Abstract
Senescence of cultured cells involves activation of the p19Arf-p53 and the p16Ink4a-Rb tumor suppressor pathways. This, together with the observation that p19Arf and p16Ink4a expression increases with age in many tissues of humans and rodents, led to the speculation that these pathways drive in vivo senescence and natural aging. However, it has been difficult to test this hypothesis using a mammalian model system because inactivation of either of these pathways results in early death from tumors. One approach to bypass this problem would be to inactivate these pathways in a murine segmental progeria model such as mice that express low amounts of the mitotic checkpoint protein BubR1 (BubR1 hypo-morphic mice). These mice have a five-fold reduced lifespan and develop a variety of early-aging associated phenotypes including cachetic dwarfism, skeletal muscle degeneration, cataracts, arterial stiffening, (subcutaneous) fat loss, reduced stress tolerance and impaired wound healing. Importantly, BubR1 hypomorphism elevates both p16Ink4a and p19Arf expression in skeletal muscle and fat. Inactivation of p16Ink4a in BubR1 mutant mice delays both cellular senescence and aging specifically in these tissues. Surprisingly, however, inactivation of p19Arf has the opposite effect; it exacerbates in vivo senescence and aging in skeletal muscle and fat. These mouse studies suggest that p16Ink4a is indeed an effector of aging and in vivo senescence, but p19Arf an attenuator. Thus, the role of the p19Arf-p53 pathway in aging and in vivo senescence seems far more complex than previously anticipated.
Keywords: BubR1, p16Ink4a, p19Arf, spindle assembly checkpoint, premature aging, cellular senescence
Introduction
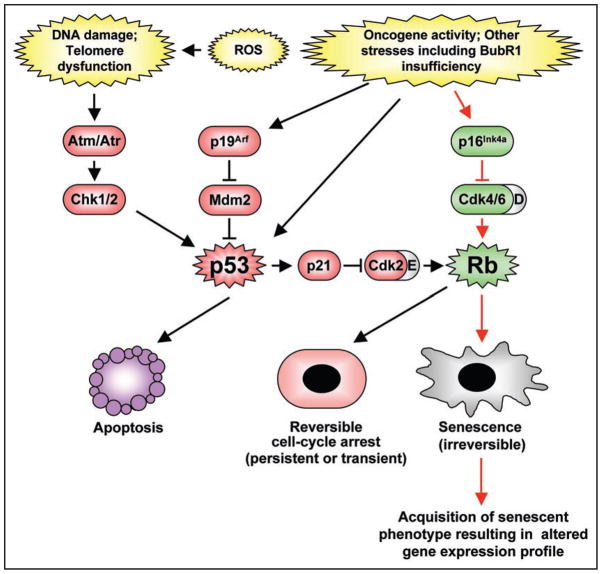
A variety of stressful stimuli, including oncogene overexpression, telomere maintenance defects, oxidative stress, DNA damage and nutrient deprivation, lead to the onset of senescence, defined as a state of permanent cell cycle arrest.1,2 Two important cell cycle inhibitors p16Ink4a and p19Arf, which are both encoded at the Cdkn2a locus, are expressed at high levels in senescing cultured cells and are believed to play a central role in establishing the senescent state.3,4 Although both proteins block cell cycle progression when expressed above a certain level, the molecular mechanisms by which they operate are quite distinct. p16Ink4a prevents progression through G1 phase by inhibiting the activity of D-type cyclins, thus rendering Rb active (Fig. 1). Short-term signaling through the p16Ink4a-Rb pathway results in a transient G1 arrest, but chronic, long-term p16Ink4a induction leads to cellular senescence, which is characterized by dramatic morphological and transcriptional changes.2,5 In contrast, p19Arf does not act directly on cyclins, but instead stabilizes the tumor suppressor p53.6,7 This stabilization can lead to one of two cellular fates: apoptosis or growth arrest.8 Under conditions of high stress causing cellular damage that is beyond repair, p53 induces a robust transcriptional response that mediates apoptosis and the cell is removed entirely.9 However, when cycle arrest is desired to allow for repair of minor stress-related damage, p53 induces p21Cip1 (referred to hereafter as p21) to transiently inhibit the activity of all cyclin-Cdk complexes present at the time stress is inflicted.10–12 Long-term signaling through the p53 pathway in G1 in cases where the damage is more substantial causes a more permanent cycle arrest mediated by p21 inhibition of cyclin E-Cdk2, resulting in Rb hypophosphorylation and E2F inactivation. Cells in this state are often referred to as senescent cells, but in contrast to p16Ink4a-Rb driven senescence, this arrest is reversible upon the removal of either p53 or p21.13
Figure 1.
Senescence controlled by the p16Ink4a-Rb and p19Arf-p53 pathways. Various intrinsic and extrinsic stress-inducing signals are able to engage the tumor suppressor pathways containing p53, p16Ink4a-Rb or both. Activation of p53 triggers cell death by inducing expression of a number of downstream target genes implicated in apoptosis (e.g., Bim, Puma, Noxa, Bid, Bax and Apaf1) or a transient or permanent (though reversible) cell cycle arrest via induction of p21. On the other hand, Rb is capable of inducing an irreversible state of cellular senescence via repression of E2F target genes and alterations in chromatin structure.
