Abstract
Light is a central regulator of plant growth and development. Among the processes triggered by blue and UV-A light, phototropism, stomatal movement, and chloroplast orientation rely on the activation of blue-light receptors known as phototropins. So far, these photoreceptors constitute a class of light receptor kinases unique to the plant kingdom. In Arabidopsis thaliana, the two members phot1 and phot2 have been shown to display partially overlapping functions. Up to now little is known about the signaling cascade, which links these phototropins to the physiological responses downstream of blue-light perception. Here, we show that on illumination with blue light, but not red light, voltage-dependent and calcium-permeable channels activate in the plasma membrane of mesophyll cells. Blue-light stimulation in the presence of the photosynthetic electron transport inhibitor, 3-(3,4-dichlorophenyl)-1,1-dimethylurea, indicates that blue-light receptors rather than photosynthesis control channel activity. Sensitivity toward the protein kinase inhibitor K252a further pointed to the possible involvement of light receptor kinases. In support of this hypothesis, in the photoreceptor mutant phot1-5, blue-light induction of calcium currents was dramatically reduced and was eliminated in the double mutant phot1-5 phot2-1. By contrast, in cry1-304 cry2-1, an Arabidopsis mutant lacking another class of plant blue-light receptors, the channel remained sensitive to blue light. We thus conclude that blue light triggers calcium fluxes via the phototropin-activated calcium-permeable channel.
Keywords: Ca2+ current‖ion channel‖photoreceptor
Plants are able to respond to changes in light quality, quantity, and direction. Since the early observation that blue light triggers phototropism (1), many studies aimed to explore the physiology of blue-light-dependent movements in plants (2–6). Among the photoreceptors, phot1 (7) and phot2 (8) from Arabidopsis thaliana represent a new class of receptor kinases unique to the plant kingdom. Phototropins possess flavin mononucleotides as chromophores and become autophosphorylated on blue-light irradiation via their C-terminal serine/threonine kinase domain (9–11). Phototropins have been shown to control blue-light-dependent processes, such as phototropism, chloroplast orientation, and stomatal opening (3). In addition to these photomovements, phot1 seems to control the rapid, transient inhibition of stem growth, when dark-grown seedlings are transferred to blue light (12). A second slow phase of stem-growth inhibition, which is observed ≈30 min after blue-light illumination, however, depends on the action of cryptochromes (12), the second class of blue-light receptors in plants (4, 6, 13).
In Arabidopsis mutants defective in phot1 (phot1), hypocotyls lose their ability to bend toward weak blue light (7, 14). A less pronounced but significant loss of sensitivity toward low blue-light regimes was observed for the reorientation of chloroplasts and opening of stomata (15, 16). By contrast, mutants lacking phot2 (phot2) showed normal photomovements under these light conditions (11, 17, 18). phot2 develops a blue-light-dependent phenotype when exposed to high light intensities. In wild type, chloroplasts are able to avoid these strong light intensities by moving to the anticlinal walls of the mesophyll cell, which are oriented parallel to the direction of light. The chloroplast avoidance reaction impaired in phot2 allows minimizing irradiation of these organelles and photo-damage of the photosynthetic apparatus (17–19).
Finally, the double mutant phot1 phot2 is defective in all phototropin-dependent movements (11, 16). Together, these findings indicate that both receptors exhibit partially overlapping functions with respect to phototropism, chloroplast movement, and stomatal opening (ref. 3 and references therein).
To date, little is known about the signal transduction elements following the blue-light perception by phot1 and phot2 (2, 16, 20–24). In guard cells, blue-light activation of the plasma membrane H+-ATPase depends on phototropins, driving turgor formation and stomatal opening (16). By using apoaequorin-expressing Arabidopsis, Ca2+ signals have been shown as downstream elements of phototropin action, too (24). In seedlings from phot1-5, these blue-light-induced calcium signals were reduced, raising the question about the mechanism of blue-light-dependent calcium fluxes. In the present study we thus aimed to identify Ca2+ channels involved in blue-light signaling. For this purpose, we applied the patch-clamp technique to mesophyll protoplasts from A. thaliana wild type and photoreceptor mutants. We demonstrate that hyperpolarization-dependent calcium-permeable channels are activated by blue light, and that the activation mechanism is mediated by phototropins rather than cryptochrome blue-light receptors or photosynthesis.
Materials and Methods
Plant Material and Protoplast Isolation.
