Abstract
In the retina, dopaminergic amacrine (interplexiform) cells establish multiple synapses on the perikarya of AII amacrines, the neurons that distribute rod signals to on- and off-cone bipolars. We used triple-label immunocytochemistry and confocal microscopy to identify the receptors contained within the postsynaptic active zone of these synapses in both mouse and rat retinas. We found that at the interface between the dendrites of the dopaminergic neurons and the AII amacrine cell perikarya clusters of postsynaptic γ-aminobutyric acid type A (GABAA) receptors are situated in register with aggregates of presynaptic organelles immunoreactive for GABA, the GABA vesicular transporter, and the vesicular monoamine transporter-2. D1 and D2/3 dopamine receptors, on the other hand, do not form clusters on the surface of the perikarya of AII amacrine cells. We suggest that the synapses between retinal dopaminergic neurons and AII amacrine cells are GABAergic and that both GABA and dopamine are released by the presynaptic endings. GABA acts on the ionotropic receptors clustered at the postsynaptic active zone, whereas dopamine diffuses to more distant, slower-acting metabotropic receptors.
Keywords: dopaminergic amacrines‖interplexiform cells‖AII amacrines‖ GABAA receptors
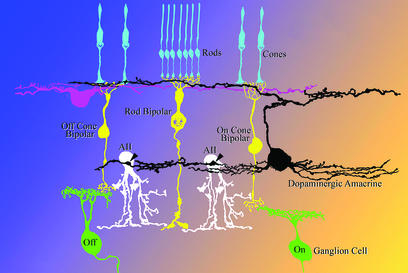
The dopaminergic neurons of the retina [dopaminergic amacrine (DA) cells], either amacrine or interplexiform cells, establish synapses on AII amacrines (1–4), a neuronal type inserted in series along the pathway that carries dim light signals to ganglion cells (Fig. 1). The neurotransmitter released at these synapses is not known but, in addition to dopamine, γ-aminobutyric acid (GABA) is a candidate, because both this molecule and its synthetic enzyme glutamic acid decarboxylase are present in the perikarya of DA cells (5–7). In addition to this conventional synaptic output onto AII amacrines, DA cells act on more distant targets, because the released dopamine, diffusing throughout the intercellular spaces of the retina, binds to a family of metabotropic receptors distributed on the surface of most retinal neurons and thus participates in setting the gain of the retina for vision in bright light (8).
Figure 1.
Connections of dopaminergic and AII amacrine cells. AII amacrine cells transfer rod signals from rod bipolars to the axonal endings of on- and off-cone bipolars. The synapses of DA cells onto the perikaryon of AII amacrines (arrowheads) are situated near the origin of the primary dendrite(s). Because there is no comprehensive morphological analysis of the rodent retina, neurons in the diagram are drawn in the style of Polyak (42).
Colocalization of dopamine with other transmitters seems to be the rule in the central nervous system: GABA is contained in periglomerular cells of the olfactory bulb (5) and in a subpopulation of neurons of the substantia nigra (9), whereas glutamate may be present in the remaining nigral neurons and in those of the ventral tegmental area (VTA) (10, 11). VTA neurons make excitatory glutamatergic autapses when maintained as microcultures (11); however, in the intact tissue it is not known which transmitter is released at the synapse and the identity of the postsynaptic receptors.
The contacts between DA cells and AII amacrines in the rodent retina represent an ideal site to identify the postsynaptic receptors by immunocytochemistry and triple-label confocal microscopy. First, the synaptic partners can be stained with antibodies to different cell-filling antigens: DA cells contain tyrosine hydroxylase (TH), the rate-limiting enzyme in dopamine biosynthesis (12–14), whereas AII amacrines contain Dab1, the product of the disabled-1 gene, in the mouse (15) and the calcium-binding protein parvalbumin (PV) in the rat (16). Second, AII amacrine cells are the most common amacrine cell type in the mammalian retina (17). Third, in stratum S1 of the inner plexiform layer (IPL) the dendro-somatic DA-to-AII amacrine cell synapses are numerous (3, 18) and easily identified, because they occur at the site where the processes of DA cells form a ring around the origin of the primary dendrite(s) of AII amacrines (2, 19, 20).
In this article, we report that the relative distribution of pre- and postsynaptic markers strongly suggests that the DA-to-AII amacrine cell synapses are GABAergic.
Materials and Methods
Electron Microscopy.