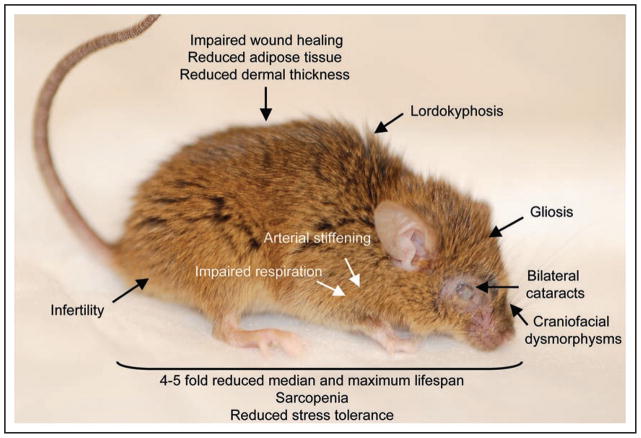
While mechanisms mediating in vitro senescence have been well characterized, whether the same pathways are involved in promoting in vivo cellular senescence and aging has been unclear. Additionally, although cells with characteristics of senescence accumulate in tissues during normal aging and in tissues that exhibit certain age-related pathologies, whether senescent cells contribute directly to organismal aging remains a matter of debate.5,14 Studies aimed at understanding the molecular mechanisms of cellular senescence and its role in aging have been hampered by the fact that inactivation of many members of the p19Arf-p53 and p16Ink4a-Rb pathway in mice results in early death from tumors.1,2 An alternative way to determine how these pathways are implicated in organismal aging could be to utilize mutant mouse strains that develop age-related (progeroid) phenotypes at an early age. Mice that express low amounts of the mitotic checkpoint protein BubR1 develop a wide-variety of age-related pathologies affecting multiple tissue types within 3 to 5 months (Fig. 2).15–18 Furthermore, the expression of both p16Ink4a and p19Arf is elevated in certain tissues susceptible to early aging in this model, specifically eye, fat and skeletal muscle.18 Therefore this model seems well suited for investigating the role that p16Ink4a and p19Arf play in the establishment of these characteristics and whether in vivo cellular senescence is to blame.
Figure 2.
Summary of tissues affected with age-related pathologies in BubR1 hypomorphic mice. Shown is a 5-month-old BubR1 insufficient animal. Highlighted are several of the progeroid features commonly observed in this model.
The Yin: p16Ink4a Drives Aging in Certain Tissues of BubR1 Mutant Mice
First, BubR1 hypomorphic mice were bred onto a p16Ink4a nullizygous background and monitored for the rate of aging. In the absence of p16Ink4a, certain, but not all, age-related characteristics of BubR1 mutant mice were delayed, including sarcopenia, cataracts, fat loss and median survival,18 suggesting that p16Ink4a promotes aging only in certain tissues in response to BubR1 hypomorphism. None of the early age-related phenotypes of BubR1 hypomorphic mice were accelerated, suggesting that p16Ink4a has no anti-aging properties. One explanation for the differential corrective effect of p16Ink4a loss might be that BubR1 hypomorphism engages the p16Ink4a-Rb pathway in a tissue specific fashion. Indeed, only tissues with delayed aging when p16Ink4a is lacking, such as eye, adipose tissue and skeletal muscle, exhibit induction of this protein in response to BubR1 insufficiency.
So how might BubR1 hypomorphism trigger p16Ink4a expression? Currently, the only well established BubR1 functions are all related to mitosis. BubR1 is primarily known for its inhibition of Cdc20, a key activator of a multiprotein ubiquitin E3 ligase complex called the anaphase-promoting complex (APC/C) that regulates mitotic progression by targeting various mitotic substrates for degradation by the proteosome.19 In addition, BubR1 controls the rate with which cells progress from prophase to anaphase (referred to as mitotic timing)20 and mediates stable attachment of kinetochores to spindle microtubules.21 As cells enter G1 phase, BubR1 levels drop considerably. Whether these low residual amounts of BubR1 have additional functions outside of mitosis remains an open question. In vitro studies have implied a role for the BubR1-binding protein Bub3 in gene transcription through its ability to interact with certain histone deacetylases.22 An attractive hypothesis would be that BubR1 might repress the p16Ink4a promoter. However, this seems improbable because, unlike Bub3, BubR1 does not exert transcriptional repressive activity in vitro.
Recent data suggests that cells expressing low amounts of BubR1 that aberrantly segregate their chromosomes undergo Mek and Erk phosphorylation,23 two kinases of the canonical Ras-Raf-Mek-Erk mitogenic cascade.24 Oncogenic activation of this pathway is known to induce the expression of many genes, including p16Ink4a.25,26 So perhaps BubR1 hypomorphism might activate Erk, which in turn might induce p16Ink4a. Whether Erk is indeed aberrantly activated and how low amounts of BubR1 trigger this response is an area requiring further exploration using hypomorphic BubR1 mice.
It is important to note that while knocking out p16Ink4a significantly delayed the development of certain age-related diseases in BubR1 hypomorphic mice, eventually these animals exhibited progeroid features in these tissues. In addition, other tissues that develop age-related diseases that are not influenced by inactivation of p16Ink4a, including dermis, brain, aorta, testis and ovary, exhibit normal levels of p16Ink4a. These results imply that additional pathways besides p16Ink4a-Rb are activated in response to BubR1 insufficiency to drive the aging process. The identity of other genes and pathways that may also be activated in response to BubR1 hypomorphism remains elusive and is clearly a venue for further experimentation.
