Abstract
With data from recently available selective antagonists for the 5-HT7 receptor, it has been hypothesized that 5-hydroxytryptamine (5-HT)-induced hypothermia is mediated by the 5-HT7 receptor, an effect previously attributed to other receptor subtypes. It has been established that the biologically active lipid oleamide allosterically interacts with the 5-HT7 receptor to regulate its transmission. The most well characterized effects of oleamide administration are induction of sleep and hypothermia. Here, we demonstrate, by using mice lacking the 5-HT7 receptor, that 5-HT-induced hypothermia is mediated by the 5-HT7 receptor. Both 5-HT and 5-carboxamidotryptamine, a 5-HT1 and 5-HT7 receptor agonist, in physiological doses fail to induce hypothermia in 5-HT7 knockout mice. In contrast, oleamide was equally effective in inducing hypothermia in mice lacking the 5-HT7 receptors as in wild-type mice. When administered together, 5-HT and oleamide showed additive or greater than additive effects in reducing body temperature. Taken together, the results show that 5-HT-induced hypothermia is mediated by the 5-HT7 receptor, and that oleamide may act through an independent mechanism as well as at an allosteric 5-HT7 receptor site to regulate body temperature.
The 5-HT7 receptor is one of the most recently described members of the large family of serotonin [5-hydroxytryptamine (5-HT)] receptors (1, 2). cDNA clones of the mRNAs encoding this receptor have been isolated from several different species, including mouse, rat, guinea pig, and human. In all species, the 5-HT7 receptor shows a consistent mRNA distribution pattern within the brain (1, 3, 4) and ligand binding profile (5–8). The 5-HT7 receptor is mainly localized to the hypothalamus, hippocampus, and frontal cortex.
Functionally, the 5-HT7 receptor has been shown to stimulate cAMP formation (1, 2, 6, 7). This stimulation has been linked to the activation of a calmodulin-regulated adenylyl cyclase (9). The existence of several splice variants of the 5-HT7 receptor mRNA has been clearly demonstrated (10–12). The variants encode receptors that have slightly different lengths of the C termini, but with no detectable difference in tissue distributions or functional couplings (13). The 5-HT7 receptor has also been detected in the periphery, where it is found primarily in smooth muscle cells of blood vessels (2, 14), but also in the gastrointestinal tract (2).
Early studies established a pharmacological profile that distinguished 5-HT7 receptors from other 5-HT receptors. The 5-HT7 receptor shows a high affinity for 5-HT, 5-carboxamidotryptamine (5-CT), and methiothepin, relatively high affinity for 8-hydroxy-[N-n-dipropyl-N-(3′-iodo-2′propenyl)amino]tetralin (8-OH-DPAT) and ritanserin, and low affinity for pindolol and buspirone (1, 15). Recently, more selective antagonists have been described (16–19), developments that will facilitate the characterization of the 5-HT7 receptor.
The 5-HT7 receptor has been linked to a number of physiological and pathophysiological phenomena. Early evidence supported by the pharmacological profile suggested that the 5-HT7 receptor mediates the 5-HT-induced phase resetting of the circadian clock within the suprachiasmatic nucleus of the hypothalamus (1). The ability of 5-HT7 receptors to mediate smooth muscle relaxation (14, 20) has led to the suggestion that ligands may have therapeutic value in migraine (21). The 5-HT7 receptor has also been implicated in endocrine regulation and neuropsychiatric disorders (22). Especially intriguing is the possible involvement in disregulated circadian rhythms and depression, as antidepressants induce c-fos expression and down-regulate 5-HT7 receptor binding in the suprachiasmatic nucleus (23).
It is well established that 5-HT plays a role in thermoregulation and that systemic administration of 5-HT leads to hypothermia in rats, mice, guinea pigs, and rabbits (24–26). Several studies have attempted to establish which 5-HT receptors mediate this regulation. Early evidence suggested that a 5-HT1 receptor subtype was involved (25). A subsequent study suggested the involvement of peripheral and central 5-HT2 receptors (24), a finding that was disputed in a later study demonstrating that the 5-HT2A receptor most likely is not involved (27). At the time, it was instead speculated that 5-HT1A and 5-HT2A receptors had opposite effects, at least in the rat. The evidence supporting a role for the 5-HT1A receptor in 5-HT-induced hypothermia was largely based on data from using 8-OH-DPAT as a ligand (28). Because 8-OH-DPAT has affinity also for the 5-HT7 receptor, it can be hypothesized that this receptor is involved in 5-HT-mediated thermoregulation. A study using a new selective 5-HT7 receptor antagonist, SB-269970-A, supported this idea (19); this report demonstrated that hypothermia induced by 5-CT could be antagonized by the selective antagonist, but not by antagonists selective for 5-HT1A/1B and 5-HT1B/1D receptors. Furthermore, the unselective antagonist metergoline, which has high affinity for the 5-HT7 receptor, was able to block the hypothermia induced by 5-CT. The fact that both peripheral (24) and central (25) administration of 5-HT yields similar hypothermic responses indicates that the effect is mainly centrally mediated (29). Such a mechanism is further supported by the finding that peripherally administered 5-HT can penetrate the blood–brain barrier (30). Similar conclusions have been drawn with regard to 5-CT, which also exhibits similar hypothermic responses after central and peripheral administration (19).