A transgenic mouse line was used in which DA cells in the retina expressed human placental alkaline phosphatase (PLAP) on the outer surface of the cell membrane. These animals were obtained by introducing into the mouse genome PLAP cDNA linked to a promoter sequence of the gene for TH (21). Details of the technique of specimen preparation and staining for alkaline phosphatase activity were described (21). Briefly, adult mice, homozygous for PLAP cDNA, were anesthetized by i.p. injection of 0.1 ml of a solution containing 5% ketamine HCl (Ketaset; Fort Dodge Laboratories, Fort Dodge, IA) and 1% xylazine (Rompun; Bayer, Shawnee Mission, KS). They were perfused through the heart with 2% formaldehyde and 1% glutaraldehyde in Sörensen phosphate buffer (pH 7.4), after rinsing the vascular tree with carboxygenated Ames medium (Sigma) containing 40 mM glucose. Whole retinas were kept in the fixative fluid for 2 h at room temperature, heated in PBS at 65°C for 30 min, and carefully rinsed with 5% sucrose in 0.2 M cacodylate buffer (pH 7.4) to eliminate phosphate ions. Specimens were then incubated for 8–24 h at room temperature under constant, mild agitation in a β-glycerophosphate, alkaline lead citrate solution (21). They were subsequently postfixed in 3% glutaraldehyde, followed by osmium-ferrocyanide and staining en bloc with uranyl acetate. After embedding and thin sectioning, micrographs were obtained with a JEOL 1200EX electron microscope.
Immunocytochemistry.
Adult C57BL/6J mice and Long–Evans rats were given a lethal dose of sodium pentobarbital and their eyes were enucleated. Posterior eyecups were immersed in Ames medium, and retinas were separated from the remaining ocular tunics. The specimens were immersed in 2% formaldehyde in 0.15 M Sörenson phosphate buffer (pH 7.4) in 30-mm petri dishes and fixed for 15–17 s in a microwave oven (Pelco; Ted Pella, Inc., Redding, CA). Upon irradiation, the temperature of the fixative increased by 30–40°C. Retinas were subsequently rinsed in PBS (pH 7.4), cryoprotected in 20% sucrose, and frozen in the liquid phase of partially solidified monochlorodifluoromethane. Radial and horizontal sections 5–10 μm in thickness were obtained in a cryostat and stained with the indirect fluorescence antibody technique.
Antibodies and Dilutions.
Primary.
Rabbit polyclonal to Dab1, a gift from B. Howell (Neurogenetics, National Institute of Neurological Disorders and Stroke/National Institutes of Health, Bethesda), 1:500; rabbit polyclonal to PV (no. PC255L, Oncogene Research Products, Boston), 1:4,000; mouse monoclonal to PV clone PARV-19 (no. P3088, Sigma), 1:1,000; sheep polyclonal to TH (no. NB 300-110, Novus Biologicals, Littleton, CO), 1:500; rabbit polyclonal to TH (no. AB152, Chemicon), 1:500; mouse monoclonal to TH (no. 22941, DiaSorin, Stillwater, MN), 1:100; guinea pig polyclonal to the α3 subunit of the GΑΒΑ type A (GABAA) receptor, a gift from J.-M. Fritschy (University of Zurich, Zurich), 1:1,000; rabbit polyclonal to the α1 subunit of the GABAA receptor, a gift from W. Sieghart (University of Vienna, Vienna), 1.8 μg/ml; rabbit polyclonal to GABA (no. AB141, Chemicon), 1:300; rabbit polyclonal to the vesicular GABA transporter, a gift from R. H. Edwards (University of California, San Francisco), 1:2,000; rabbit polyclonal to the vesicular monoamine transporter-2, a gift from R. H. Edwards, 1:1,000; rat monoclonal antibody to dopamine receptor D1, clone 1-1-F11 s.E6 (no. D-187, Sigma), 1:400; and rabbit polyclonal to dopamine receptors D2/3 (no. 3949-1007, Biogenesis, Kingston, NH), 1:100. Primary antibodies were diluted in 2% BSA (Sigma) in PBS.
Secondary.
FITC donkey anti-guinea pig (no. 706-095-148, Jackson ImmunoResearch), 1:200; Oregon green 488 donkey anti-rat (no. A-21208, Molecular Probes), 1:150; Oregon green 488 goat anti-rabbit (no. 0-638, Molecular Probes), 1:200; Alexafluor 568 goat anti-mouse (no. A-11031, Molecular Probes), 1:200; Alexafluor 568 goat anti-rabbit (no. A-1103, Molecular Probes), 1:200; Alexafluor 660 donkey anti-sheep (no. A-21101, Molecular Probes), 1:200; Alexafluor 660 goat anti-mouse (no. A-21054, Molecular Probes), 1:200; and Alexafluor 660 goat anti-rabbit (no. A-21054, Molecular Probes), 1:200. Secondary antibodies were diluted with 2% BSA, 1% normal goat serum (Vector Laboratories), and 0.2% fish gelatin (Goldmark Biologicals, Phillipsburg, NJ) in PBS.
Staining.
Sections were preincubated in 2% BSA, 10% normal goat serum, and 2% fish gelatin in PBS; incubated overnight in the mixture of primary antibodies; rinsed in PBS; and stained with the mixture of secondary antibodies for 3 h. After rinsing, the sections were mounted in Vectashield (Vector Laboratories).