A. thaliana plants (Columbia ecotype) were grown in a growth chamber at a photoperiod of 8 h and a photon flux density of 100 μmol·m−2·s−1 (Philips TL70 and Osram 25W, Osram, Munich). The temperature was set to 22°C in the light and 16°C in the dark. The humidity ranged between 50% and 60%. Mesophyll protoplasts were enzymatically isolated from 3- to 10-week-old leaves according to the method of Sando and Goto (25) and stored on ice before experiments. The mutant phot1-5 is a kind gift of W. R. Briggs (Stanford University, Stanford, CA). Cry1-304 cry2-1 plants were used by kind permission of C. Lin (University of California, Los Angeles).
Patch–Clamp Recordings.
Currents were recorded with an EPC-9 patch-clamp amplifier (HEKA Electronics, Lambrecht/Pfalz, Germany) as described (26). To determine the activation state of channels, 10 repetitive voltage ramps of 1-s duration were applied with an interval of 3 s, after a preactivation period of 50 ms at hyperpolarized potentials, and the current responses were averaged (Figs. 3–5). Voltage protocols are given in the figure legends. Standard solutions contained (in mM) 40 Ca-gluconate2 and 10 Mes/Tris (pH 5.6) in the bath; and 10 Ba-gluconate2, 4 KCl, 4 EGTA, and 10 Hepes/Tris (pH 7.1) in the pipette. Alternatively, 40 CaCl2 and 10 BaCl2 (without 4 KCl) were used in the bath and pipette, respectively, without noticeable effect on cation currents. Solutions were adjusted to 400 milliosmol kg−1, using d-sorbitol. For illumination experiments, protoplasts were kept in darkness, and the sealing procedure was done under dim light (10 μmol·m−2·s−1) provided by the microscope halogen lamp (12 V, 50 W). After establishment of the cell-attached configuration the light was turned off and background currents were recorded. For subsequent light treatments, blue light was generated by a 75-W xenon lamp and directed through a band-pass filter (450–490 nm) of an inverted microscope (Zeiss Axiovert 35). Photon flux density was adjusted to 275 μmol·m−2· s−1 by using neutral density filters (Schott, Mainz, Germany). Red light was provided by the microscope lamp with an RG630 filter (Schott), and the photon flux density was set to 125 or 300 μmol·m−2·s−1. Fluence rates were measured with a quantum meter (Li-250, Li-Cor, Lincoln, NE).
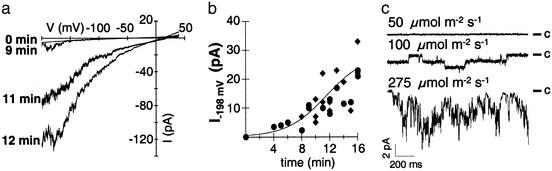
Figure 3.
Blue-light regulation of calcium-permeable channels. (a) Current responses to 1-s voltage ramps from −198 to +42 mV before and during continuous illumination with 275 μmol·m−2·s−1 of blue light. The holding potential was −38 mV, and channels were preactivated for 50 ms at −198 mV. Note that channel activation was obtained in 18 cell-attached experiments with (n = 4) or without (n = 14) 0.1 mM DTT in standard bath and pipette solutions, without noticeable difference. DTT was initially applied to solutions to prevent spontaneous activation of voltage-dependent cation channels, as was reported for guard cells (28). (b) Time course of channel activation by blue light. Blue-light-induced currents were recorded during ramps as shown in a. Data points represent the current at −198 mV as a function of time after the onset of blue-light illumination. The line represents a sigmoidal fit of the activation time course. For comparability, protoplasts with similar current amplitudes are shown only [results from all cells tested are shown in Fig. 5; wild type (circles) and cry1-304 cry2-1 (diamonds)]. (c) Light dose dependence of channel activation. Starting from a holding potential of −38 mV, single channels were recorded during voltage pulses to −198 mV after a blue-light illumination period of 12 min at the fluence rates indicated. Measurements shown in a–c were performed in the cell-attached configuration.
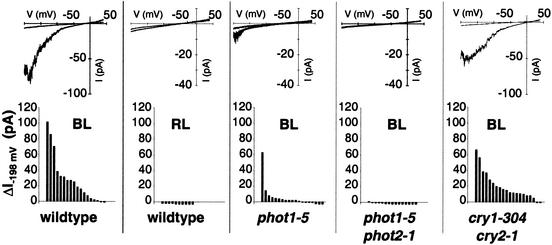
Figure 5.
