- 1.Roozendaal B. Psychoneuroendocrinology. 2000;25:213–238. doi: 10.1016/s0306-4530(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 2.McGaugh J L, Roozendaal B. Curr Opin Neurobiol. 2002;12:205–210. doi: 10.1016/s0959-4388(02)00306-9. [DOI] [PubMed] [Google Scholar]
- 3.Roozendaal B. Neurobiol Learn Mem. 2002;78:578–595. doi: 10.1006/nlme.2002.4080. [DOI] [PubMed] [Google Scholar]
- 4.Oitzl M S, de Kloet E R. Behav Neurosci. 1992;108:62–71. doi: 10.1037//0735-7044.106.1.62. [DOI] [PubMed] [Google Scholar]
- 5.Roozendaal B, Portillo-Marquez G, McGaugh J L. Behav Neurosci. 1996;110:1074–1083. doi: 10.1037//0735-7044.110.5.1074. [DOI] [PubMed] [Google Scholar]
- 6.Pugh C R, Tremblay D, Fleshner M, Rudy J W. Behav Neurosci. 1997;111:502–511. [PubMed] [Google Scholar]
- 7.Sandi C, Loscertales M, Guanza C. Eur J Neurosci. 1997;9:637–642. doi: 10.1111/j.1460-9568.1997.tb01412.x. [DOI] [PubMed] [Google Scholar]
- 8.Cordero M I, Sandi C. Brain Res. 1998;786:11–17. doi: 10.1016/s0006-8993(97)01420-0. [DOI] [PubMed] [Google Scholar]
- 9.Conrad C D, Lupien S J, McEwen B S. Neurobiol Learn Mem. 1999;72:39–46. doi: 10.1006/nlme.1998.3898. [DOI] [PubMed] [Google Scholar]
- 10.Cottrell G A, Nakajima S. Pharmacol Biochem Behav. 1977;7:277–280. doi: 10.1016/0091-3057(77)90146-0. [DOI] [PubMed] [Google Scholar]
- 11.Micheau J, Destrade C, Soumireu-Mourat B. Eur J Pharmacol. 1985;106:39–46. doi: 10.1016/0014-2999(84)90675-7. [DOI] [PubMed] [Google Scholar]
- 12.de Kloet E R, de Kock S, Schild V, Veldhuis H D. Neuroendocrinology. 1988;47:109–115. doi: 10.1159/000124900. [DOI] [PubMed] [Google Scholar]
- 13.Korte S M, de Kloet E R, Buwalda B, Bouman S D, Bohus B. Eur J Pharmacol. 1996;301:19–25. doi: 10.1016/0014-2999(96)00064-7. [DOI] [PubMed] [Google Scholar]
- 14.Roozendaal B, McGaugh J L. Eur J Neurosci. 1997;9:76–83. doi: 10.1111/j.1460-9568.1997.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 15.Roozendaal B, Nguyen B T, Power A E, McGaugh J L. Proc Natl Acad Sci USA. 1999;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roozendaal B, de Quervain D J-F, Ferry B, Setlow B, McGaugh J L. J Neurosci. 2001;21:2518–2525. doi: 10.1523/JNEUROSCI.21-07-02518.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Roozendaal B, McGaugh J L. Neurobiol Learn Mem. 1997;67:176–179. doi: 10.1006/nlme.1996.3765. [DOI] [PubMed] [Google Scholar]
- 18.Roozendaal B, Quirarte G L, McGaugh J L. Eur J Neurosci. 2002;15:553–560. doi: 10.1046/j.0953-816x.2001.01876.x. [DOI] [PubMed] [Google Scholar]
- 19.Packard M G, Cahill L, McGaugh J L. Proc Natl Acad Sci USA. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGaugh J L, Cahill L, Roozendaal B. Proc Natl Acad Sci USA. 1996;93:13508–13514. doi: 10.1073/pnas.93.24.13508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McGaugh J L. Trends Neurosci. 2002;25:456–461. doi: 10.1016/s0166-2236(02)02211-7. [DOI] [PubMed] [Google Scholar]
- 22.de Quervain D J-F, Roozendaal B, McGaugh J L. Nature. 1998;394:787–790. doi: 10.1038/29542. [DOI] [PubMed] [Google Scholar]
- 23.de Quervain D J-F, Roozendaal B, Nitsch R M, McGaugh J L, Hock C. Nat Neurosci. 2000;3:313–314. doi: 10.1038/73873. [DOI] [PubMed] [Google Scholar]
- 24.Wolf O T, Convit A, McHugh P F, Kandil E, Thorn E L, de Santi S, McEwen B S, Leon M J. Behav Neurosci. 2001;115:1002–1011. doi: 10.1037//0735-7044.115.5.1002. [DOI] [PubMed] [Google Scholar]