Confocal Microscopy.
Fluorescence was detected with a Bio-Rad MRC-1024 confocal imaging system equipped with an argon-krypton laser and a Nikon microscope. Sections were viewed with ×40 plan-apochromat objective, 1.4 numerical aperture. Images (1,024 × 1,024 pixels) were obtained sequentially from two or three channels and stored as tiff files. Brightness and contrast of the final images were adjusted by using photoshop software (Adobe Systems, Mountain View, CA).
Results
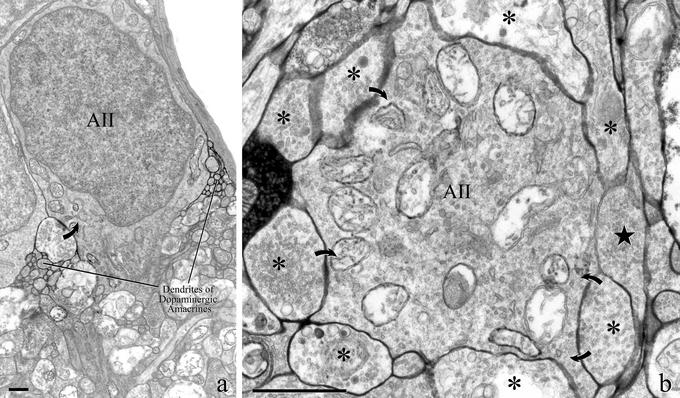
With the electron microscope, in the retina of transgenic mice expressing PLAP under control of the TH promoter, the histochemical technique for phosphatase activity labeled the surface of DA cells with a dense precipitate of lead phosphate. In radial sections, small groups of DA cell dendrites contacted the deep surface of amacrine cell bodies on either side of the origin of their primary dendrite (Fig. 2a). Frequently, these dendrites were presynaptic to the amacrine cell perikaryon. In horizontal sections, labeled endings completely surrounded the postsynaptic amacrine cell and established with it multiple synapses (Fig. 2b). Clearly, these images represented the ultrastructural counterpart of the rings formed by DA cell dendrites around the collar of AII amacrine cells. This identification was confirmed by the complement of organelles contained within the postsynaptic cell: large mitochondria, Golgi complexes, and a profusion of vesicles and tubules are in fact typical features of the cytoplasm of AII amacrines (3, 18). As expected, the cell body of AII amacrines did not contain presynaptic active zones.
Figure 2.
Fine structure of DA-to-AII amacrine cell synapses. (a) DA cells were labeled in transgenic mice with PLAP, an enzyme that resides on the outer surface of the cell membrane. With the electron microscope, after staining with the histochemical reaction for phosphatase activity, a dense precipitate of lead phosphate occupies the intercellular spaces surrounding the processes of DA cells. In a vertical section of the retina, labeled DA cell dendrites form a plexus at the scleral margin of the inner plexiform layer. Bundles of labeled dendrites occur near the vitreal surface of the perikaryon of an AII amacrine cell. One of such processes is presynaptic (curved arrow). (b) In a horizontal section of the retina, numerous dendrites of DA cells (asterisks) surround the vitreal surface of an AII amacrine cell, forming a ring around the origin of its primary dendrite. Three of the labeled endings are presynaptic (curved arrows). The abundance of large mitochondria and the profusion of vesicle and tubules are typical of the cytoplasm of AII amacrine cells. A process belonging to a neuron that does not express PLAP is indicated by a star. (Bar = 1 μm.)
With the confocal microscope, DA cells or type 1 catecholaminergic amacrines were intensely stained by antibodies to TH. Their large perikarya resided in the vitreal tier of the cell bodies of the inner nuclear layer (Fig. 3a, rat) and gave rise to a dense dendritic arbor, rigorously stratified in the outermost stratum (S1) of the IPL. Numerous varicosities, 0.5–2 μm in diameter, were distributed at irregular intervals along the length of the dendrites; they represented the light microscope counterpart of the endings observed with the electron microscope. In C57BL/6J mice, type 2 catecholaminergic amacrines did not react with antibodies to TH. In the rat, they were stained, but less intensely than DA cells, and sent their dendrites to the middle of the IPL. This cell type will not be considered further in this study. AII amacrine cells in the rat were stained in their entirety by antibodies to PV (Fig. 3a), whereas in the mouse the staining with antibodies to Dab1 was less complete. They exhibited the distinctive morphological features that are conserved throughout the mammalian retinas: a small cell body situated at the very border between inner nuclear layer and IPL and therefore nested in the dendritic plexus of DA cells; one or, less commonly, two primary dendrites originating from the perikaryon and descending vertically into the IPL; and finally two parallel dendritic arbors, one in each of the sublaminae of the IPL. In contrast with the antibody to Dab1, which exclusively stained AII amacrines in the mouse, antibodies to PV also labeled with varying intensity a class of cone bipolars, other amacrine cells, and some ganglion cells. AII amacrines, however, were easily identified on account of the intensity of their staining, their unmistakable shape, and the position and size of their cell bodies.