Light-induced cation currents in wild type and photoreceptor mutants. (Upper) Current responses of individual cell-attached patches to voltage ramps as shown in Fig. 3a before and 12–15 min after illumination. (Lower) Channel activation by light is expressed as the current increase within 12–15 min of light treatment [ΔI−198mV = (Ibefore − Iafter)]. Each bar corresponds to the blue-light-dependent current difference obtained from one experiment. Current amplitudes were measured at a command voltage of −198 mV and averaged from 10 ramps. BL, blue-light irradiation of 275 μmol·m−2·s−1; RL, red-light irradiation of 300 μmol·m−2·s−1.
Conventions.
Because blue-light-induced currents were measured in the cell-attached mode of the patch-clamp technique, the potential (V) in Figs. 2–5 denotes the command voltage corrected for liquid junction potentials. In whole-cell and outside-out measurements (Fig. 1), V indicates the actual membrane potential.
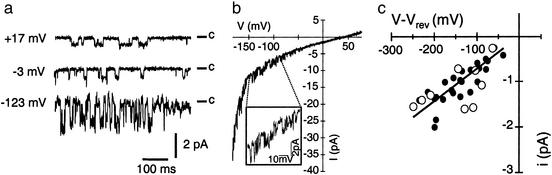
Figure 2.
Single-channel recordings of cation channels. (a) Single-channel fluctuations in one cell-attached patch during pulses to command voltages indicated, starting from a holding potential of −23 mV. (b) Single-channel activity during a voltage ramp from −183 mV to +77 mV in 1.2 s, after a 50-ms preactivation period at −183 mV. (c) Single-channel amplitudes plotted against the driving force (V–Vrev). Data were obtained in seven experiments, and currents were fitted by linear regressions, resulting in a mean single-channel conductance of 7.2 ± 1 pS (n = 7). For comparability, the data in c were plotted against (V–Vrev), where Vrev was determined from linear regressions in the individual experiments. The continuous line represents the average of the seven linear fits, with a slope of 7.2 pS and (V–Vrev) = 0 mV. Currents in a–c were recorded in light-adapted cells. Note that similar results were obtained in cell-attached patches on protoplasts continuously illuminated with white light (filled circles in c) and on predarkened protoplasts after illumination with blue light (open circles in c; for light treatments, see Materials and Methods). In all cell-attached measurements (Figs. 2–5), V indicates the command voltage.
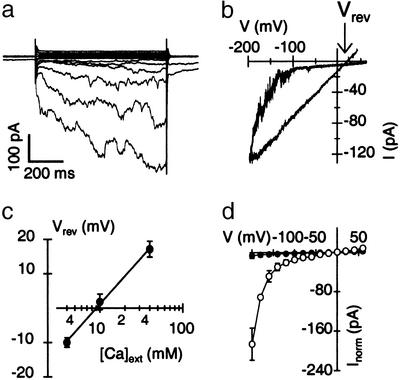
Figure 1.
Hyperpolarization-activated inward currents in Arabidopsis mesophyll cell protoplasts. (a) Whole-cell currents in response to voltage pulses. Traces represent mean currents from three consecutively applied voltage protocols. From a holding potential of −23 mV, 800-ms test pulses with an interval of 3 s were applied from +57 mV to −183 mV in 20-mV decrements. (b) Current response of an outside-out patch to voltage ramps. From a holding potential of −38 mV, a 3-s voltage ramp was applied from +62 mV to −198 mV, followed by a faster, 1-s ramp back to +42 mV. (c) Reversal potentials of cation currents as a function of the external Ca2+ concentration measured in outside-out patches. Standard bath solution with 40 mM Ca-gluconate2 was exchanged with solutions containing either 10 or 4 mM Ca-gluconate2. Reversal potentials were obtained from the crossing of current traces during fast (100 ms) ramps from −218 mV to +82 mV (cf. b), applied from a holding potential of −38 mV with 3-s intervals. Data points are mean values ± SD (n ≥ 5). The line represents a fit according to the Nernst equation for divalent cations, with RT/zF × 2.303 = 27 mV. (d) Current–voltage relation before (open circles, n = 5) and after (filled circles, n = 3) application of 100 μM LaCl3 to outside-out patches. Current amplitudes were determined at the end of 800-ms pulses as shown in a and normalized to the current recorded at −178 mV. Holding potential was −38 mV; test pulses were separated by 3-s intervals. Error bars indicate SD.
Results
Voltage-Dependent Cation Currents.