The Yang: p19Arf Applies the Brakes to p16Ink4a-Mediated Aging
Eye, adipose tissue and skeletal muscle, all of which express high p16Ink4a in response to BubR1 hypomorphism, also have high p19Arf levels,18 suggesting a coordinated activation of both genes encoded by the Cdkn2a locus. Inactivation of p16Ink4a in BubR1 hypomorphic mice restores normal p19Arf levels, implying that increased p19Arf expression is dependent on p16Ink4a induction. Given this dependence, it is unlikely that BubR1 controls p19Arf expression directly. Unexpectedly, inactivation of p19Arf in BubR1 mutant mice led to faster rather than slower aging, specifically in eye, adipose tissue and skeletal muscle, indicating that p19Arf induction in response to BubR1 hypomorphism acts to attenuate the aging proces.18 One possible explanation for the accelerated aging following the inactivation of p19Arf might be that p16Ink4a expression is further elevated in these tissues. Indeed, p16Ink4a is superinduced in skeletal muscle, fat and eye of BubR1 hypomorphic mice lacking p19Arf.18 This, combined with the observation that BubR1 mutants lacking p16Ink4a have normal levels of p19Arf, suggests that p19Arf exerts its anti-aging effect, at least in part, through a currently unknown ability to negatively regulate p16Ink4a.
How might results obtained via the use of BubR1 hypomorphic mice combined with deficiencies in p16Ink4a or p19Arf relate to natural aging? Several mouse tissues show a substantial reduction in BubR1 during the course of natural aging,15,18 suggesting that BubR1 has a potential role in the regulation of organismal aging. Critical testing of this idea would require the use of mice with forced overexpression of BubR1 via transgenes to determine if BubR1 is indeed a modulator of aging. The link between mitotic checkpoint impairment and aging is further supported by the finding that a high proportion of individuals with mosaic variegated aneuploidy (MVA) syndrome exhibit mono- or biallelic mutations in BubR1.27,28 Carriers of such mutations show various progeroid features, including short lifespan, dwarfism, cataracts and facial dysmorphisms.29,30 Whether these patients develop additional progeroid features seen in hypomorphic mice is not entirely clear, mainly because MVA syndrome is such a rare disorder. It will thus be imperative to develop a mouse to model human BubR1 mutations to learn more about the pathology of this syndrome and its link to premature aging. MVA patients with biallelic BubR1 mutations carry one missense mutation in the BubR1 kinase domain and a truncating mutation upstream of the kinase domain.27 On the other hand, MVA patients with monoallelic BubR1 mutations harbor a truncating mutation and a non-mutated BubR1 allele that yields subnormal amounts of wildtype BubR1 protein.27 The latter finding is interesting, as it suggests that hypomorphic BubR1 alleles that produce less protein exist within the human population.28 It would be interesting to identify these alleles and determine whether individuals that carry them are prone to certain age-related pathologies.
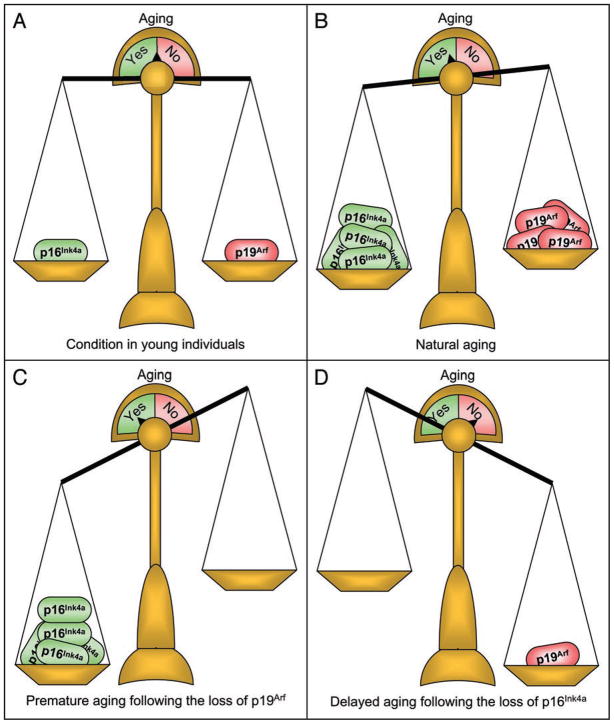
Many tissues that show a decline in BubR1 with age have elevated amounts of both p16Ink4a and p19Arf in naturally aged mice.18,31 Because p19Arf appears to counteract the pro-aging effects of p16Ink4a, a delicate balance between these two proteins may be required to prevent the acquisition of age-related pathologies. Young animals express relatively low amounts of both p16Ink4a and p19Arf, and therefore aging is not promoted (Fig. 3A). During the course of natural aging, the elevation of these two genes appears in concert with each other, but ultimately the balance might tip towards p16Ink4a and aging ensues (Fig. 3B). If the balance would be tipped more towards p19Arf then aging might be attenuated. Consistent with this idea is the recent observation that transgenic mice with an extra copy of both p19Arf and p53 are protected from aging-associated damage and live longer than normal mice.32 Under conditions where the level of p16Ink4a is not being balanced by p19Arf, organismal aging might occur at much faster rates, as evidenced by BubR1 hypomorphic mice lacking p19Arf (Fig. 3C). A converse to this would be that mice lacking p16Ink4a do not have the driving force needed for certain tissues to develop aging (Fig. 3D). In accordance with this idea, aging is delayed following the loss of p16Ink4a in mice with insufficient amounts of BubR1. It is important to note that this model fits within the results obtained by using BubR1 hypomorphic mice lacking p16Ink4a or p19Arf, but additional experimentation with other progeroid mouse models will be necessary to validate it.