Figure 3.
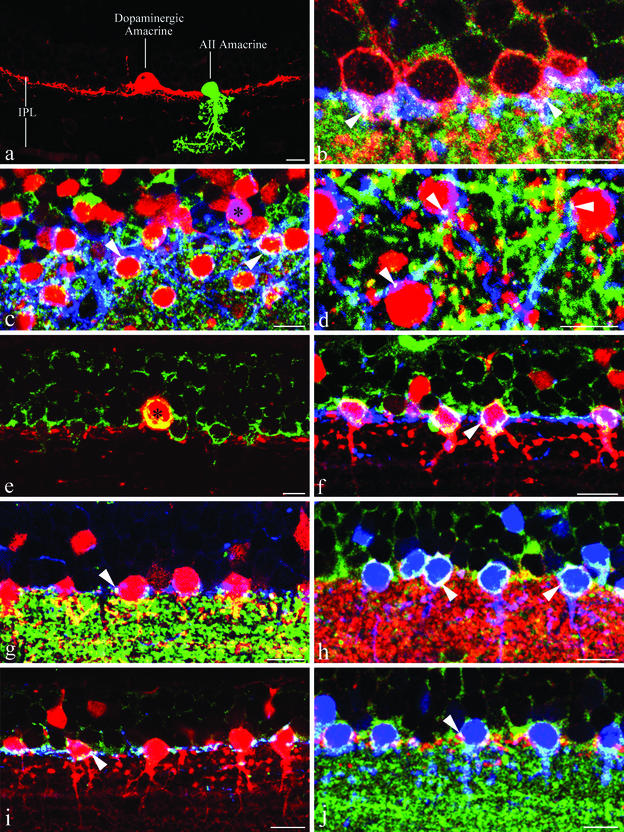
Distribution of pre- and postsynaptic markers at the DA-to-AII amacrine cell synapses. A 10-μm bar is included in all images of this figure. (a) Morphology of a DA cell and an AII amacrine in the rat retina. The two neurons were selected from PHOTOSHOP images of specimens stained for TH and PV and pasted into a single image. (b) Dab1, red; TH, blue; α3 subunits of the GABAA receptor, green. In a radial section of the mouse retina, the perikarya of AII amacrine cells (red) are nested in the plexus formed by the dendrites of DA cells (blue) in the most superficial stratum (S1) of the IPL. The white spots at the periphery of the cell bodies of AII amacrines (arrowheads) represent clusters of α3 subunits. The white color indicates coincidence of the three labels; therefore, the clusters occur in the region of apposition between DA cell dendrites and cell body of AII amacrine cells. (c) PV, red; TH, blue; α3 subunits of the GABAA receptor, green. In an oblique section through the rat retina, the dendrites of DA cells (blue) form a dense plexus whose meshes are predominantly occupied by the perikarya of AII amacrine cells (red). Clusters of α3 subunits (white, arrowheads) occur in the region of overlap between DA cell dendrites and AII amacrine cell bodies. One cell body is stained purple (black asterisk), because it contains both TH and PV. It probably belongs to a type 2 catecholaminergic amacrine. (d) PV, red; TH, blue; α1 subunits of the GABAA receptor, green. In a horizontal section through the rat retina, clusters of α1 subunits (white, arrowheads) occur in the region of overlap between DA cell dendrites (blue) and AII amacrine cell bodies (red). Thus, the presence of clusters of GABAA receptors at the interface between the two types of neurons is confirmed by the staining with the antibody to another subunit of the receptor. (e) TH, red; GABA, green. The cytoplasm of a DA cell (red) is intensely stained by antibody to GABA (yellow). The contrast of this micrograph was enhanced for clarity. (f) PV, red; TH, blue; GABA, green. Foci of immunoreactivity for GABA (white, arrowhead) occur at the site of apposition between DA cell dendrites (blue) and AII amacrine cell bodies (red). (g) PV, red; TH, blue; VGAT, green. In this high-contrast image, aggregates of VGAT-containing organelles (white, arrowhead) are situated on the surface of AII amacrine cell bodies (red), at the site where they are contacted by the dendrites of DA cells (blue). (h) PV, blue; VGAT, red; α3 subunits of the GABAA receptor, green. Aggregates of VGAT-positive organelles are situated in register with clusters of α3 subunits of the GABAA receptor (white, arrowheads) at the surface of AII amacrine cells (blue). (i) PV, red; TH, blue; VMAT2, green. Aggregates of VMAT2-positive organelles (white, arrowhead) are situated within DA cell dendrites (blue) at the site where they contact the cell bodies of AII amacrines (red). (j) PV, blue; VMAT2, red; α3 subunits of the GABAA receptor, green. At the surface of the cell bodies of AII amacrines (blue), the aggregates of VMAT2-containing organelles are situated in register with clusters of α3 subunits of the GABAA receptor (white, arrowhead).