A large body of evidence has established an interaction between the 5-HT7 receptor and the lipid oleamide (31–35), suggesting that oleamide allosterically regulates the 5-HT7 receptor (32, 34, 35). Oleamide is an amidated fatty acid that was originally discovered in the cerebrospinal fluid of sleep-deprived cats (36, 37). Oleamide belongs to a family of lipids that includes the endogenous ligands for the cannabinoid receptors (38). There is evidence suggesting that oleamide interacts with these receptors, but this has not been demonstrated conclusively (38, 39). Neither has a specific receptor for oleamide been demonstrated. Oleamide has been demonstrated to induce sleep in several mammals (37, 40, 41). Furthermore, it has been established that oleamide has a hypothermia-inducing effect after systemic administration (38–40). In cells expressing the 5-HT7 receptor subtype, oleamide has been shown to increase cAMP accumulation in a concentration-dependent fashion, but with a lower efficacy than that observed for 5-HT (32). In the presence of 5-HT, oleamide was shown to have the opposite effect on cAMP, causing insurmountable antagonism of the concentration-effect curve to 5-HT. These results have been interpreted to indicate that oleamide acts at an apparent allosteric site on the 5-HT7 receptor and elicits functional responses via activation of this site. Receptor binding studies have supported this hypothesis unambiguously (34, 35). Furthermore, it has been demonstrated that oleamide induces increases in c-fos mRNA expression within distinct nuclei of the thalamus and hypothalamus, where the majority of neurons express the 5-HT7 receptor (31).
We have generated a mouse strain with a targeted disruption of the 5-HT7 receptor gene (5-HT7−/− mice). Here, we have used this mouse strain to test the dual hypotheses that 5-HT and oleamide induce hypothermia by acting at the 5-HT7 receptor.
Materials and Methods
Targeted Disruption of the 5-HT7 Receptor Gene.
The mouse 5-HT7 receptor gene was isolated from a library made from genomic DNA from strain 129/SvJ. The sequence of a 4.5-kb BamHI–SacI fragment of the 5-HT7 gene containing its 0.76-kb exon II was fully determined. The neomycin expression cassette pMC1NeoPolyA (Stratagene), which directs neo expression from the TK promoter, was inserted into an MluI site within exon II, at the 3′ end of a region encoding the fifth transmembrane domain of 5-HT7 (Fig. 1A). A thymidine kinase expression cassette, derived from pMC1tk (42), was attached 5′ of the short arm of the knockout construct so that nonhomologous integrants could be selected against. The sequence of this construct, which should direct expression of a nonfunctional, truncated 5-HT7 protein, was verified. This construct should inactivate all splice forms of the receptor. The construct was transfected into 129Sv/Ev embryonic stem cells. Cells in which one copy of the 5-HT7 receptor gene had been inactivated by homologous recombination were isolated, and these cells were used to produce mice that gave germ-line transmission of the modified gene, all using standard methods (43). These were successfully bred with the C57BL/6J strain, and offspring in expected ratios for various crossings were obtained. Homozygotes were viable and fertile. The animals used in the present study were back-crossed for at least eight generations on a C57BL/6J background.
Figure 1.
Generation and characterization of 5-HT7−/− mice. (A) The genomic structure surrounding the interrupted exon II of the 5-HT7 knockout construct. The exon was interrupted by inserting a neomycin cassette at a genomic MluI restriction site. The MluI and other relevant restriction sites used in generating the construct or for diagnostic use are shown. (B) Southern blot analysis of EcoRI/BglII-digested genomic DNA from six individual animals by using the external probe indicated to visualize ≈7-kb and 4.9-kb bands. (C) Northern blot analysis of total RNA extracted from six individual animals by using a probe recognizing exon II sequence to visualize wild-type mRNA. A large aberrant message is present in the 5-HT7+/− and 5-HT7−/− mice. (D) The same blot as in C showing that this message contains the neomycin cassette as visualized by a probe recognizing a 300-bp piece of the cassette.
Experimental Animals.