Figure 3.
The Cdkn2a locus and aging. (A) In young mice, the amount of p16Ink4a and p19Arf is quite low and in balance. (B) As animals age, p16Ink4a and p19Arf accumulate in the same tissue, with p19Arf attempting to counter-balance the pro-aging effects of p16Ink4a. With time, the balance shifts towards a state of aging. (C) In scenarios where p19Arf is not present to counteract p16Ink4a, the absolute levels of p16Ink4a increase dramatically, further promoting a pro-aging microenvironment. (D) When p16Ink4a is absent, p19Arf does not accumulate. Aging is delayed and the induction of p19Arf is not required.
Linking cellular senescence in vivo to aging
It has long been suspected that senescent cells contribute to age-related pathologies.5,14 However, this has been difficult to prove experimentally. Skeletal muscle and fat tissue of BubR1 hypomorphic mice, two tissues that undergo accelerated aging, accumulate vast amounts of senescent cells in a p16Ink4a dependent fashion, indicating that p16Ink4a contributes to aging by promoting cellular senescence.18 The role of senescence in driving the aging process is further underscored by the observation that p19Arf not only delays the onset of age-related phenotypes in skeletal muscle and fat of BubR1 hypomorphic mice, but also protects cells of these same tissues from entering the senescent state.18 It is important to note that the pathways that drive senescence in response to BubR1 hypomorphism in cultured cells do not necessarily represent those that occur in vivo. Senescence of BubR1 hypomorphic MEFs in culture can be completely prevented by p19Arf inactivation, whereas p16Ink4a loss has no effect. The discrepancy between the effects of the p19Arf-p53 and p16Ink4a-Rb pathway on in vitro and in vivo senescence requires further validation using alternative prematurely aged mouse models, preferably those that exhibit increased rates of cellular senescence and in which both signaling pathways are engaged.
p53 and Aging: a Complex Relationship
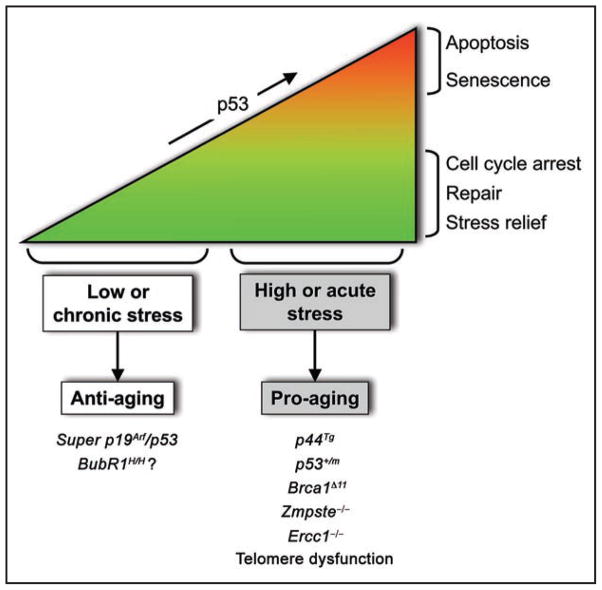
Given the importance of p19Arf for prevention of senescence in eye, skeletal muscle and fat in BubR1 hypomorphic tissues, it will be important to examine whether p53 induction is required for attenuating senescence and aging of these tissues. If so, the next question that arises is which are the critical downstream p53 targets. Various mouse studies have characterized p53 as an effector of organismal aging.8,34,35 Mutant mouse strains harboring certain p53 mutations or expressing high levels of the p53 splice variant p44 show accelerated aging, presumably due to constitutive hyper-activation of p53.36,37 Furthermore, several mouse models of premature aging, including Zmpste24−/−, Ercc1−/− and Brca1Δ11 show elevated p53 levels, with inactivation of p53 in these models delaying aging.38–40 However, one complication of this idea that p53 drives aging is the finding that mice harboring an extra genomic copy of both the p53 and the p19Arf gene locus accumulate less aging-associated damage and live longer than normal mice.32 Perhaps, the level of induction of p53 may influence organismal aging to vastly different degrees (Fig. 4). High/acute stressors might induce a robust p53 transcriptional response that leads to apoptosis or senescence, both of which promote tissue deterioration and aging. However, in situations where the stress is mild and more chronic, perhaps as in natural aging, p53 might trigger a long-term cell cycle arrest without the adverse consequences of cellular senescence that functionally impair neighboring cells. Conceivably, less robust p53 transcriptional responses might alleviate the impact of chronic stress, thereby perhaps extending cell functionality and thus organ function.
Figure 4.