Radial sections of the mouse retina, fixed for 15–17 s with 2% formaldehyde under microwave irradiation, were triple-labeled with a sheep polyclonal antibody to TH, a rabbit polyclonal to Dab-1, and a guinea pig polyclonal to the α3 subunit of the GABAA receptor (22) (Fig. 3b). In these sections, confocal microscopy showed that clusters of α3 subunits were present at the site of overlap between the dendrites of DA cells and the surface of the cell bodies of AII amacrines in the stratum S1 of the IPL. The clusters appeared as round or elliptical plaques, 0.2–0.5 μm in diameter when seen en face, and as short, thin lines when seen in profile at the edge of the cell. Most commonly, they were situated either at the equator or on the vitreal surface of the AII amacrine cell bodies. In places, however, they occurred on the scleral surface of the perikarya, a finding not unexpected because DA-to-AII amacrine cell synapses are seen occasionally on the scleral pole of the postsynaptic neuron. Because of their topography, size, and the fact that the cell body of AII amacrines does not contain presynaptic active zones, the clusters of subunits were interpreted as postsynaptic specializations of the DA-to-AII amacrine cell synapses. Their frequency, however, was lower than that reported in previous studies with the electron microscope (2, 20). We attribute this discrepancy to the denaturation of the epitopes that bind the antibody to the α3 subunit, because the sensitivity of the GABAA receptor subunits to aldehyde fixation is well known (23). In fact, no clusters were present after a conventional fixation with 2% formaldehyde for 2 h.
Results with the α3 subunit were confirmed in the rat by staining with a guinea pig polyclonal to the α3 subunit of the GABAA receptor, in combination with a sheep polyclonal to TH and a rabbit polyclonal to PV (Fig. 3c). Clusters of α3 subunits were present in the region of overlap between the dendrites of DA cells and the cell bodies of AII amacrines. Similar results were obtained with a rabbit polyclonal to the α1 subunit (24) (Fig. 3d). Because PV is a better cell-filling marker for AII amacrines than Dab-1, the rat retina was used for the remaining experiments of the present study.
We sought further evidence that the clusters of GABAA receptors were the postsynaptic specialization of the DA-to-AII amacrine cell synapses by examining the distribution of the immunoreactivity for GABA at the interface between DA cell dendrites and perikarya of AII amacrine cells. Staining with a rabbit polyclonal to glutaraldehyde-linked GABA in combination with a mouse monoclonal to TH confirmed the colocalization of GABA and TH in DA cells (5–7). In the perikaryon, the transmitter was distributed throughout the perinuclear cytoplasm (Fig. 3e). In the dendrites, after triple-labeling with sheep polyclonal to TH, mouse monoclonal to PV, and rabbit polyclonal to GABA, foci of stained material, 0.5–1 μm in size, probably GABA-containing organelles, were consistently observed at the site of apposition between DA cell dendrites and cell body of AII amacrines, near the origin of their primary dendrite(s) (Fig. 3f). AII amacrines are glycinergic (25, 26) and do not contain GABA; thus, the clusters had to be contained within the cytoplasm of the dendrites of DA cells.
Vesicular GABA transporter (VGAT) is a transporter that delivers both GABA and glycine to synaptic vesicles (27) and is contained in AII amacrines (28). Assuming that VGAT was especially concentrated in clusters of synaptic vesicles, we analyzed its distribution at the DA cell/AII amacrine interface in high contrast images of 5-μm sections after triple-labeling with sheep polyclonal to TH, mouse monoclonal to PV, and rabbit polyclonal to VGAT (Fig. 3g). In these conditions of image sampling, the lobular appendages of AII amacrines were intensely immunoreactive but the staining of the perinuclear cytoplasm was either absent or weak. A notable exception, however, was represented by a necklace of bright spots, 0.5–1 μm in size, at the interface between DA cell dendrites and surface of the AII amacrine cell body either at the equator or near the origin of the primary dendrite(s). To establish whether these spots or clusters of membranous organelles coincided with the DA-to-AII amacrine cell synapses, we examined their topographic relations with the clusters of GABAA receptors. After triple-labeling with mouse monoclonal to PV, rabbit polyclonal to VGAT, and guinea pig polyclonal to the α3 subunit, aggregates of VGAT-containing organelles were found in register with clusters of receptor subunits on the surface of the perikarya of AII amacrines (Fig. 3h).