In total, 44 6- to 8-week-old mice were used, 21 male and 23 female. 5-HT7+/+ siblings were used as controls. Because of the limited availability of animals, there was some overlap between the experimental groups resulting in individual animals being given more than one of the drugs or doses tested. In such cases, at least 72 h was allowed between injections to the same animal. A minimum of six animals was included in each experimental group.
Drug Treatments.
5-Hydroxytryptamine (Sigma), 5-carboxamidotryptamine (Sigma), or oleamide (gift from Dale Boger, The Scripps Research Institute) was given as a single intraperitoneal injection by using the doses indicated in a volume of 0.5 ml; 5-HT and 5-CT were dissolved in 0.9% saline, and 0.9% saline was used as control. Oleamide was dissolved in 100% ethanol to a 10× concentration and then diluted in saline immediately before injection, and 10% ethanol in saline was used as control.
Temperature Recordings.
Core body temperature was measured by using a rectal thermometer probe. A basal value was measured at the time of injection, and then measurements were made every 30 min for 2 h. All experiments were started at 0900 h. There was no difference in basal body temperature between 5-HT7+/+ and 5-HT7−/− mice (35.24 ± 0.14°C and 35.59 ± 0.15°C, respectively).
Statistical Analysis.
Analysis of variance was used to analyze the rectal temperature data. Dunnett's test was used for post hoc comparisons. Differences were considered significant at P < 0.05.
Results
Targeted Disruption of the 5-HT7 Receptor Gene.
To generate mice lacking functional 5-HT7 receptors, the second exon of the 5-HT7 gene was disrupted by homologous recombination (Fig. 1A). The modified gene including flanking sequence from the 5-HT7 gene was cloned from 5-HT7−/− mice and sequenced to verify correct insertion of the construct. Southern blots (Fig. 1B) also verify the correct insertion and were routinely (in combination with a PCR assay) used to genotype animals used for breeding and the present study. A Northern blot indicates that an aberrant message is present in the 5-HT7−/− mice (Fig. 1 C and D). Its size is consistent with it representing the wild-type transcript bearing the inactivating insertion. Because of the presence of multiple stop codons within the construct, it should not be possible to translate this message into a functional receptor.
Effects of 5-HT and 5-CT on Body Temperature.
There were no differences observed between males and females in any of the experiments (not shown) allowing data from both sexes to be pooled. There was no difference in basal temperature between 5-HT7+/+ and 5-HT7−/− mice at the beginning of each experiment. Saline injections had no effect on core body temperature in either 5-HT7+/+ or 5-HT7−/− mice. 5-HT (5 mg/kg) induced significant hypothermia in 5-HT7+/+ mice with a peak effect at 30 min after the injection (Fig. 2). The maximum reduction in body temperature was ≈2.5°C. After 2 h, the body temperature had returned to base level. In contrast, 5-HT had no significant effect on body temperature in the 5-HT7−/− mice. Thus, 5-HT-induced hypothermia is at least primarily mediated by the 5-HT7 receptor.
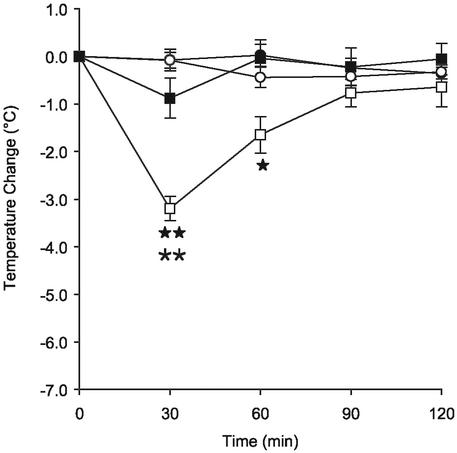
Figure 2.
5-HT fails to induce hypothermia in 5-HT7−/− mice. Time course of hypothermia after an i.p. injection of 5-HT (5 mg/kg) or vehicle in 5-HT7+/+ or 5-HT7−/− mice. The symbols represent 5-HT7+/+ vehicle (○), 5-HT7−/− vehicle (●), 5-HT7+/+ 5-HT (□), and 5-HT7−/− 5-HT (■), respectively. **, P < 0.01 for 5-HT-treated vs. vehicle-treated of the same genotype. ★, P < 0.05; ★★, P < 0.01 for 5-HT7+/+ vs. 5-HT7−/− mice treated with 5-HT. Data represent mean ± SEM; n = 6–10 mice per group.
