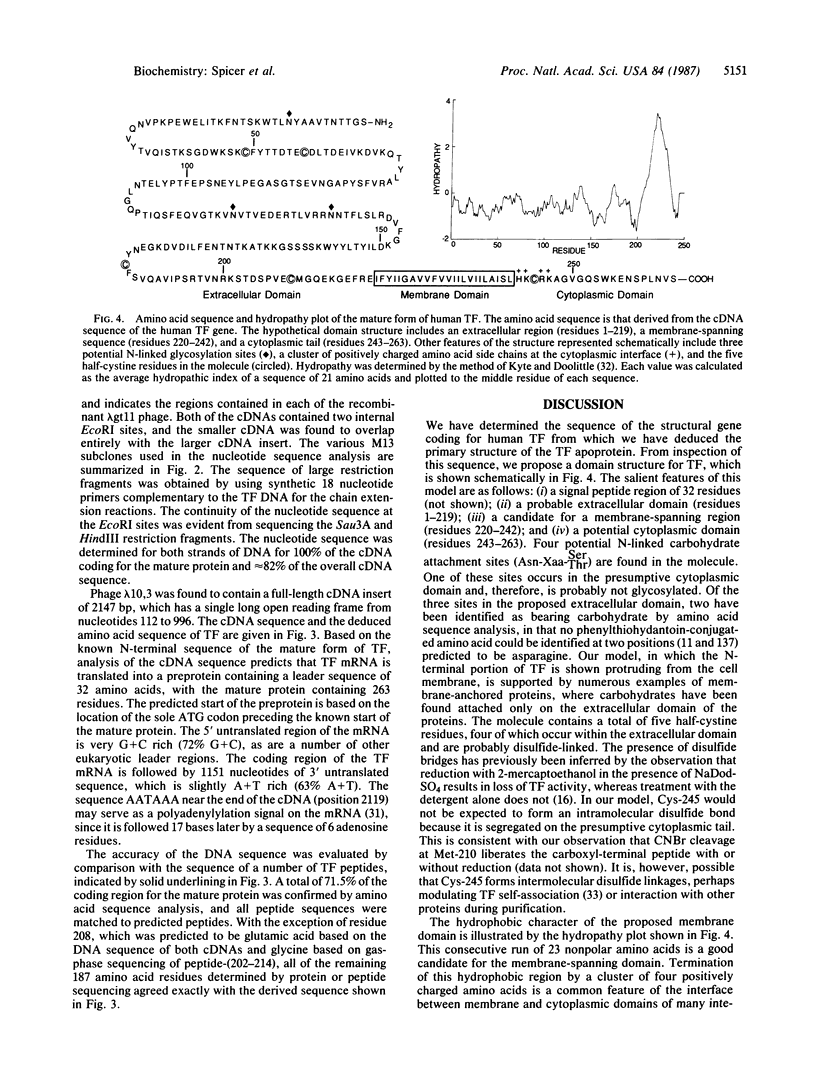
Abstract
Tissue factor is a membrane-bound procoagulant protein that activates the extrinsic pathway of blood coagulation in the presence of factor VII and calcium. lambda Phage containing the tissue factor gene were isolated from a human placental cDNA library. The amino acid sequence deduced from the nucleotide sequence of the cDNAs indicates that tissue factor is synthesized as a higher molecular weight precursor with a leader sequence of 32 amino acids, while the mature protein is a single polypeptide chain composed of 263 residues. The derived primary structure of tissue factor has been confirmed by comparison to protein and peptide sequence data. The sequence of the mature protein suggests that there are three distinct domains: extracellular, residues 1-219; hydrophobic, residues 220-242; and cytoplasmic, residues 243-263. Three potential N-linked carbohydrate attachment sites occur in the extracellular domain. The amino acid sequence of tissue factor shows no significant homology with the vitamin K-dependent serine proteases, coagulation cofactors, or any other protein in the National Biomedical Research Foundation sequence data bank (Washington, DC).