To investigate the distribution of dopamine-containing organelles in the dendrites of DA cells, we resorted to triple-labeling with sheep polyclonal to TH, mouse monoclonal to PV, and rabbit polyclonal to the vesicular monoamine transporter-2 (VMAT2) (29). Staining with the antibody to VMAT2 was confined to the cytoplasm of DA cells (30) and was absent in AII amacrines. Prominent aggregates of VMAT2-immunoreactive organelles, 0.5–1 μm in size, were observed in juxtaposition with the cell bodies of AII amacrines: in a vertical section of the retina, three to four aggregates per cell were often seen (Fig. 3i). Again, we studied their topographic relationships with the postsynaptic clusters of GABAA receptor subunits. After triple-labeling with mouse monoclonal to PV, rabbit polyclonal to VMAT2, and guinea pig polyclonal to the α3 subunit, the aggregates of VMAT2-containing organelles were positioned in register with the clusters of receptor subunits (Fig. 3j). Thus, aggregates of organelles containing VGAT and VMAT2 are both situated in register with clusters of postsynaptic GABAA receptors on the surface of the AII amacrine cell bodies. Both must therefore coincide with the DA-to-AII amacrine cell synapses. Because the perinuclear cytoplasm of the AII amacrine cells is not stained by antibodies to VGAT in our conditions of image sampling and does not contain presynaptic clusters of vesicles, it seems likely that both VGAT and VMAT2 are localized within the DA cell presynaptic endings.
We finally examined the distribution of dopamine receptors in AII amacrines. No discrete clusters were observed on the vitreal surface of the cell bodies (data not shown), suggesting that dopamine receptors do not cluster at the DA-to-AII amacrine cell synapses.
Discussion
Convergence of multiple lines of reasoning supports the notion that the synapses established by DA cells onto the cell body of AII amacrines are GABAergic. First and foremost, this conclusion is grounded on solid anatomical facts: the synapses are located in the rings formed by the dendrites of DA cells around the bodies of AII amacrines, both very distinctive regions of the pre- and postsynaptic neurons. They represent the only synapses received by the perikarya of AII amacrines, and these perikarya, in turn, do not contain presynaptic active zones. All these facts are well established in the literature (3, 18) and have been confirmed in the present article by examining with the electron microscope the retina of transgenic mice in which the membrane of DA cells was labeled with PLAP. At the site of the synapses, confocal microscopy demonstrates the presence of clusters of GABAA receptor subunits, which have the size of the postsynaptic active zones. Finally, dopamine receptors do not form clusters on the perikarya of AII amacrines, in agreement with previous studies that reported the absence of either D1 (31, 32) or D2/3 (33) receptors on the soma of AII amacrine cells.
The second line of evidence concerns the distribution of three markers: GABA, VGAT, and VMAT2. GABA and VMAT2 are expressed in DA cells and are absent in AII amacrines, which use glycine as a transmitter (25, 26). VGAT is present in AII amacrines, particularly in their lobular appendages. In addition, however, VGAT is highly concentrated in spots that coincide with the rings formed by the DA cell dendrites around the bodies of AII amacrine cells. These foci of VGAT immunoreactivity are situated in register with the clusters of GABAA receptors, suggesting that they correspond to the DA-to-AII amacrine cell synapses. Because the antibodies to VGAT and VMAT2 were obtained from the same animal species, it was impossible to combine them in a single immunocytochemical reaction. By using the cluster of postsynaptic GABAA receptor subunits as a marker, however, we observed that both molecules were colocalized and could reasonably reside within the presynaptic active zone of the DA-to-AII amacrine cell contacts. This finding suggests that both dopamine and GABA are released at this synapse.
A third line of reasoning is more circumstantial. DA cells do contain GABAA receptors, uniformly distributed throughout their surface (30) as well as concentrated at the site of the synapses that DA cells receive from other GABAergic amacrines (23). Although examples exist in the literature of presynaptic inhibition mediated by presynaptic GABAA receptors (34, 35), it seems unlikely that such receptors are concentrated at the release sites of the DA cell synapses. Finally, GABAA receptors are present in AII amacrine cells, because a Cl− conductance is activated in these neurons by muscimol, a GABA receptor agonist (36).
In conclusion, this article provides evidence in favor of the hypothesis that in the dopaminergic neurons of the retina, the transmitter responsible for synaptic transmission is not a catecholamine. There is evidence that dopamine is released by exocytosis over the entire surface of the cell (30) and binds to distant metabotropic receptors that activate slow-acting second messenger cascades (8). In addition, DA cells release GABA, and this transmitter diffuses across the narrow intercellular cleft of the synapses to bind fast-acting ionotropic receptors that are confined to the postsynaptic active zone. It remains to be established whether dopamine and GABA reside within the same or different membrane-bounded organelles.