Working model for regulation of aging by the p19Arf-p53 pathway. Various stresses engage and stabilize p53. Acute situations mobilize an intense p53 response, leading to apoptosis or cellular senescence and the establishment of early aging. Chronic/low amounts of stress on the other hand, might activate p53 to provide a transient cell cycle arrest that provides an opportunity to repair damage or relieve cellular stress, thereby improving cell function in the presence of stress. Stress encountered with natural aging might engage a response similar to that of chronic stress.
BubR1 Hypomorphism: What is the Primary Lesion Causing Premature Aging?
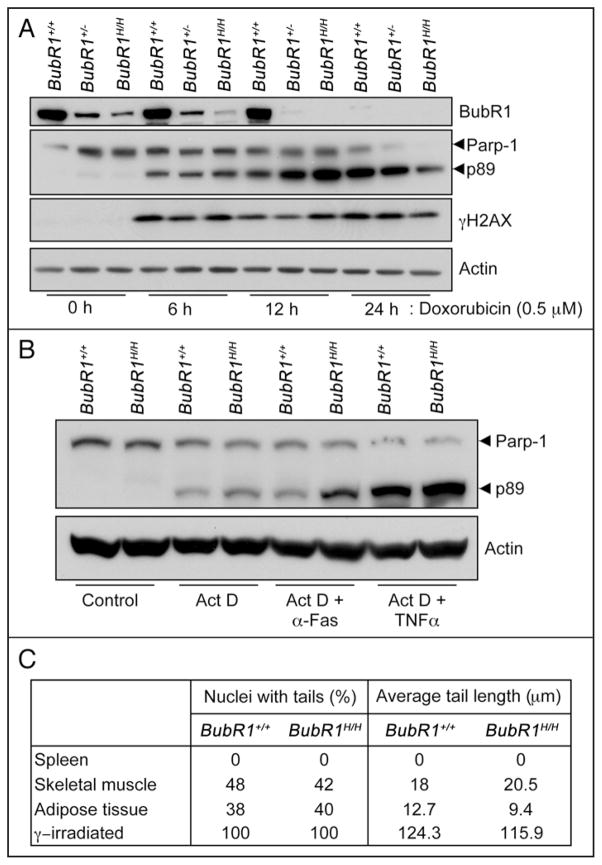
So, what could be the nature of the primary lesion (or stress) causing the activation of senescence and aging in response to BubR1 hypomorphism? Because accurate chromosome segregation is highly dependent on BubR1, premature aging of BubR1 hypomorphic mice was initially believed to be due to the accumulation of aneuploid cells.15 However, subsequent studies using other mitotic checkpoint defective mouse models did not support this hypothesis.41–43 Outside of its role in the mitotic checkpoint surveillance machinery, mitotic timing and stable microtubule-kinetochore attachment, BubR1 has been implicated in mediating an adequate DNA damage response.44 Specifically, BubR1 haploinsufficient MEFs were reported to be defective in γH2AX phosphorylation and Parp-1 stabilization following exposure to DNA damaging agents such as doxorubicin and ultraviolet light. However, BubR1 haploinsufficient MEFs from an independent strain as well as BubR1 hypomorphic MEFs showed normal levels of γH2AX and Parp-1 cleavage upon doxorubicin exposure, implying that their DNA damage repair pathways were unperturbed (Fig. 5A). This was further confirmed by data showing that survival and colony formation ability of BubR1 hypomorphic MEFs was normal upon treatment with various kinds of DNA damaging agents.15 Additional agents used to promote apoptosis in cultured cells, TNFα and α-Fas, induced Parp-1 cleavage and γH2AX to a similar degree in wildtype and BubR1 hypomorphic cells (Fig. 5B). In addition, single cell gel electrophoresis, or comet, assays performed on BubR1 hypomorphic tissues with early age-related disease revealed no evidence for increased single-strand or double strand breaks, apurinic or apyrimidinic sites, or DNA adducts (Fig. 5C). Thus, although deficiencies in the DNA damage repair machinery have been linked to premature aging in mice,45 there is currently no evidence for impaired DNA damage repair ability in BubR1 hypomorphic cells. Recent evidence suggests that BubR1 is implicated in the cellular response to dysfunctional telomeres.46 However, the typical cellular phenotypes observed in cells deficient for adequate telomere maintenance, such as end-to-end fusions and increased rates of γH2AX stabilization, are not seen in BubR1 hypomorphic MEFs, suggesting that telomere dysfunction is unlikely to underlie premature aging in BubR1 mutant mice. BubR1 is a modular protein that has various functional domains. Thus, although several lesions implicated in the aging process do not seem to apply to BubR1 hypomorphic mice, the primary lesion causing premature aging in this model remains to be identified. One approach that might provide crucial insight would be to determine which functional domains of the BubR1 protein are required for prevention of premature aging in mice.
Figure 5.