- 25.Moser M B, Moser E I. J Neurosci. 1998;18:7535–7542. doi: 10.1523/JNEUROSCI.18-18-07535.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holt W, Maren S. J Neurosci. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Riedel G, Micheau J, Lam A G M, Roloff E v L, Martin S J, Bridge H, de Hoz L, Poeschel B, McCulloch J, Morris R G M. Nat Neurosci. 1999;2:898–905. doi: 10.1038/13202. [DOI] [PubMed] [Google Scholar]
- 28.Izquierdo L A, Barros D M, Ardenghi P G, Pereira P, Rodrigues C, Choi H, Medina J H, Izquierdo I. Behav Brain Res. 2000;111:93–98. doi: 10.1016/s0166-4328(00)00137-6. [DOI] [PubMed] [Google Scholar]
- 29.Corcoran K A, Maren S. J Neurosci. 2001;21:1720–1726. doi: 10.1523/JNEUROSCI.21-05-01720.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roozendaal B, Phillips R G, Power A E, Brooke S M, Sapolsky R M, McGaugh J L. Nat Neurosci. 2001;4:1169–1171. doi: 10.1038/nn766. [DOI] [PubMed] [Google Scholar]
- 31.Steffenach H A, Sloviter R S, Moser E I, Moser M B. Proc Natl Acad Sci USA. 2002;99:3194–3198. doi: 10.1073/pnas.042700999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schacter D L, Wagner A D. Hippocampus. 1999;9:7–24. doi: 10.1002/(SICI)1098-1063(1999)9:1<7::AID-HIPO2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 33.Cabeza R, Nyberg L. J Cognit Neurosci. 2000;12:1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- 34.Eldridge L L, Knowlton B J, Furmanski C S, Bookheimer S Y, Engel S A. Nat Neurosci. 2000;3:1149–1152. doi: 10.1038/80671. [DOI] [PubMed] [Google Scholar]
- 35.Reul J M H M, de Kloet E R. Endocrinology. 1985;117:2505–2512. doi: 10.1210/endo-117-6-2505. [DOI] [PubMed] [Google Scholar]
- 36.Oitzl M S, Fluttert M, de Kloet E R. Eur J Neurosci. 1994;6:1072–1079. doi: 10.1111/j.1460-9568.1994.tb00604.x. [DOI] [PubMed] [Google Scholar]
- 37.de Kloet E R, Oitzl M S, Joëls M. Trends Neurosci. 1999;22:422–426. doi: 10.1016/s0166-2236(99)01438-1. [DOI] [PubMed] [Google Scholar]
- 38.Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd Ed. San Diego: Academic; 1997. [DOI] [PubMed] [Google Scholar]
- 39.Teutsch G, Costerousse G, Deraedt R, Benzoni J, Fortin M, Philibert D. Steroids. 1981;38:651–665. doi: 10.1016/0039-128x(81)90084-2. [DOI] [PubMed] [Google Scholar]
- 40.Parent M B, McGaugh J L. Brain Res. 1994;661:97–103. doi: 10.1016/0006-8993(94)91186-x. [DOI] [PubMed] [Google Scholar]
- 41.Diamond D M, Park C R, Heman K L, Rose G M. Hippocampus. 1999;9:542–552. doi: 10.1002/(SICI)1098-1063(1999)9:5<542::AID-HIPO8>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 42.Bats S, Thoumas J L, Lordi B, Tonon M C, Lalonde R, Caston J. Behav Brain Res. 2000;118:11–15. doi: 10.1016/s0166-4328(00)00285-0. [DOI] [PubMed] [Google Scholar]
- 43.Bohus B, de Wied D. In: General, Comparative and Clinical Endocrinology of the Adrenal Cortex. Chester-Jones I, Henderson I W, editors. London: Academic; 1980. pp. 265–347. [Google Scholar]
- 44.Izquierdo I. Behav Neural Biol. 1989;51:171–202. doi: 10.1016/s0163-1047(89)90812-1. [DOI] [PubMed] [Google Scholar]
- 45.Bohus B. In: The Memory System of the Brain. Delacour J, editor. Singapore: World Scientific; 1994. pp. 337–364. [Google Scholar]
- 46.Vianna M R M, Szapiro G, McGaugh J L, Medina J H, Izquierdo I. Proc Natl Acad Sci USA. 2001;98:12251–12254. doi: 10.1073/pnas.211433298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Izquierdo L A, Barros D M, Medina J H, Izquierdo I. Behav Pharmacol. 2002;13:203–213. doi: 10.1097/00008877-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 48.Morimoto M, Morita N, Ozawa H, Yokoyama K, Kawata M. Neurosci Res. 1996;26:235–269. doi: 10.1016/s0168-0102(96)01105-4. [DOI] [PubMed] [Google Scholar]