To resolve calcium channels involved in blue-light signaling, we applied the patch-clamp technique to single light-adapted mesophyll protoplasts in the presence of 40 mM extracellular Ca2+ and 10 mM intracellular Ba2+. In the whole-cell configuration, hyperpolarizing voltage pulses elicited slowly activating inward currents (Fig. 1a). During a 3-s voltage ramp from +62 mV to −198 mV, inward currents appeared negative of about −100 mV (Fig. 1b). Due to their slow kinetics, these channels did not deactivate during the subsequent fast depolarizing ramp to +42 mV, which allowed assessing the reversal potential of channel currents in standard solutions in the outside-out configuration (arrow in Fig. 1b). A value of +16.5 ± 3 mV (n = 21) under these conditions was near the equilibrium potential for divalent cations (ECa/Ba = +17.8 mV), pointing to significant cation permeability. According to the Goldman–Hodgkin–Katz voltage equation, from this value a relative permeability for Ba2+ over Ca2+ of PBa:PCa = 1.2 was determined, neglecting the presence of 4 mM KCl in the pipette solution. Changing the external Ca2+ concentrations between 4 mM and 40 mM, reversal potentials followed the Nernst prediction for a calcium-permeable channel, with a change of 27 mV per 10-fold increase in Ca2+ concentration (Fig. 1c). In line with the low discrimination between Ba2+ and Ca2+, the sequence of relative current amplitudes was Ba2+ (131.8 ± 20, n = 7) > Ca2+ (100, n = 7) > Mg2+ (84.3 ± 18, n = 5, determined at −198 mV) and indicated a cation selectivity similar to other Ca2+-permeable channels in plants (27–30). The currents were furthermore sensitive to cation channel blockers (Fig. 1d). A reduction of current amplitudes by 95.2 ± 7% (n = 4) and 97.9 ± 2% (n = 3) was observed after the application of extracellular 100 μM gadolinium (Gd3+) or lanthanum (La3+), respectively.
In the cell-attached configuration, hyperpolarization-activated single channels were analyzed (Fig. 2). The single-channel activity increased on hyperpolarization induced by voltage pulses (Fig. 2a) and voltage ramps (Fig. 2b), resembling the macroscopic whole-cell currents (Fig. 1 a and b). In the presence of 100 μM LaCl3 in the pipette solution, these single channels were not monitored (data not shown), most probably due to their La3+ sensitivity. From the current-voltage plot of the single-channel amplitudes, a conductance of 7.2 ± 1 pS (n = 7, Fig. 2c) was determined. With respect to their conductance and voltage-dependence these channels resemble abscisic acid (ABA)- and H2O2-activated calcium channels from guard cells (27). In contrast to the fast gating of guard cell channels (31) the mesophyll channel is characterized by its slower activation kinetics (Fig. 1a). Based on its biophysical fingerprint, the channel identified in mesophyll protoplasts can be classified as a hyperpolarization-activated, calcium-permeable cation channel.
Blue-Light Activation of Cation Currents.
When mesophyll cell protoplasts were kept in darkness before the experiments and illuminated with dim light during the process of patch-pipette attachment, no pronounced channel activity could be recorded in the cell-attached configuration (Fig. 3a, 0 min). After the onset of blue-light illumination, a time-dependent increase of inward currents could be monitored (Fig. 3a). In the small membrane patch under investigation, initially single-channel fluctuations appeared, which increased in number to finally generate a macroscopic current (Fig. 3 a and b). Between 11–16 min, the activation process tended to saturate. In this mode of the patch-clamp technique the biochemical machinery of the cytoplasm remains intact, allowing even a photosynthetic electron transport similar to that in intact leaves (32). In excised outside-out patches, most likely due to the loss of transduction elements, blue light did not stimulate voltage-dependent currents (data not shown; n = 3). Analysis of the blue-light quantities required for activation of cation currents revealed a fluence rate-dependence (Fig. 3c). It should be noted that the protoplast isolation procedure may have caused a reduction in light sensitivity (33). In this context, it has been shown that, compared with isolated protoplasts, guard cells in intact leaves are characterized by a higher blue-light sensitivity (34).
Insensitivity to 3-(3,4-Dichlorophenyl)-1,1-Dimethylurea (DCMU).