A massive GABAergic input at a strategic site such as the origin of the dendritic tree is bound to play a prominent role in the response properties of AII amacrine cells, the neuron that carries rod signals to cone bipolars. Surprisingly, however, the function of these synapses is unclear. Perhaps the appropriate physiological experiments have not been done, because of the expectation that the synaptic input from DA cells was mediated by dopamine, which is known to modulate the permeability to tracers of the AII-to-AII gap junctions (37, 38).
The physiological behavior of AII amacrines is complex and strikingly different from that of the rod bipolars presynaptic to them. AII amacrines respond over a 6–7 log unit range of light intensities (39), whereas rod bipolars have an operating range of about 3 log units, similar to that of rods (40). As a result, photopic stimuli that are saturating for rod bipolars cause vigorous responses in AII amacrines. Furthermore, it is controversial whether AII amacrines possess an off-surround in photopic illumination (39, 41). Because dopamine efflux from the retina is maximal with photopic stimuli, especially flickering light (8), it is likely that GABA is released in the same conditions of illumination. Thus, the GABAergic input of DA cells onto AII amacrines may subserve important functions in bright light.
Acknowledgments
This work was made possible by the generosity of the individuals who contributed critical antibodies: Drs. R. H. Edwards, J.-M. Fritschy, B. Howell, and W. Sieghart. This work was supported by National Institutes of Health Grant EY-01344.
Abbreviations
- DA
dopaminergic amacrine (interplexiform)
- GABA
γ-aminobutyric acid
- GABAA
GABA type A
- TH
tyrosine hydroxylase
- PV
parvalbumin
- IPL
inner plexiform layer
- PLAP
human placental alkaline phosphatase
- VGAT
vesicular GABA transporter
- VMAT2
vesicular monoamine transporter-2
References
- 1.Pourcho R G. Brain Res. 1982;252:101–109. doi: 10.1016/0006-8993(82)90982-9. [DOI] [PubMed] [Google Scholar]
- 2.Voigt T, Wässle H. J Neurosci. 1987;7:4115–4128. doi: 10.1523/JNEUROSCI.07-12-04115.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kolb H, Cuenca N, Wang H-H, Dekorver L. J Neurocytol. 1990;19:343–366. doi: 10.1007/BF01188404. [DOI] [PubMed] [Google Scholar]
- 4.Kolb H, Cuenca N, Dekorver L. J Comp Neurol. 1991;310:267–284. doi: 10.1002/cne.903100210. [DOI] [PubMed] [Google Scholar]
- 5.Kosaka T, Kosaka K, Hataguchi Y, Nagatsu I, Wu J-Y, Ottersen O P, Storm-Mathisen J, Hama K. Exp Brain Res. 1987;66:191–210. doi: 10.1007/BF00236215. [DOI] [PubMed] [Google Scholar]
- 6.Wässle H, Chun M H. J Neurosci. 1988;8:3383–3394. doi: 10.1523/JNEUROSCI.08-09-03383.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wulle I, Wagner H-J. J Comp Neurol. 1990;296:173–178. doi: 10.1002/cne.902960111. [DOI] [PubMed] [Google Scholar]
- 8.Witkovsky P, Dearry A. Prog Ret Res. 1991;11:247–292. [Google Scholar]
- 9.Campbell K J, Takada M, Hattori T. Brain Res. 1991;558:239–244. doi: 10.1016/0006-8993(91)90774-p. [DOI] [PubMed] [Google Scholar]
- 10.Kaneko T, Akiyama H, Nagatsu I, Mizuno N. Brain Res. 1990;507:151–154. doi: 10.1016/0006-8993(90)90535-j. [DOI] [PubMed] [Google Scholar]
- 11.Sulzer D, Joyce M P, Lin L, Geldwert D, Haber S N, Hattori T, Rayport S. J Neurosci. 1998;18:4588–4602. doi: 10.1523/JNEUROSCI.18-12-04588.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brecha N C, Oyster C W, Takahashi E S. Invest Ophthalmol Visual Sci. 1984;25:66–70. [PubMed] [Google Scholar]
- 13.Nguyen-Legros J, Botteri C, Phuc L H, Vigny A, Gay M. Brain Res. 1984;295:145–153. doi: 10.1016/0006-8993(84)90825-4. [DOI] [PubMed] [Google Scholar]
- 14.Oyster C W, Takahashi E S, Cilluffo M, Brecha N C. Proc Natl Acad Sci USA. 1985;82:6335–6339. doi: 10.1073/pnas.82.18.6335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice D S, Curran T. J Comp Neurol. 2000;424:327–338. doi: 10.1002/1096-9861(20000821)424:2<327::aid-cne10>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 16.Wässle H, Grünert U, Röhrenbeck J. J Comp Neurol. 1993;332:407–420. doi: 10.1002/cne.903320403. [DOI] [PubMed] [Google Scholar]