A dose-dependent decrease in body temperature was caused by 5-CT in 5-HT7+/+ mice (Fig. 3). A submaximal dose (0.5 mg/kg 5-CT) had a peak effect at 30 min with a 2.0°C reduction (Fig. 3A). At the end of the recording period, the temperature had returned to the base level. A higher dose (3 mg/kg) reduced the temperature by 3.4°C at 30 min and then continued to potentiate this decrease during the 2-h recording period (Fig. 3B). The lower dose of 5-CT had no effect on body temperature in the 5-HT7−/− mice. However, a significant decrease was observed in the 5-HT7−/− animals with the high dose (3 mg/kg), although this decrease was significantly less pronounced than that in the 5-HT7+/+ mice. Thus, the hypothermia-inducing effect of 5-CT is also primarily mediated by the 5-HT7 receptor, particularly at lower doses of 5-CT.
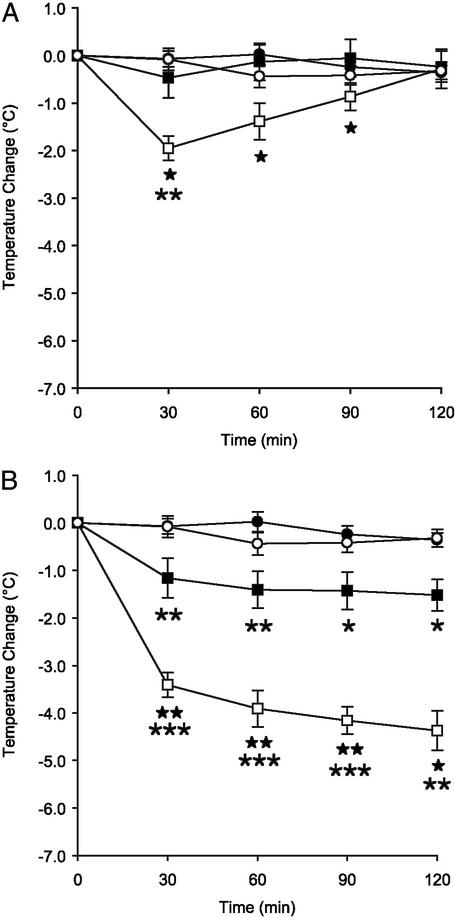
Figure 3.
Effects of 5-CT on body temperature. Time course of hypothermia following an i.p. injection of 5-CT (0.5 mg/kg, A; or 3 mg/kg, B) or vehicle in 5-HT7+/+ or 5-HT7−/− mice. The symbols represent 5-HT7+/+ vehicle (○), 5-HT7−/− vehicle (●), 5-HT7+/+ 5-CT (□), and 5-HT7−/− 5-CT (■), respectively. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for 5-CT-treated vs. vehicle-treated in the same genotype. ★, P < 0.05; ★★, P < 0.01 for 5-HT7+/+ vs. 5-HT7−/− mice treated with 5-CT. Data represent mean ± SEM, n = 6 mice per group.
Effects of Oleamide on Body Temperature.
Oleamide dose-dependently reduced body temperature in both the 5-HT7+/+ and 5-HT7−/− mice (Fig. 4). The vehicle 10% ethanol in saline did not significantly alter body temperature. At 30 min, 1 mg/kg oleamide reduced the temperature by 1.5°C in the 5-HT7+/+ mice and by 1.9°C in the 5-HT7−/− mice. The 25 mg/kg dose reduced body temperature by 4.0°C and 3.8°C in the 5-HT7+/+ and 5-HT7−/− mice, respectively. At the lower dose, temperature had returned to base level at the end of the recording period, whereas it remained significantly reduced, at least in the 5-HT7+/+ mice, with the higher dose (Fig. 4B). Thus, the 5-HT7 receptor does not play a major role in the hypothermia-inducing effect of oleamide.
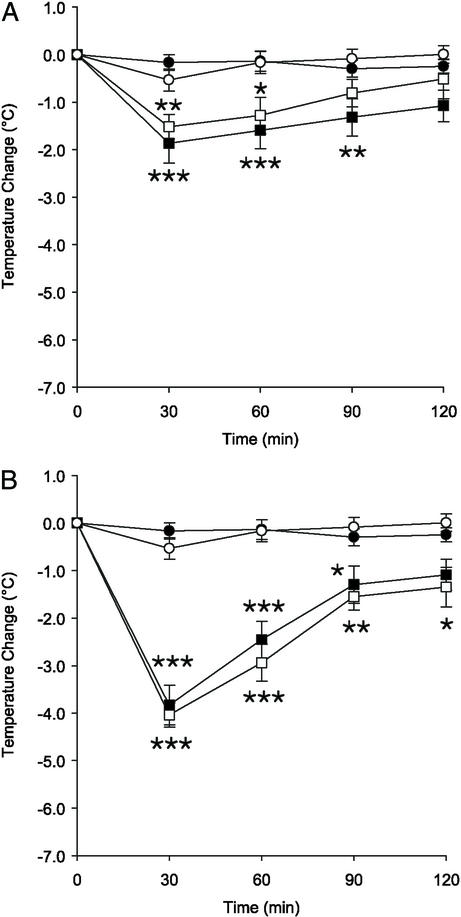
Figure 4.