To test whether channel activation involved photosynthesis or followed blue-light perception by photoreceptors (6, 35), protoplasts were illuminated with red light. In these experiments, fluence rates of 125 (data not shown) and 300 μmol·m−2·s−1 (Fig. 5) failed to induce cation currents. Furthermore, blue light was able to activate this channel type even in the presence of 100 μM DCMU, an inhibitor of photosynthetic electron transport (Fig. 4a). Thus, the blue-light response does not depend on an active photosystem II. This blue-light-activated cation channel was also clearly distinguishable from light-activated and DCMU-sensitive anion channels in this cell type (36). Furthermore, blue-light activation was not sensitive to the anion channel blocker 4,4′-diisothiocyano-2,2′-disulfonic stilbene (DIDS; Fig. 4b). Voltage-dependence, La3+ sensitivity, and single-channel conductance (open circles in Fig. 2c) of the blue-light-dependent cation channels recorded in the cell-attached configuration correspond with the properties of calcium-permeable channels recorded in light-adapted mesophyll cells (Fig. 1a) and calcium-permeable channels from other cell types (27–30, 37). Although blue light triggers proton extrusion in guard cells (38), activation of Ca2+-dependent chloride channels in hypocotyl cells (39), as well as K+ channels in motor cells (40), in mesophyll cells calcium-permeable channels seem to represent targets of blue-light signaling. Because hypocotyl chloride channels are activated via cryptochromes, and blue-light-dependent pumping of the H+-ATPase very likely relies on phototropins (16), we further analyzed the mechanism and responsible photoreceptors for activation of cation channels in mesophyll cells.
Figure 4.
Sensitivity of blue-light-mediated cation currents toward inhibitors. Shown is the current response of representative cell-attached patches to voltage ramps as shown in Fig. 3a before and 12–15 min after blue-light illumination, in the presence of 100 μM 3-(3,4-dichlorophenyl)-1,1-dimethylurea (DCMU; a) and 200 μM 4,4′-diisothiocyano-2,2′-disulfonic stilbene (DIDS; b). In contrast, preincubation with 200 nM K252a (c) inhibited channel activation. Experiments with similar results as shown in a–c were repeated at least four times.
Phototropin and Cryptochrome Mutants.
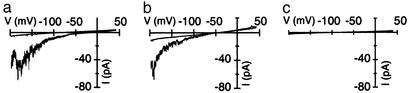
When mesophyll protoplasts were incubated with 200 nM of the protein kinase inhibitor K252a, blue-light-dependent inward currents were suppressed (Fig. 4c). It was therefore tempting to speculate that a blue-light receptor-based signaling cascade triggers the plasma membrane calcium-permeable channel. To provide direct evidence for this hypothesis, we compared blue-light-dependent channel activities in mesophyll protoplasts derived from Arabidopsis wild type and phototropin mutants (Fig. 5). In the phototropin mutant phot1-5, which develops chloroplasts with a reduced accumulation response and lacks the phototropic curvature (14, 15), blue-light-induced calcium currents were significantly smaller than in wild-type cells. In a next step, we performed similar experiments on the double mutant phot1 phot2, which is impaired in accumulation and avoidance response of chloroplasts, phototropism, blue-light-dependent stomatal movement, and leaf expansion, as well as rapid inhibition of stem growth (11, 12, 16, 41). In line with the hypothesis of phototropin-controlled calcium channels, blue light failed to activate voltage-dependent cation currents in the double mutant phot1-5 phot2-1 (Fig. 5).
Studies have shown that cryptochromes, representing the second class of blue-light receptors in plants, do not play a major role in blue-light-induced cytosolic Ca2+ signals in Arabidopsis seedlings (24). It should be noted, however, that the activity of cryptochromes is at least partially dependent on the calcium-binding protein SUB1, which seems to be associated with the nuclear envelope and/or endoplasmic reticular membranes (42). In mesophyll cells of the cryptochrome double mutant cry1-304 cry2-1 (43), cation channels were still able to activate in response to blue-light treatment (Fig. 5). Therefore we propose that in agreement with the overlapping functions of phot1 and phot2 in phototropism, chloroplast movement, and stomatal function (3, 11, 16), both photokinase-dependent pathways converge to generate a cytoplasmic calcium signal via activation of voltage-dependent cation channels. Thus we named this plasma membrane Ca2+ gate phototropin-activated calcium-permeable channel (PACC).
Discussion
We have shown that blue light triggers the opening of calcium-permeable channels. The activation mechanism was independent of photosynthesis and cryptochromes but involved the action of light receptor kinases. In mesophyll cells this phototropin-activated calcium-permeable channel, PACC, represents a likely candidate to mediate calcium increases, which accompany chloroplast movements. Although the blue-light sensitivity of PACC in isolated mesophyll protoplasts is reduced compared with the chloroplast movement in intact leaves, the protein kinase inhibitor K252a inhibits both processes (data not shown). Whether and how PACC could be involved in either the movement control, which relies on the cytoskeleton (44–46), or within the signal transduction from the photoreceptor to the “motor unit,” however, has to be elucidated. In addition, phototropins trigger blue-light-dependent stomatal opening as well as phototropism and are involved in the growth inhibition of hypocotyls (3), raising the question as to whether PAC-like channels play a role in the blue-light response of these cell types, too.