- 17.Strettoi E, Masland R H. Proc Natl Acad Sci USA. 1996;93:14906–14911. doi: 10.1073/pnas.93.25.14906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Strettoi E, Raviola E, Dacheux R F. J Comp Neurol. 1992;325:152–168. doi: 10.1002/cne.903250203. [DOI] [PubMed] [Google Scholar]
- 19.Törk I, Stone J. Brain Res. 1979;169:261–273. doi: 10.1016/0006-8993(79)91029-1. [DOI] [PubMed] [Google Scholar]
- 20.Sterling P. Annu Rev Neurosci. 1983;6:149–185. doi: 10.1146/annurev.ne.06.030183.001053. [DOI] [PubMed] [Google Scholar]
- 21.Gustincich S, Feigenspan A, Wu D-K, Koopman L J, Raviola E. Neuron. 1997;18:723–736. doi: 10.1016/s0896-6273(00)80313-x. [DOI] [PubMed] [Google Scholar]
- 22.Fritschy J-M, Mohler H. J Comp Neurol. 1995;359:154–194. doi: 10.1002/cne.903590111. [DOI] [PubMed] [Google Scholar]
- 23.Gustincich S, Feigenspan A, Sieghart W, Raviola E. J Neurosci. 1999;19:7812–7822. doi: 10.1523/JNEUROSCI.19-18-07812.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperk G, Schwarzer C, Tsunashima K, Fuchs K, Sieghart W. Neuroscience. 1997;80:987–1000. doi: 10.1016/s0306-4522(97)00146-2. [DOI] [PubMed] [Google Scholar]
- 25.Pourcho R G, Goebel D J. J Comp Neurol. 1985;33:473–480. doi: 10.1002/cne.902330406. [DOI] [PubMed] [Google Scholar]
- 26.Pourcho R G, Goebel D J. J Neurosci. 1987;7:1189–1197. doi: 10.1523/JNEUROSCI.07-04-01189.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhry F A, Reimer R J, Bellocchio E E, Danbolt N C, Osen K K, Edwards R H, Storm-Mathisen J. J Neurosci. 1998;18:9733–9750. doi: 10.1523/JNEUROSCI.18-23-09733.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cueva J G, Haverkamp S, Reimer R J, Edwards R, Wässle H, Brecha N C. J Comp Neurol. 2002;445:227–237. doi: 10.1002/cne.10166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nirenberg M J, Chan J, Liu Y, Edwards R H, Pickel V M. J Neurosci. 1996;16:4135–4145. doi: 10.1523/JNEUROSCI.16-13-04135.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Puopolo M, Hochstetler S E, Gustincich S, Wightman R M, Raviola E. Neuron. 2001;30:211–225. doi: 10.1016/s0896-6273(01)00274-4. [DOI] [PubMed] [Google Scholar]
- 31.Veruki M L, Wässle H. Eur J Neurosci. 1996;8:2286–2297. doi: 10.1111/j.1460-9568.1996.tb01192.x. [DOI] [PubMed] [Google Scholar]
- 32.Nguyen-Legros J, Simon A, Caille I, Bloch B. Visual Neurosci. 1997;14:545–551. doi: 10.1017/s0952523800012207. [DOI] [PubMed] [Google Scholar]
- 33.Derouiche A, Asan E. Eur J Neurosci. 1999;11:1391–1402. doi: 10.1046/j.1460-9568.1999.00557.x. [DOI] [PubMed] [Google Scholar]
- 34.Parnas I, Rashkovan G, Ravin R, Fischer Y. J Neurophysiol. 2000;84:1240–1246. doi: 10.1152/jn.2000.84.3.1240. [DOI] [PubMed] [Google Scholar]
- 35.Jang I-S, Jeong H-J, Katsurabayashi S, Akaike N. J Physiol (London) 2002;541:423–434. doi: 10.1113/jphysiol.2001.016535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Boos R, Schneider H, Wässle H. J Neurosci. 1993;13:2874–2888. doi: 10.1523/JNEUROSCI.13-07-02874.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hampson E C, Vaney I, Weiler R. J Neurosci. 1992;12:4911–4922. doi: 10.1523/JNEUROSCI.12-12-04911.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mills S L, Massey S C. Nature. 1995;377:734–737. doi: 10.1038/377734a0. [DOI] [PubMed] [Google Scholar]
- 39.Xin D, Bloomfield S A. Visual Neurosci. 1999;16:653–665. doi: 10.1017/s0952523899164058. [DOI] [PubMed] [Google Scholar]
- 40.Dacheux R F, Raviola E. J Neurosci. 1986;6:331–345. doi: 10.1523/JNEUROSCI.06-02-00331.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dacey D M. Prog Ret Eye Res. 1999;18:737–763. doi: 10.1016/s1350-9462(98)00013-5. [DOI] [PubMed] [Google Scholar]
- 42.Polyak S L. The Retina. Chicago: Univ. of Chicago Press; 1941. [Google Scholar]