Oleamide induces hypothermia in both 5-HT7+/+ and 5-HT7−/− mice. Time course of hypothermia after an i.p. injection of oleamide (1 mg/kg, A; or 25 mg/kg, B), or vehicle in 5-HT7+/+ or 5-HT7−/− mice. The symbols represent 5-HT7+/+ vehicle (○), 5-HT7−/− vehicle (●), 5-HT7+/+ oleamide (□), and 5-HT7−/− oleamide (■), respectively. *, P < 0.05; **, P < 0.01; ***, P < 0.001 for oleamide-treated vs. vehicle-treated in the same genotype. Data represent mean ± SEM, n = 6–12 mice per group.
Effects of 5-HT in Combination with Oleamide on Body Temperature.
A dose of 1 mg/kg oleamide in combination with 5-HT (5 mg/kg) caused a significant decrease in body temperature of both 5-HT7+/+ and 5-HT7−/− mice (Fig. 5). The effect in the 5-HT7+/+ mice was significantly greater than that in the 5-HT7−/− mice. At 60 min postinjection, the body temperature was decreased by 5.5°C in the 5-HT7+/+ mice and remained significantly reduced during the length of the recording period. These data are similar to the sum of the data from Figs. 2 and 4A (Fig. 5B).
Figure 5.
5-HT and oleamide have additive and sustained effects on body temperature. (A) Time course of hypothermia after an i.p. injection of 5-HT (5 mg/kg) in combination with oleamide (1 mg/kg), or vehicle in 5-HT7+/+ or 5-HT7−/− mice. (B) A representation of the data in Figs. 2 and 4A added together for comparison. The symbols represent 5-HT7+/+ vehicle (○), 5-HT7−/− vehicle (●), 5-HT7+/+ 5-HT + oleamide (□), and 5-HT7−/− 5-HT + oleamide (■), respectively. **, P < 0.01; ***, P < 0.001 for 5-HT/oleamide-treated vs. vehicle-treated of the same genotype. ★, P < 0.05; ★★, P < 0.01; ★★★, P < 0.001 for 5-HT7+/+ versus 5-HT7−/− mice treated with 5-HT/oleamide. Data represent mean ± SEM; n = 6 mice per group.
Discussion
There are two groups of major findings in the present study. First, 5-HT and 5-CT at physiological doses could not induce hypothermia in 5-HT7−/− mice. Second, oleamide induces hypothermia similarly in both 5-HT7+/+ and 5-HT7−/− mice.
In 5-HT7+/+ mice, 5-HT (5 mg/kg) caused a reduction of body temperature with a magnitude and time course similar to that found in previous studies (24). Hence, it is unlikely that there are strain differences with regard to thermoregulation in the background strain (C57BL/6J) of mice used for breeding the mice in the present study and mice used in other studies. Nevertheless, littermates were used as controls throughout this study to control for possible strain differences. In the 5-HT7−/− mice, 5-HT (5 mg/kg) failed to elicit a hypothermic response. The 5-HT7−/− mice do not express any overt behavioral phenotype, and the control temperature recordings show that the basal thermoregulation is normal in these mice. Thus, it can be concluded that the hypothermic response to 5-HT in normal mice is mediated mainly by the 5-HT7 receptor.
Similarly, 5-CT induced hypothermia in the 5-HT7+/+ mice with magnitudes and time courses similar to that found in previous reports within the dose range used in the present study (19). As has been observed earlier (19), the higher dose (3 mg/kg) elicited a prolonged hypothermia that did not return to basal levels within the 2-h recording period. The lower dose (0.5 mg/kg) of 5-CT did not affect body temperature in the 5-HT7−/− mice. In these mice, the higher dose induced a small, but significant and sustained, hypothermia. The mechanism behind this reduction in body temperature may be because of several factors. In addition to acting at 5-HT7 receptors, 5-CT is an unselective agonist for 5-HT1 type receptors. It has previously been hypothesized that 5-HT/5-CT-induced hypothermia is mediated by 5-HT1 receptors (19, 24, 25), and thus the observed hypothermia could be explained by stimulation of 5-HT1 receptors. A previous study has shown that a selective 5-HT7 receptor antagonist was able to fully block hypothermia induced by a low dose of 5-CT (0.3 mg/kg) (19), a finding consistent with the present result. It is, however, not known whether the antagonist is able to block the sustained hypothermia induced by higher doses of 5-CT or if this effect can partly be attributed to 5-HT1 receptors. Although some controversy persists, it has also been suggested that 5-HT2 receptors may be involved in 5-HT-induced hypothermia (26, 27). Based on the selective antagonist data, such an explanation also seems unlikely (19). It is also possible that the prolonged hypothermia observed with high doses of 5-CT is because of nonphysiological and unspecific interactions.