BubR1 deficient cells have normal DNA damage responses. (A) Passage 5 mouse embryonic fibroblasts (MEFs) of the indicated genotypes were treated with doxorubicin (Dox) for the indicated times as described.44 Cell lysates were generated and western blotting was performed as described.47 Blots were probed for BubR1, Parp-1 and γH2AX. Position of p89, the major degradation product of Parp-1 is indicated. Antibodies for BubR1,15 Parp-1,44 and γH2AX48 were as described. Actin was used as a loading control. These results suggest an intact response to doxorubicin-induced damage in MEFs expressing low amounts of BubR1. (B) Additional DNA damage-induced cell death analysis in BubR1 hypomorphic cells. Passage 5 MEFs were treated with actinomycin D (Act D), or a combination of Act D and anti-Fas or TNFα and compared to non-treated MEFs as described.49 These blots suggest that signals that activate apoptosis are interpreted similarly in wildtype and BubR1 hypomorphic MEFs. (C) Comet assay results from 5-month-old tissues of wildtype and BubR1 hypomorphic mice. Comet assays were performed according to manufacturer’s protocol (Trevigen). In this assay, both the percentage of nuclei demonstrating DNA damage as well as the amount of damage reflected by tail length, are equal irrespective of genotype, suggesting that BubR1 hypomorphic mice exhibit a normal DNA damage response to in vivo stresses. γ-irradiation was used as a positive control in the Comet assays.
Acknowledgments
We thank Meelad Dawlaty, Liviu Malureanu and Robin Ricke for helpful discussions and comments on the manuscript. This work was supported by grants from the NIH (CA96985), the Ted Nash Long Life Foundation and the Ellison Medical Foundation.
References
- 1.Collado M, Blasco MA, Serrano M. Cellular senescence in cancer and aging. Cell. 2007;130:223–33. doi: 10.1016/j.cell.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 2.Campisi J, d’Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8:729–40. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 3.Kamijo T, Zindy F, Roussel MF, Quelle DE, Downing JR, Ashmun RA, Grosveld G, Sherr CJ. Tumor suppression at the mouse INK4a locus mediated by the alternative reading frame product p19ARF. Cell. 1997;91:649–59. doi: 10.1016/s0092-8674(00)80452-3. [DOI] [PubMed] [Google Scholar]
- 4.Sherr CJ. The INK4a/ARF network in tumour suppression. Nat Rev Mol Cell Biol. 2001;2:731–7. doi: 10.1038/35096061. [DOI] [PubMed] [Google Scholar]
- 5.Campisi J. Senescent cells, tumor suppression and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–22. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 6.Sharpless NE, DePinho RA. The INK4A/ARF locus and its two gene products. Curr Opin Genet Dev. 1999;9:22–30. doi: 10.1016/s0959-437x(99)80004-5. [DOI] [PubMed] [Google Scholar]
- 7.Sherr CJ, Weber JD. The ARF/p53 pathway. Curr Opin Genet Dev. 2000;10:94–9. doi: 10.1016/s0959-437x(99)00038-6. [DOI] [PubMed] [Google Scholar]
- 8.Vousden KH, Lane DP. p53 in health and disease. Nat Rev Mol Cell Biol. 2007;8:275–83. doi: 10.1038/nrm2147. [DOI] [PubMed] [Google Scholar]
- 9.Fridman JS, Lowe SW. Control of apoptosis by p53. Oncogene. 2003;22:9030–40. doi: 10.1038/sj.onc.1207116. [DOI] [PubMed] [Google Scholar]
- 10.Harper JW, Adami GR, Wei N, Keyomarsi K, Elledge SJ. The p21 Cdk-interacting protein Cip1 is a potent inhibitor of G1 cyclin-dependent kinases. Cell. 1993;75:805–16. doi: 10.1016/0092-8674(93)90499-g. [DOI] [PubMed] [Google Scholar]
- 11.Xiong Y, Hannon GJ, Zhang H, Casso D, Kobayashi R, Beach D. p21 is a universal inhibitor of cyclin kinases. Nature. 1993;366:701–4. doi: 10.1038/366701a0. [DOI] [PubMed] [Google Scholar]
- 12.Chang BD, Watanabe K, Broude EV, Fang J, Poole JC, Kalinichenko TV, Roninson IB. Effects of p21Waf1/Cip1/Sdi1 on cellular gene expression: implications for carcinogenesis, senescence and age-related diseases. Proc Natl Acad Sci USA. 2000;97:4291–6. doi: 10.1073/pnas.97.8.4291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Beausejour CM, Krtolica A, Galimi F, Narita M, Lowe SW, Yaswen P, Campisi J. Reversal of human cellular senescence: roles of the p53 and p16 pathways. Embo J. 2003;22:4212–22. doi: 10.1093/emboj/cdg417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Krtolica A, Campisi J. Cancer and aging: a model for the cancer promoting effects of the aging stroma. Int J Biochem Cell Biol. 2002;34:1401–14. doi: 10.1016/s1357-2725(02)00053-5. [DOI] [PubMed] [Google Scholar]
