Abstract
Myocardin (Mycd), which is essential for the differentiation of the smooth muscle cell lineage, is constitutively located in the nucleus, although its family members, myocardin-related transcription factors A and B (MRTF-A/B), mostly reside in the cytoplasm and translocate to the nucleus in response to Rho signaling. The mechanism for their nuclear import is unclear. Here we investigated the mechanism for the nuclear import of Mycd family members and demonstrated any correlation between such mechanism and the phenotype of vascular smooth muscle cells (VSMCs). In cultured VSMCs, the knockdown of importin β1 inhibited the nuclear import of Mycd and MRTF-A/B. Their NH2-terminal basic domain was identified as a binding site for importin α/β1 by in vitro analyses. However, Mycd had a higher affinity for importin α/β1 than did MRTF-A/B, even in the absence of G-actin, and Mycd affinity for importin α1/β1 was stronger than for any other importin α/β1 heterodimers. The binding of Mycd to importin α/β1 was insensitive to G-actin, whereas that of MRTF-A/B was differently inhibited by G-actin. In dedifferentiated VSMCs, the levels of importins α1 and β1 were reduced concomitant with down-regulation of Mycd, serum response factor, and smooth muscle cell markers. By contrast, in differentiated VSMCs, their expressions were up-regulated. Thus, the nuclear import of Mycd family members in VSMCs depends on importin α/β1, and their relative affinities for importin α/β1 heterodimers determine Mycd nuclear import. The expression of Mycd nuclear import machineries is related to the expression levels of VSMC phenotype-dependent smooth muscle cell markers.
Keywords: Actin, Cell Differentiation, Nuclear Transport, Smooth Muscle, Transcription Coactivators, MRTF-A/B, Importin α/β1, Myocardin, Nuclear Import, Vascular Smooth Muscle Cells
Introduction
The myocardin (Mycd)3 family includes Mycd, myocardin-related transcription factor A (MRTF-A/MAL/MKL1), and MRTF-B (MRTF-B/MAL16/MKL2). The expression of Mycd is restricted to cardiac and smooth muscles (1), whereas MRTF-A/B are ubiquitously expressed (2–4). All the family members bind to serum response factor (SRF) and function as its specific coactivator (4, 5). Mycd is suggested to play key roles in the differentiation of the cardiac and smooth muscle cell lineages. In support of this idea, Mycd knock-out mice die by embryonic day 10.5 (E10.5) with a lack of vascular smooth muscle cells (VSMCs) (6), and the ectopic expression of Mycd can induce the SMC differentiation program in a mesenchymal cell line, such as 10T1/2 cells (7). In contrast, SMC gene expression is abrogated by the suppression of Mycd function (8, 9). Thus, Mycd is critically required for VSMC differentiation.
Mycd appears to be constitutively located in the nucleus (1), whereas MRTF-A/B mainly reside in the cytoplasm and transiently translocate to the nucleus in response to Rho signaling (4, 10, 11). Although the Mycd family members contain an RPEL motif that acts as a G-actin binding site, MRTF-A/B RPEL motifs have a much higher affinity for G-actin than does Mycd (12). This relative affinity for G-actin is closely related to the subcellular localization of the Mycd family members (12–15). For example, in serum-starved cells, the steady-state cytoplasmic localization of MRTF-A depends on the activation of its Crm1-mediated nuclear export through an increase in the G-actin pool. When the G-actin pool is reduced by Rho signaling, the Crm1-dependent nuclear export of MRTF-A is suppressed, and MRTF-A is transiently retained in the nucleus (14). However, the nuclear accumulation of Mycd is not affected by actin dynamics in VSMCs or other heterologous cells. Recently, Guettler et al. (12) published a model for such actin dynamics-dependent regulation of the MRTF-A nucleocytoplasmic shuttling. The basic assumption of this model is as follows; the degrees of nuclear import of MRTF-A and Mycd are identical under the G-actin-depleted conditions, and actin binding to MRTF-A interferes its nuclear import at high G-actin concentrations. However, they have not yet identified the nuclear import machinery. Although nuclear localization (import) signals (NLSs) consisting of basic amino acid clusters have been identified in Mycd (1) and MRTF-A (13), neither their nuclear import machinery nor any correlation between such machinery and the VSMC phenotype has been demonstrated.