Oleamide induced hypothermia in a dose-dependent manner and with time courses and magnitudes similar to those previously observed (38–40) in both the 5-HT7+/+ and 5-HT7−/− mice. These findings make it unlikely that the primary mechanism of action for oleamide is a direct activation the 5-HT7 receptor, but rather suggest that oleamide acts on a site different from the 5-HT7 receptor to induce hypothermia. A receptor or other specific site of action for oleamide has not been established. As oleamide is a member of the family of compounds believed to be the endogenous ligands for the cannabinoid receptors, it may be hypothesized that oleamide reduces body temperature by acting at these receptors. It has been shown that other cannabinoid agonists induce hypothermia by acting on CB1 cannabinoid receptors (44). Cannabinoid receptor antagonists have also been shown to block the hypnotic effect of oleamide (39, 45). Thus, we propose that 5-HT and oleamide induce hypothermia by independent mechanisms.
When oleamide (1 mg/kg) was given in combination with 5-HT (5 mg/kg), a small but significant hypothermic response could be observed in the 5-HT7−/− mice. In view of the results above, this effect is most likely attributable to oleamide alone. Although a comparison of the results where 5-HT and oleamide were given alone and in combination mainly indicate additive effects, another possibility is that oleamide potentiated 5-HT acting on a 5-HT receptor other than 5-HT7. Oleamide is known to potentiate the 5-HT1A, 5-HT2A, and 5-HT2C receptors (46, 47), also by acting on cannabinoid receptors (48). In fact, when oleamide and 5-HT were given together, they induced a hypothermia in the 5-HT7+/+ mice that was greater than an additive effect, at least at the 60 min and later time point. Although one study has found that oleamide decreases the affinity of 5-HT7 receptors (34) and another that oleamide inhibits 5-HT-induced cAMP formation mediated by 5-HT7 receptors (32), there is also evidence that oleamide promotes high affinity 5-HT binding at the 5-HT7 receptor (35) and that 5-HT7 receptor-expressing neurons are activated by oleamide (31), as determined by c-fos expression. Except for the c-fos study, these results were obtained by using cell lines transfected with the 5-HT7 receptor. Thus, it may be hypothesized that in the intact animal, oleamide acts at an allosteric site on the 5-HT7 receptor to mediate an enhanced hypothermic response.
Acknowledgments
We thank Professor Dale Boger (The Scripps Research Institute) for generously providing oleamide, Professor Floyd Bloom for helpful suggestions on the manuscript, and D. Krishun for technical assistance. This work was supported by National Institutes of Health Grant GM32355.
Abbreviations
- 5-CT
5-carboxamidotryptamine
- 5-HT
5-hydroxytryptamine
- 8-OH-DPAT
8-hydroxy-[N-n-dipropyl-N-(3′-iodo-2′propenyl)amino]tetralin
References
- 1.Lovenberg T W, Baron B M, de Lecea L, Miller J D, Prosser R A, Rea M A, Foye P E, Racke M, Slone A L, Siegel B W, et al. Neuron. 1993;11:449–458. doi: 10.1016/0896-6273(93)90149-l. [DOI] [PubMed] [Google Scholar]
- 2.Bard J A, Zgombick J, Adham N, Vaysse P, Branchek T A, Weinshank R L. J Biol Chem. 1993;268:23422–23426. [PubMed] [Google Scholar]
- 3.Gustafson E L, Durkin M M, Bard J A, Zgombick J, Branchek T A. Br J Pharmacol. 1996;117:657–666. doi: 10.1111/j.1476-5381.1996.tb15241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mengod G, Vilaro M T, Raurich A, Lopez-Gimenez J F, Cortes R, Palacios J M. Histochem J. 1996;28:747–758. doi: 10.1007/BF02272148. [DOI] [PubMed] [Google Scholar]