Blue-light activation of PACC could be measured only in cell-attached patches, showing that this process required an intact cytoplasm. Theoretically, the activation mechanism could involve a membrane hyperpolarization, which in turn activates voltage-dependent PACCs. In mesophyll cells, blue light induces a transient depolarization rather than the hyperpolarization observed in guard cells, rendering this hypothesis unlikely (M. R. G. Roelfsema and R.H., unpublished results). Because phototropins are associated with the plasma membrane by an as yet unknown mechanism (47) and seem evenly distributed within the membrane of mesophyll cells (41), blue-light stimulation of phototropins might translate into PACC activity via a cytosolic or membrane-delimited signal transduction cascade rather than a change in membrane potential only.
PAC-Like Channels in Guard Cells.
Hyperpolarization-activated calcium channels have previously been recorded in guard cells. These channels activate on treatment with the wilting hormone ABA, indicating that the guard cell channel plays a role in ABA-induced stomatal closure (27, 28). In the present study on mesophyll cells, we did not observe an increase in cation currents within 30 min after application of 50 μM ABA (data not shown; n = 8). Furthermore, 12.5 and 50 μM diphenylene iodonium (DPI), which in guard cells effectively blocks ABA induction of cation channels (28), had no effect on blue-light stimulation of PACC in mesophyll cells (preincubation up to 3 h, n = 6). It thus remains to be determined whether the same hyperpolarization-activated calcium-channel type is involved in cell type-specific signaling, or whether blue-light-activated PACCs and ABA-dependent channels represent different members of a cation channel family in Arabidopsis.
PAC-Like Channels in Hypocotyls.
Surface potential measurements on hypocotyls of Arabidopsis revealed a biphasic response of the membrane potential to blue-light treatment. A rapid and transient depolarization is followed by a second, slower depolarization, which starts after ≈2 min of illumination (figure 1 in ref. 12). Interestingly, phot1 and phot1 cry1 lack the second depolarization phase and show a reduced fast response. In contrast, cry1cry2 plants are impaired only in the fast response. Thus, phototropins as well as cryptochromes are likely to control fast changes in the membrane potential. In this context, cryptochromes have been shown to cause fast membrane depolarizations via activation of anion-selective channels (12, 39, 48). In contrast, the slow depolarization phase appears to rely only on phototropins (figure 1 in ref. 12). Measurements of Ca2+ changes in hypocotyls showed that blue light induced a Ca2+ signal, which peaked around 3–12 min (23). Further support for the involvement of Ca2+ signals in the phototropin-controlled growth of hypocotyls was provided by the observation that most of the Ca2+ signal is eliminated in phot1 (23). Similar to the present results obtained on mesophyll cells, hypocotyls in phot1 phot2 were characterized by a complete loss of blue-light activation. It is therefore tempting to speculate that PAC-like channels present in this tissue are involved in phototropin-dependent calcium signals, slow membrane depolarizations, and growth inhibition.
Compared with the light quantities required to induce phototropin-dependent chloroplast movement, phototropism, or stomatal movement in intact tissues, PACC in isolated mesophyll protoplasts exhibits a significantly lower blue-light sensitivity. Whether this discrepancy is a result of the experimental conditions or protoplast isolation procedure, or points to a distinct role of PACC in high-light responses, awaits studies of PACC in intact tissue and the availability of PACC mutants.
The blue-light-induced calcium response in mesophyll cells originates from calcium influx across the plasma membrane. Because cryptochrome-deficient Arabidopsis plants are not impaired in blue-light stimulation of cation currents in mesophyll cells and in the generation of cytoplasmic calcium signals in seedlings (24), this class of blue-light receptors thus far does not seem to play an important role in generation of blue-light-dependent cytosolic calcium signals via plasma membrane calcium channels. Evidence is accumulating that blue-light-induced Ca2+ signals are involved in photomovements and growth inhibition triggered by phototropins. Unraveling the responsible genes and their cell type-specific roles is therefore one of the future challenges in the research on blue-light signaling in plants.
Acknowledgments
We thank W. Briggs, M. R. G. Roelfsema, and B. Lacombe for helpful comments on the manuscript. This work was supported by grants from the Ministry of Education, Culture, Sports, Science, and Technology (to M.W.), Precursory Research for Embryonic Science and Technology (to T.K.), and Deutsche Forschungsgemeinschaft (to R.H. and P.D.).