- 15.Baker DJ, Jeganathan KB, Cameron JD, Thompson M, Juneja S, Kopecka A, Kumar R, Jenkins RB, de Groen PC, Roche P, van Deursen JM. BubR1 insufficiency causes early onset of aging-associated phenotypes and infertility in mice. Nat Genet. 2004;36:744–9. doi: 10.1038/ng1382. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto T, Baker DJ, d’Uscio LV, Mozammel G, Katusic ZS, van Deursen JM. Aging-associated vascular phenotype in mutant mice with low levels of BubR1. Stroke. 2007;38:1050–6. doi: 10.1161/01.STR.0000257967.86132.01. [DOI] [PubMed] [Google Scholar]
- 17.Hartman TK, Wengenack TM, Poduslo JF, van Deursen JM. Mutant mice with small amounts of BubR1 display accelerated age-related gliosis. Neurobiol Aging. 2007;28:921–7. doi: 10.1016/j.neurobiolaging.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 18.Baker DJ, Perez-Terzic C, Jin F, Pitel K, Niederlander NJ, Jeganathan K, Yamada S, Reyes S, Rowe L, Hiddinga HJ, Eberhardt NL, Terzic A, van Deursen JM. Opposing roles for p16 Ink4a and p19 Arf in senescence and ageing caused by BubR1 insufficiency. Nat Cell Biol. 2008;10:825–36. doi: 10.1038/ncb1744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu H. Regulation of APC-Cdc20 by the spindle checkpoint. Curr Opin Cell Biol. 2002;14:706–14. doi: 10.1016/s0955-0674(02)00382-4. [DOI] [PubMed] [Google Scholar]
- 20.Meraldi P, Draviam VM, Sorger PK. Timing and checkpoints in the regulation of mitotic progression. Dev Cell. 2004;7:45–60. doi: 10.1016/j.devcel.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Chan GK, Liu ST, Yen TJ. Kinetochore structure and function. Trends Cell Biol. 2005;15:589–98. doi: 10.1016/j.tcb.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 22.Yoon YM, Baek KH, Jeong SJ, Shin HJ, Ha GH, Jeon AH, Hwang SG, Chun JS, Lee CW. WD repeat-containing mitotic checkpoint proteins act as transcriptional repressors during interphase. FEBS Lett. 2004;575:23–9. doi: 10.1016/j.febslet.2004.07.089. [DOI] [PubMed] [Google Scholar]
- 23.Yang YL, Duan Q, Guo TB, Wang XX, Ruan Q, Xu GT, Zhang JW, Lu ZY, Xu M, Lu L, Dai W. BubR1 deficiency results in enhanced activation of MEK and ERKs upon microtubule stresses. Cell Prolif. 2007;40:397–410. doi: 10.1111/j.1365-2184.2007.00443.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 25.Chang F, Steelman LS, Lee JT, Shelton JG, Navolanic PM, Blalock WL, Franklin RA, McCubrey JA. Signal transduction mediated by the Ras/Raf/MEK/ERK pathway from cytokine receptors to transcription factors: potential targeting for therapeutic intervention. Leukemia. 2003;17:1263–93. doi: 10.1038/sj.leu.2402945. [DOI] [PubMed] [Google Scholar]
- 26.Serrano M, Lin AW, McCurrach ME, Beach D, Lowe SW. Oncogenic ras provokes premature cell senescence associated with accumulation of p53 and p16INK4a. Cell. 1997;88:593–602. doi: 10.1016/s0092-8674(00)81902-9. [DOI] [PubMed] [Google Scholar]
- 27.Hanks S, Coleman K, Reid S, Plaja A, Firth H, Fitzpatrick D, Kidd A, Mehes K, Nash R, Robin N, Shannon N, Tolmie J, Swansbury J, Irrthum A, Douglas J, Rahman N. Constitutional aneuploidy and cancer predisposition caused by biallelic mutations in BUB1B. Nat Genet. 2004;36:1159–61. doi: 10.1038/ng1449. [DOI] [PubMed] [Google Scholar]
- 28.Matsuura S, Matsumoto Y, Morishima K, Izumi H, Matsumoto H, Ito E, Tsutsui K, Kobayashi J, Tauchi H, Kajiwara Y, Hama S, Kurisu K, Tahara H, Oshimura M, Komatsu K, Ikeuchi T, Kajii T. Monoallelic BUB1B mutations and defective mitotic-spindle checkpoint in seven families with premature chromatid separation (PCS) syndrome. Am J Med Genet A. 2006;140:358–67. doi: 10.1002/ajmg.a.31069. [DOI] [PubMed] [Google Scholar]
- 29.Kajii T, Ikeuchi T, Yang ZQ, Nakamura Y, Tsuji Y, Yokomori K, Kawamura M, Fukuda S, Horita S, Asamoto A. Cancer-prone syndrome of mosaic variegated aneuploidy and total premature chromatid separation: report of five infants. Am J Med Genet. 2001;104:57–64. doi: 10.1002/ajmg.1580. [DOI] [PubMed] [Google Scholar]
- 30.Plaja A, Vendrell T, Smeets D, Sarret E, Gili T, Catala V, Mediano C, Scheres JM. Variegated aneuploidy related to premature centromere division (PCD) is expressed in vivo and is a cancer-prone disease. Am J Med Genet. 2001;98:216–23. doi: 10.1002/1096-8628(20010122)98:3<216::aid-ajmg1091>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 31.Krishnamurthy J, Torrice C, Ramsey MR, Kovalev GI, Al-Regaiey K, Su L, Sharpless NE. Ink4a/Arf expression is a biomarker of aging. J Clin Invest. 2004;114:1299–307. doi: 10.1172/JCI22475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Matheu A, Maraver A, Klatt P, Flores I, Garcia-Cao I, Borras C, Flores JM, Vina J, Blasco MA, Serrano M. Delayed ageing through damage protection by the Arf/p53 pathway. Nature. 2007;448:375–9. doi: 10.1038/nature05949. [DOI] [PubMed] [Google Scholar]