- 5.Tsou A P, Kosaka A, Bach C, Zuppan P, Yee C, Tom L, Alvarez R, Ramsey S, Bonhaus D W, Stefanich E, et al. J Neurochem. 1994;63:456–464. doi: 10.1046/j.1471-4159.1994.63020456.x. [DOI] [PubMed] [Google Scholar]
- 6.Plassat J L, Amlaiky N, Hen R. Mol Pharmacol. 1993;44:229–236. [PubMed] [Google Scholar]
- 7.Ruat M, Traiffort E, Leurs R, Tardivel-Lacombe J, Diaz J, Arrang J M, Schwartz J C. Proc Natl Acad Sci USA. 1993;90:8547–8551. doi: 10.1073/pnas.90.18.8547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen Y, Monsma F J, Jr, Metcalf M A, Jose P A, Hamblin M W, Sibley D R. J Biol Chem. 1993;268:18200–18204. [PubMed] [Google Scholar]
- 9.Baker L P, Nielsen M D, Impey S, Metcalf M A, Poser S W, Chan G, Obrietan K, Hamblin M W, Storm D R. J Biol Chem. 1998;273:17469–17476. doi: 10.1074/jbc.273.28.17469. [DOI] [PubMed] [Google Scholar]
- 10.Heidmann D E, Metcalf M A, Kohen R, Hamblin M W. J Neurochem. 1997;68:1372–1381. doi: 10.1046/j.1471-4159.1997.68041372.x. [DOI] [PubMed] [Google Scholar]
- 11.Jasper J R, Kosaka A, To Z P, Chang D J, Eglen R M. Br J Pharmacol. 1997;122:126–132. doi: 10.1038/sj.bjp.0701336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stam N J, Roesink C, Dijcks F, Garritsen A, van Herpen A, Olijve W. FEBS Lett. 1997;413:489–494. doi: 10.1016/s0014-5793(97)00964-2. [DOI] [PubMed] [Google Scholar]
- 13.Krobert K A, Levy F O. Br J Pharmacol. 2002;135:1563–1571. doi: 10.1038/sj.bjp.0704588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schoeffter P, Ullmer C, Bobirnac I, Gabbiani G, Lübbert H. Br J Pharmacol. 1996;117:993–994. doi: 10.1111/j.1476-5381.1996.tb16687.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.To Z P, Bonhaus D W, Eglen R M, Jakeman L B. Br J Pharmacol. 1995;115:107–116. doi: 10.1111/j.1476-5381.1995.tb16327.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Forbes I T, Dabbs S, Duckworth D M, Jennings A J, King F D, Lovell P J, Brown A M, Collin L, Hagan J J, Middlemiss D N, et al. J Med Chem. 1998;41:655–657. doi: 10.1021/jm970519e. [DOI] [PubMed] [Google Scholar]
- 17.Kikuchi C, Nagaso H, Hiranuma T, Koyama M. J Med Chem. 1999;42:533–535. doi: 10.1021/jm980519u. [DOI] [PubMed] [Google Scholar]
- 18.Thomas D R, Middlemiss D N, Taylor S G, Nelson P, Brown A M. Br J Pharmacol. 1999;128:158–164. doi: 10.1038/sj.bjp.0702759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagan J J, Price G W, Jeffrey P, Deeks N J, Stean T, Piper D, Smith M I, Upton N, Medhurst A D, Middlemiss D N, et al. Br J Pharmacol. 2000;130:539–548. doi: 10.1038/sj.bjp.0703357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Eglen R M, Jasper J R, Chang D J, Martin G R. Trends Pharmacol Sci. 1997;18:104–107. doi: 10.1016/s0165-6147(97)01043-2. [DOI] [PubMed] [Google Scholar]
- 21.Terron J A, Falcon-Neri A. Br J Pharmacol. 1999;127:609–616. doi: 10.1038/sj.bjp.0702580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vanhoenacker P, Haegeman G, Leysen J E. Trends Pharmacol Sci. 2000;21:70–77. doi: 10.1016/s0165-6147(99)01432-7. [DOI] [PubMed] [Google Scholar]
- 23.Mullins U L, Gianutsos G, Eison A S. Neuropsychopharmacology. 1999;21:352–367. doi: 10.1016/S0893-133X(99)00041-X. [DOI] [PubMed] [Google Scholar]
- 24.Sugimoto Y, Yamada J, Horisaka K. Life Sci. 1991;48:419–423. doi: 10.1016/0024-3205(91)90497-y. [DOI] [PubMed] [Google Scholar]
- 25.Yamada J, Sugimoto Y, Wakita H, Horisaka K. Jpn J Pharmacol. 1988;48:145–148. doi: 10.1254/jjp.48.145. [DOI] [PubMed] [Google Scholar]