Abbreviations
- ABA
abscisic acid
- PACC
phototropin-activated calcium-permeable channel
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Darwin C, Darwin F. The Power of Movement in Plants. New York: Appleton; 1881. [Google Scholar]
- 2.Briggs W R, Huala E. Annu Rev Cell Dev Biol. 1999;15:33–62. doi: 10.1146/annurev.cellbio.15.1.33. [DOI] [PubMed] [Google Scholar]
- 3.Briggs W R, Christie J M. Trends Plant Sci. 2002;7:204–210. doi: 10.1016/s1360-1385(02)02245-8. [DOI] [PubMed] [Google Scholar]
- 4.Lin C. Trends Plant Sci. 2000;5:337–342. doi: 10.1016/s1360-1385(00)01687-3. [DOI] [PubMed] [Google Scholar]
- 5. Lin, C. (2002) Sci. STKE ( http://stke.sciencemag.org/cgi/content/full/sigtrans;2002/118/pe5). [DOI] [PubMed]
- 6.Cashmore A R, Jarillo J A, Wu Y J, Liu D. Science. 1999;284:760–765. doi: 10.1126/science.284.5415.760. [DOI] [PubMed] [Google Scholar]
- 7.Huala E, Oeller P W, Liscum E, Han I S, Larsen E, Briggs W R. Science. 1997;278:2120–2123. doi: 10.1126/science.278.5346.2120. [DOI] [PubMed] [Google Scholar]
- 8.Jarillo J A, Ahmad M, Cashmore A R. Plant Physiol. 1998;117:719. [Google Scholar]
- 9.Swartz T E, Corchnoy S B, Christie J M, Lewis J W, Szundi I, Briggs W R, Bogomolni R A. J Biol Chem. 2001;276:36493–36500. doi: 10.1074/jbc.M103114200. [DOI] [PubMed] [Google Scholar]
- 10.Christie J M, Reymond P, Powell G K, Bernasconi P, Raibekas A A, Liscum E, Briggs W R. Science. 1998;282:1698–1701. doi: 10.1126/science.282.5394.1698. [DOI] [PubMed] [Google Scholar]
- 11.Sakai T, Kagawa T, Kasahara M, Swartz T E, Christie J M, Briggs W R, Wada M, Okada K. Proc Natl Acad Sci USA. 2001;98:6969–6974. doi: 10.1073/pnas.101137598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folta K M, Spalding E P. Plant J. 2001;26:471–478. doi: 10.1046/j.1365-313x.2001.01038.x. [DOI] [PubMed] [Google Scholar]
- 13.Briggs W R, Olney M A. Plant Physiol. 2001;125:85–88. doi: 10.1104/pp.125.1.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liscum E, Briggs W R. Plant Cell. 1995;7:473–485. doi: 10.1105/tpc.7.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kagawa T, Wada M. Plant Cell Physiol. 2000;41:84–93. doi: 10.1093/pcp/41.1.84. [DOI] [PubMed] [Google Scholar]
- 16.Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K-I. Nature. 2001;414:656–660. doi: 10.1038/414656a. [DOI] [PubMed] [Google Scholar]
- 17.Kagawa T, Sakai T, Suetsugu N, Oikawa K, Ishiguro S, Kato T, Tabata S, Okada K, Wada M. Science. 2001;291:2138–2141. doi: 10.1126/science.291.5511.2138. [DOI] [PubMed] [Google Scholar]
- 18.Jarillo J A, Gabrys H, Capel J, Alonso J M, Ecker J R, Cashmore A R. Nature. 2001;410:952–954. doi: 10.1038/35073622. [DOI] [PubMed] [Google Scholar]
- 19.Kagawa T, Wada M. Plant Cell Physiol. 2002;43:367–371. doi: 10.1093/pcp/pcf049. [DOI] [PubMed] [Google Scholar]
- 20.Schroeder J I, Allen G J, Hugouvieux V, Kwak J M, Waner D. Annu Rev Plant Physiol Plant Mol Biol. 2001;52:627–658. doi: 10.1146/annurev.arplant.52.1.627. [DOI] [PubMed] [Google Scholar]
- 21.Shimazaki K, Goh C-H, Kinoshita T. Physiol Plant. 1999;105:554–561. [Google Scholar]
- 22.Tlalka M, Fricker M. Plant J. 1999;20:461–473. doi: 10.1046/j.1365-313x.1999.00621.x. [DOI] [PubMed] [Google Scholar]