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bach R., Gentry R., Nemerson Y. Factor VII binding to tissue factor in reconstituted phospholipid vesicles: induction of cooperativity by phosphatidylserine. Biochemistry. 1986 Jul 15;25(14):4007–4020. doi: 10.1021/bi00362a005. [DOI] [PubMed] [Google Scholar]
- Bach R., Nemerson Y., Konigsberg W. Purification and characterization of bovine tissue factor. J Biol Chem. 1981 Aug 25;256(16):8324–8331. [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Birnstiel M. L., Busslinger M., Strub K. Transcription termination and 3' processing: the end is in site! Cell. 1985 Jun;41(2):349–359. doi: 10.1016/s0092-8674(85)80007-6. [DOI] [PubMed] [Google Scholar]
- Broze G. J., Jr, Leykam J. E., Schwartz B. D., Miletich J. P. Purification of human brain tissue factor. J Biol Chem. 1985 Sep 15;260(20):10917–10920. [PubMed] [Google Scholar]
- Carson S. D. Tissue factor-initiated blood coagulation. Prog Clin Pathol. 1984;9:1–14. [PubMed] [Google Scholar]
- Degen S. J., MacGillivray R. T., Davie E. W. Characterization of the complementary deoxyribonucleic acid and gene coding for human prothrombin. Biochemistry. 1983 Apr 26;22(9):2087–2097. doi: 10.1021/bi00278a008. [DOI] [PubMed] [Google Scholar]
- Edge A. S., Faltynek C. R., Hof L., Reichert L. E., Jr, Weber P. Deglycosylation of glycoproteins by trifluoromethanesulfonic acid. Anal Biochem. 1981 Nov 15;118(1):131–137. doi: 10.1016/0003-2697(81)90168-8. [DOI] [PubMed] [Google Scholar]
- Gitschier J., Wood W. I., Goralka T. M., Wion K. L., Chen E. Y., Eaton D. H., Vehar G. A., Capon D. J., Lawn R. M. Characterization of the human factor VIII gene. Nature. 1984 Nov 22;312(5992):326–330. doi: 10.1038/312326a0. [DOI] [PubMed] [Google Scholar]
- Guha A., Bach R., Konigsberg W., Nemerson Y. Affinity purification of human tissue factor: interaction of factor VII and tissue factor in detergent micelles. Proc Natl Acad Sci U S A. 1986 Jan;83(2):299–302. doi: 10.1073/pnas.83.2.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagen F. S., Gray C. L., O'Hara P., Grant F. J., Saari G. C., Woodbury R. G., Hart C. E., Insley M., Kisiel W., Kurachi K. Characterization of a cDNA coding for human factor VII. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2412–2416. doi: 10.1073/pnas.83.8.2412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackman R. W., Beeler D. L., VanDeWater L., Rosenberg R. D. Characterization of a thrombomodulin cDNA reveals structural similarity to the low density lipoprotein receptor. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8834–8838. doi: 10.1073/pnas.83.23.8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane W. H., Davie E. W. Cloning of a cDNA coding for human factor V, a blood coagulation factor homologous to factor VIII and ceruloplasmin. Proc Natl Acad Sci U S A. 1986 Sep;83(18):6800–6804. doi: 10.1073/pnas.83.18.6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaul R. K., Hildebrand B., Roberts S., Jagadeeswaran P. Isolation and characterization of human blood-coagulation factor X cDNA. Gene. 1986;41(2-3):311–314. doi: 10.1016/0378-1119(86)90112-5. [DOI] [PubMed] [Google Scholar]