- 49.Ratka A, Sutanto W, Bloemers M, de Kloet E R. Neuroendocrinology. 1989;50:117–123. doi: 10.1159/000125210. [DOI] [PubMed] [Google Scholar]
- 50.van Haarst A D, Oitzl M S, de Kloet E R. Neurochem Res. 1997;22:1323–1328. doi: 10.1023/a:1022010904600. [DOI] [PubMed] [Google Scholar]
- 51.Feldman S, Weidenfeld J. Brain Res. 1999;821:33–37. doi: 10.1016/s0006-8993(99)01054-9. [DOI] [PubMed] [Google Scholar]
- 52.Pfaff D W, Silva M T A, Weiss J M. Science. 1971;171:394–395. doi: 10.1126/science.172.3981.394. [DOI] [PubMed] [Google Scholar]
- 53.Joëls M. J Neuroendocrinol. 2001;13:657–669. doi: 10.1046/j.1365-2826.2001.00688.x. [DOI] [PubMed] [Google Scholar]
- 54.Starkman M N, Gebarski S S, Berent S, Schteingart D E. Biol Psychiatry. 1992;32:756–765. doi: 10.1016/0006-3223(92)90079-f. [DOI] [PubMed] [Google Scholar]
- 55.Sheline Y I, Wang P W, Gada M H, Csernansky J G, Vannier M W. Proc Natl Acad Sci USA. 1996;93:3908–3913. doi: 10.1073/pnas.93.9.3908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lupien S J, de Leon M, de Santi S, Convit A, Tarshish C, Nair N P, Thakur M, McEwen B S, Hauger R L, Meaney M J. Nat Neurosci. 1998;1:69–73. doi: 10.1038/271. [DOI] [PubMed] [Google Scholar]
- 57.Sapolsky R M, Uno H, Rebert C S, Finch C E. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Watanabe Y, Gould E, McEwen B S. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- 59.Szapiro G, Izquierdo L A, Alonso M, Barros D, Paratcha G, Ardenghi P, Pereira P, Medina J H, Izquierdo I. Neuroscience. 2000;99:1–5. doi: 10.1016/s0306-4522(00)00236-0. [DOI] [PubMed] [Google Scholar]
- 60.Hall J, Thomas K L, Everitt B J. J Neurosci. 2001;21:2186–2193. doi: 10.1523/JNEUROSCI.21-06-02186.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kim J J, Lee H J, Han J S, Packard M G. J Neurosci. 2001;21:5222–5228. doi: 10.1523/JNEUROSCI.21-14-05222.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Barros D M, Izquierdo L A, Mello e Souza T, Ardenghi P G, Pereira P, Medina J H, Izquierdo I. Behav Brain Res. 2000;114:183–192. doi: 10.1016/s0166-4328(00)00226-6. [DOI] [PubMed] [Google Scholar]
- 63.Kim M, Davis M. Behav Neurosci. 1993;107:1088–1092. doi: 10.1037//0735-7044.107.6.1088. [DOI] [PubMed] [Google Scholar]
- 64.Maren S, Fanselow M S. Neuron. 1996;16:237–240. doi: 10.1016/s0896-6273(00)80041-0. [DOI] [PubMed] [Google Scholar]
- 65.Fendt M. J Neurosci. 2001;21:4111–4115. doi: 10.1523/JNEUROSCI.21-11-04111.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mesches M H, Bianchin M, McGaugh J L. Neurobiol Learn Mem. 1996;66:324–340. doi: 10.1006/nlme.1996.0073. [DOI] [PubMed] [Google Scholar]
- 67.Vazdarjanova A, McGaugh J L. Proc Natl Acad Sci USA. 1998;95:15003–15007. doi: 10.1073/pnas.95.25.15003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Petrovich G D, Canteras N S, Swanson L W. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- 69.Holland P C, Gallagher M. Trends Cognit Sci. 1999;3:65–73. doi: 10.1016/s1364-6613(98)01271-6. [DOI] [PubMed] [Google Scholar]
- 70.Hasselmo M E, Wyble B P, Wallenstein G V. Hippocampus. 1996;6:693–708. doi: 10.1002/(SICI)1098-1063(1996)6:6<693::AID-HIPO12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]