- 23.Babourina O, Newman I, Shabala S. Proc Natl Acad Sci USA. 2002;99:2433–2438. doi: 10.1073/pnas.042294599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Baum G, Long J C, Jenkins G I, Trewavas A J. Proc Natl Acad Sci USA. 1999;96:13554–13559. doi: 10.1073/pnas.96.23.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sando S, Goto N. Plant Cell Rep. 1994;14:75–80. doi: 10.1007/BF00233765. [DOI] [PubMed] [Google Scholar]
- 26.Szyroki A, Ivashikina N, Dietrich P, Roelfsema M R, Ache P, Reintanz B, Deeken R, Godde M, Felle H, Steinmeyer R, et al. Proc Natl Acad Sci USA. 2001;98:2917–2921. doi: 10.1073/pnas.051616698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamilton D W, Hills A, Köhler B, Blatt M R. Proc Natl Acad Sci USA. 2000;97:4967–4972. doi: 10.1073/pnas.080068897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pei Z M, Murata Y, Benning G, Thomine S, Klusener B, Allen G J, Grill E, Schroeder J I. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- 29.Gelli A, Blumwald E. J Membr Biol. 1997;155:35–45. doi: 10.1007/s002329900156. [DOI] [PubMed] [Google Scholar]
- 30.Véry A A, Davies J M. Proc Natl Acad Sci USA. 2000;97:9801–9806. doi: 10.1073/pnas.160250397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klüsener B, Young J J, Murata Y, Allen G J, Mori I C, Hugouvieux V, Schroeder J I. Plant Physiol. 2002;130:1–12. doi: 10.1104/pp.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goh C-H, Dietrich P, Steinmeyer R, Schreiber U, Nam H-G, Hedrich R. Plant J. 2002;32:1–8. doi: 10.1046/j.1365-313x.2002.01451.x. [DOI] [PubMed] [Google Scholar]
- 33.Augustynowicz J, Lekka M, Burda K, Gabrys H. Acta Physiol Plant. 2001;23:291–302. [Google Scholar]
- 34.Roelfsema M R, Steinmeyer R, Staal M, Hedrich R. Plant J. 2001;26:1–13. doi: 10.1046/j.1365-313x.2001.01000.x. [DOI] [PubMed] [Google Scholar]
- 35.Briggs W R, Beck C F, Cashmore A R, Christie J M, Hughes J, Jarillo J A, Kagawa T, Kanegae H, Liscum E, Nagatani A, et al. Plant Cell. 2001;13:993–997. doi: 10.1105/tpc.13.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Elzenga J-T-M, Van Volkenburgh E. Plant Physiol. 1997;113:1419–1426. doi: 10.1104/pp.113.4.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kiegle E, Gilliham M, Haseloff J, Tester M. Plant J. 2000;21:225–229. doi: 10.1046/j.1365-313x.2000.00659.x. [DOI] [PubMed] [Google Scholar]
- 38.Assmann S M, Simoncini L, Schroeder J I. Nature. 1985;318:285–287. [Google Scholar]
- 39.Cho M H, Spalding E P. Proc Natl Acad Sci USA. 1996;93:8134–8138. doi: 10.1073/pnas.93.15.8134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Suh S, Moran N, Lee Y. Plant Physiol. 2000;123:833–843. doi: 10.1104/pp.123.3.833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sakamoto K, Briggs W R. Plant Cell. 2002;14:1723–1735. doi: 10.1105/tpc.003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo H, Mockler T, Duong H, Lin C. Science. 2001;291:487–490. doi: 10.1126/science.291.5503.487. [DOI] [PubMed] [Google Scholar]
- 43.Mockler T C, Guo H, Yang H, Duong H, Lin C. Development (Cambridge, UK) 1999;126:2073–2082. doi: 10.1242/dev.126.10.2073. [DOI] [PubMed] [Google Scholar]
- 44.Tlalka M, Gabrys H. Planta. 1993;189:491–498. [Google Scholar]
- 45. Kadota, A. & Wada, M. (1992) Bot. Mag. Tokyo105.
- 46.Sato Y, Wada M, Kadota A. J Cell Sci. 2001;114:269–279. doi: 10.1242/jcs.114.2.269. [DOI] [PubMed] [Google Scholar]
- 47.Short T W, Briggs W R. Annu Rev Plant Physiol Plant Mol Biol. 1994;45:143–171. [Google Scholar]
- 48.Parks B M, Folta K M, Spalding E P. Curr Opin Plant Biol. 2001;4:436–440. doi: 10.1016/s1369-5266(00)00197-7. [DOI] [PubMed] [Google Scholar]