- Kurachi K., Davie E. W. Isolation and characterization of a cDNA coding for human factor IX. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6461–6464. doi: 10.1073/pnas.79.21.6461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kyte J., Doolittle R. F. A simple method for displaying the hydropathic character of a protein. J Mol Biol. 1982 May 5;157(1):105–132. doi: 10.1016/0022-2836(82)90515-0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lathe R. Synthetic oligonucleotide probes deduced from amino acid sequence data. Theoretical and practical considerations. J Mol Biol. 1985 May 5;183(1):1–12. doi: 10.1016/0022-2836(85)90276-1. [DOI] [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Marshall R. D. The nature and metabolism of the carbohydrate-peptide linkages of glycoproteins. Biochem Soc Symp. 1974;(40):17–26. [PubMed] [Google Scholar]
- Merrill B. M., Williams K. R., Chase J. W., Konigsberg W. H. Photochemical cross-linking of the Escherichia coli single-stranded DNA-binding protein to oligodeoxynucleotides. Identification of phenylalanine 60 as the site of cross-linking. J Biol Chem. 1984 Sep 10;259(17):10850–10856. [PubMed] [Google Scholar]
- Messing J. New M13 vectors for cloning. Methods Enzymol. 1983;101:20–78. doi: 10.1016/0076-6879(83)01005-8. [DOI] [PubMed] [Google Scholar]
- Nemerson Y., Furie B. Zymogens and cofactors of blood coagulation. CRC Crit Rev Biochem. 1980;9(1):45–85. doi: 10.3109/10409238009105472. [DOI] [PubMed] [Google Scholar]
- Osterud B., Rapaport S. I. Activation of factor IX by the reaction product of tissue factor and factor VII: additional pathway for initiating blood coagulation. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5260–5264. doi: 10.1073/pnas.74.12.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragni M. V., Lewis J. H., Spero J. A., Hasiba U. Factor VII deficiency. Am J Hematol. 1981;10(1):79–88. doi: 10.1002/ajh.2830100112. [DOI] [PubMed] [Google Scholar]
- Sabatini D. D., Kreibich G., Morimoto T., Adesnik M. Mechanisms for the incorporation of proteins in membranes and organelles. J Cell Biol. 1982 Jan;92(1):1–22. doi: 10.1083/jcb.92.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenflo J., Fernlund P., Egan W., Roepstorff P. Vitamin K dependent modifications of glutamic acid residues in prothrombin. Proc Natl Acad Sci U S A. 1974 Jul;71(7):2730–2733. doi: 10.1073/pnas.71.7.2730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steube K., Gross V., Heinrich P. C. Deglycosylation of alpha 1-proteinase inhibitor by endo-beta-N-acetylglucosaminidase F. Biochemistry. 1985 Sep 24;24(20):5587–5592. doi: 10.1021/bi00341a045. [DOI] [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Toole J. J., Knopf J. L., Wozney J. M., Sultzman L. A., Buecker J. L., Pittman D. D., Kaufman R. J., Brown E., Shoemaker C., Orr E. C. Molecular cloning of a cDNA encoding human antihaemophilic factor. Nature. 1984 Nov 22;312(5992):342–347. doi: 10.1038/312342a0. [DOI] [PubMed] [Google Scholar]
- Triplett D. A., Brandt J. T., Batard M. A., Dixon J. L., Fair D. S. Hereditary factor VII deficiency: heterogeneity defined by combined functional and immunochemical analysis. Blood. 1985 Dec;66(6):1284–1287. [PubMed] [Google Scholar]
- Yamamoto T., Davis C. G., Brown M. S., Schneider W. J., Casey M. L., Goldstein J. L., Russell D. W. The human LDL receptor: a cysteine-rich protein with multiple Alu sequences in its mRNA. Cell. 1984 Nov;39(1):27–38. doi: 10.1016/0092-8674(84)90188-0. [DOI] [PubMed] [Google Scholar]
- Young R. A., Davis R. W. Yeast RNA polymerase II genes: isolation with antibody probes. Science. 1983 Nov 18;222(4625):778–782. doi: 10.1126/science.6356359. [DOI] [PubMed] [Google Scholar]
- Zur M., Radcliffe R. D., Oberdick J., Nemerson Y. The dual role of factor VII in blood coagulation. Initiation and inhibition of a proteolytic system by a zymogen. J Biol Chem. 1982 May 25;257(10):5623–5631. [PubMed] [Google Scholar]