- 33.Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, Wu EA, Horner JW, DePinho RA. Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis. Nature. 2001;413:86–91. doi: 10.1038/35092592. [DOI] [PubMed] [Google Scholar]
- 34.Kirkwood TB. p53 and ageing: too much of a good thing? Bioessays. 2002;24:577–9. doi: 10.1002/bies.10111. [DOI] [PubMed] [Google Scholar]
- 35.Sharpless NE. Ink4a/Arf links senescence and aging. Exp Gerontol. 2004;39:1751–9. doi: 10.1016/j.exger.2004.06.025. [DOI] [PubMed] [Google Scholar]
- 36.Tyner SD, Venkatachalam S, Choi J, Jones S, Ghebranious N, Igelmann H, Lu X, Soron G, Cooper B, Brayton C, Hee Park S, Thompson T, Karsenty G, Bradley A, Donehower LA. p53 mutant mice that display early ageing-associated phenotypes. Nature. 2002;415:45–53. doi: 10.1038/415045a. [DOI] [PubMed] [Google Scholar]
- 37.Maier B, Gluba W, Bernier B, Turner T, Mohammad K, Guise T, Sutherland A, Thorner M, Scrable H. Modulation of mammalian life span by the short isoform of p53. Genes Dev. 2004;18:306–19. doi: 10.1101/gad.1162404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varela I, Cadinanos J, Pendas AM, Gutierrez-Fernandez A, Folgueras AR, Sanchez LM, Zhou Z, Rodriguez FJ, Stewart CL, Vega JA, Tryggvason K, Freije JM, Lopez-Otin C. Accelerated ageing in mice deficient in Zmpste24 protease is linked to p53 signalling activation. Nature. 2005;437:564–8. doi: 10.1038/nature04019. [DOI] [PubMed] [Google Scholar]
- 39.Nunez F, Chipchase MD, Clarke AR, Melton DW. Nucleotide excision repair gene (ERCC1) deficiency causes G(2) arrest in hepatocytes and a reduction in liver binucleation: the role of p53 and p21. Faseb J. 2000;14:1073–82. doi: 10.1096/fasebj.14.9.1073. [DOI] [PubMed] [Google Scholar]
- 40.Cao L, Li W, Kim S, Brodie SG, Deng CX. Senescence, aging and malignant transformation mediated by p53 in mice lacking the Brca1 full-length isoform. Genes Dev. 2003;17:201–13. doi: 10.1101/gad.1050003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jeganathan KB, Baker DJ, van Deursen JM. Securin associates with APCCdh1 in pro-metaphase but its destruction is delayed by Rae1 and Nup98 until the metaphase/anaphase transition. Cell Cycle. 2006;5:366–70. doi: 10.4161/cc.5.4.2483. [DOI] [PubMed] [Google Scholar]
- 42.Baker DJ, Jeganathan KB, Malureanu L, Perez-Terzic C, Terzic A, van Deursen JM. Early aging-associated phenotypes in Bub3/Rae1 haploinsufficient mice. J Cell Biol. 2006;172:529–40. doi: 10.1083/jcb.200507081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeganathan K, Malureanu L, Baker DJ, Abraham SC, van Deursen JM. Bub1 mediates cell death in response to chromosome missegregation and acts to suppress spontaneous tumori-genesis. J Cell Biol. 2007 doi: 10.1083/jcb.200706015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Fang Y, Liu T, Wang X, Yang YM, Deng H, Kunicki J, Traganos F, Darzynkiewicz Z, Lu L, Dai W. BubR1 is involved in regulation of DNA damage responses. Oncogene. 2006 doi: 10.1038/sj.onc.1209392. [DOI] [PubMed] [Google Scholar]
- 45.Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW. DNA repair, genome stability and aging. Cell. 2005;120:497–512. doi: 10.1016/j.cell.2005.01.028. [DOI] [PubMed] [Google Scholar]
- 46.Musaro M, Ciapponi L, Fasulo B, Gatti M, Cenci G. Unprotected Drosophila melanogaster telomeres activate the spindle assembly checkpoint. Nat Genet. 2008;40:362–6. doi: 10.1038/ng.2007.64. [DOI] [PubMed] [Google Scholar]
- 47.Kasper LH, Brindle PK, Schnabel CA, Pritchard CE, Cleary ML, van Deursen JM. CREB binding protein interacts with nucleoporin-specific FG repeats that activate transcription and mediate NUP98-HOXA9 oncogenicity. Mol Cell Biol. 1999;19:764–76. doi: 10.1128/mcb.19.1.764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fernandez-Capetillo O, Chen HT, Celeste A, Ward I, Romanienko PJ, Morales JC, Naka K, Xia Z, Camerini-Otero RD, Motoyama N, Carpenter PB, Bonner WM, Chen J, Nussenzweig A. DNA damage-induced G2-M checkpoint activation by histone H2AX and 53BP1. Nat Cell Biol. 2002;4:993–7. doi: 10.1038/ncb884. [DOI] [PubMed] [Google Scholar]
- 49.Jones BE, Lo CR, Liu H, Srinivasan A, Streetz K, Valentino KL, Czaja MJ. Hepatocytes sensitized to tumor necrosis factor-alpha cytotoxicity undergo apoptosis through caspase-dependent and caspase-independent pathways. J Biol Chem. 2000;275:705–12. doi: 10.1074/jbc.275.1.705. [DOI] [PubMed] [Google Scholar]