- 26.Won S J, Lin M T. Naunyn-Schmiedeberg's Arch Pharmacol. 1988;338:256–261. doi: 10.1007/BF00173397. [DOI] [PubMed] [Google Scholar]
- 27.Morishima Y, Shibano T. Pharmacol Biochem Behav. 1995;52:755–758. doi: 10.1016/0091-3057(95)00172-s. [DOI] [PubMed] [Google Scholar]
- 28.Hjorth S. J Neural Transm. 1985;61:131–135. doi: 10.1007/BF01253058. [DOI] [PubMed] [Google Scholar]
- 29.Cox B, Lee T F. J Pharmacol Methods. 1981;5:43–51. doi: 10.1016/0160-5402(81)90101-7. [DOI] [PubMed] [Google Scholar]
- 30.Bulat M, Supek Z. J Neurochem. 1967;14:265–271. doi: 10.1111/j.1471-4159.1967.tb09523.x. [DOI] [PubMed] [Google Scholar]
- 31.Thomas E A, Cravatt B F, Sutcliffe J G. J Neurochem. 1999;72:2370–2378. doi: 10.1046/j.1471-4159.1999.0722370.x. [DOI] [PubMed] [Google Scholar]
- 32.Thomas E A, Carson M J, Neal M J, Sutcliffe J G. Proc Natl Acad Sci USA. 1997;94:14115–14119. doi: 10.1073/pnas.94.25.14115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas E A, Cravatt B F, Danielson P E, Gilula N B, Sutcliffe J G. J Neurosci Res. 1997;50:1047–1052. doi: 10.1002/(SICI)1097-4547(19971215)50:6<1047::AID-JNR16>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 34.Hedlund P B, Carson M J, Sutcliffe J G, Thomas E A. Biochem Pharmacol. 1999;58:1807–1813. doi: 10.1016/s0006-2952(99)00274-9. [DOI] [PubMed] [Google Scholar]
- 35.Alberts G L, Chio C L, Im W B. Mol Pharmacol. 2001;60:1349–1355. doi: 10.1124/mol.60.6.1349. [DOI] [PubMed] [Google Scholar]
- 36.Lerner R A, Siuzdak G, Prospero-Garcia O, Henriksen S J, Boger D L, Cravatt B F. Proc Natl Acad Sci USA. 1994;91:9505–9509. doi: 10.1073/pnas.91.20.9505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cravatt B F, Prospero-Garcia O, Siuzdak G, Gilula N B, Henriksen S J, Boger D L, Lerner R A. Science. 1995;268:1506–1509. doi: 10.1126/science.7770779. [DOI] [PubMed] [Google Scholar]
- 38.Lichtman A H, Hawkins E G, Griffin G, Cravatt B F. J Pharmacol Exp Ther. 2002;302:73–79. doi: 10.1124/jpet.302.1.73. [DOI] [PubMed] [Google Scholar]
- 39.Fedorova I, Hashimoto A, Fecik R A, Hedrick M P, Hanus L O, Boger D L, Rice K C, Basile A S. J Pharmacol Exp Ther. 2001;299:332–342. [PubMed] [Google Scholar]
- 40.Huitron-Resendiz S, Gombart L, Cravatt B F, Henriksen S J. Exp Neurol. 2001;172:235–243. doi: 10.1006/exnr.2001.7792. [DOI] [PubMed] [Google Scholar]
- 41.Basile A S, Hanus L, Mendelson W B. NeuroReport. 1999;10:947–951. doi: 10.1097/00001756-199904060-00010. [DOI] [PubMed] [Google Scholar]
- 42.McKnight S L. Nucleic Acids Res. 1980;8:5949–5964. doi: 10.1093/nar/8.24.5949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thomas K R, Capecchi M R. Cell. 1987;51:503–512. doi: 10.1016/0092-8674(87)90646-5. [DOI] [PubMed] [Google Scholar]
- 44.Rawls S M, Cabassa J, Geller E B, Adler M W. J Pharmacol Exp Ther. 2002;301:963–968. doi: 10.1124/jpet.301.3.963. [DOI] [PubMed] [Google Scholar]
- 45.Mendelson W B, Basile A S. NeuroReport. 1999;10:3237–3239. doi: 10.1097/00001756-199910190-00021. [DOI] [PubMed] [Google Scholar]
- 46.Boger D L, Patterson J E, Jin Q. Proc Natl Acad Sci USA. 1998;95:4102–4107. doi: 10.1073/pnas.95.8.4102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huidobro-Toro J P, Harris R A. Proc Natl Acad Sci USA. 1996;93:8078–8082. doi: 10.1073/pnas.93.15.8078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cheer J F, Cadogan A K, Marsden C A, Fone K C, Kendall D A. Neuropharmacology. 1999;38:533–541. doi: 10.1016/s0028-3908(98)00208-1. [DOI] [PubMed] [Google Scholar]