In this study we investigated the nuclear import machineries of Mycd in the context of examining Mycd function in determining the VSMC phenotype. We found that the nuclear import of Mycd was mediated by the importin α1/β1 heterodimer in VSMCs. The expression of these nuclear import machineries was closely associated with the VSMC phenotype-dependent expression levels of Mycd, SRF, and SMC markers. Although the nuclear import of MRTF-A/B could also be mediated by the importin α/β1 heterodimers, their affinities for importin α/β1 were much lower than that of Mycd even under the G-actin-depleted conditions, and their expression levels did not correlate with VSMC phenotypes. Furthermore, actin dynamics did not affect the interaction between Mycd and the importin α1/β1 heterodimer or Mycd nuclear localization, but high concentrations of G-actin significantly suppressed the interaction between MRTF-A/B and importin α1/β1 and affected MRTF-A/B nuclear import. These findings suggest that the varying degrees of nuclear import among Mycd family members in VSMCs depend on their relative affinities for importin α/β1 heterodimers and that actin dynamics play an adjunctive role in the nuclear import of MRTF-A/B.
EXPERIMENTAL PROCEDURES
Reagents and Antibodies
Leptomycin B (LMB) and MG-132 were purchased from Calbiochem. Commercially available primary antibodies were as follows: anti-FLAG M2-agarose, anti-FLAG (F7425), anti-β-actin (A5441), and anti-α-tubulin (DM 1A) antibodies (Sigma); anti-hemagglutinin (HA) affinity matrix and anti-HA (3F10) antibody (Roche Applied Science); anti-importins α1 and β1, anti-GAPDH, anti-histone H2B anti-SRF antibodies (Santa Cruz Biotechnology). Antibodies against Mycd family members and SMC markers were as follows. The anti-CaD antibody was prepared in our laboratory (16). The anti-smooth muscle α-actin actin (1A4) antibody was purchased from Sigma. The anti-MHC-SM2 (1G12) antibody and anti-SM22α (10H12) antibodies were purchased from Yamasa and Novocastra Laboratories, respectively. The antibodies against Mycd, MRTF-A, and MRTF-B were prepared in our laboratory (17, 18).
Plasmids
The cDNAs of mouse full-length Mycd (accession number AF384055), MRTF-A (accession number AK044188.1), MRTF-B (accession number AF532598), rat importins α1 (accession number NM_198726), α2 (accession number AJ130946), and α4 (accession number NM_001014793), rat importin β1 (accession number NM_017063), and human β-actin (accession number NM_001101) were amplified by RT-PCR and inserted into a mammalian expression plasmid, pCS2+ (19), with the indicated tag. Mutant Mycds (8) and β-actin (β-actin R62D) were constructed by PCR-mediated mutagenesis. The enhanced green fluorescent protein (EGFP) cDNA fused with the classical NLSs (cNLS) of simian virus 40 (SV40) T antigen (20) at the COOH terminus (EGFP-cNLS) was inserted into a mammalian expression plasmid, pCAGGS (accession number DD029702) with the FLAG tag. The sequences of these constructs were confirmed.
VSMC Culture and Transfection
The abdominal aortae were quickly removed from anesthetized 6-week-old male Sprague-Dawley rats, and the medial VSMC layers were separated from the adventitia and vascular endothelium (21). The medial VSMC layers thus obtained were used as the source of proteins and mRNAs from medial VSMCs in vivo. For the experiments using cultured VSMCs, the VSMCs were isolated from the medial VSMC layers by the enzymatic dispersion method, as described elsewhere (22). These cells were cultured in Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum. In this study VSMCs passaged 2–6 times were used under the indicated culture conditions. Transfection of the indicated expression plasmids was performed using Trans IT-LT1 (Pan Vera Corp.) under 10% serum-stimulated conditions according to the manufacturer's instructions.
Immunocytochemistry
Cells were fixed with 4% formaldehyde for 30 min, permeabilized, and blocked with 0.1% Triton X-100 and 0.2% bovine serum albumin in phosphate-buffered saline for 1 h at room temperature. The cells were then incubated with the indicated primary antibodies for 1 h followed by the indicated secondary antibodies with Hoechst 33258 for 1 h at room temperature. Fluorescent images were collected using a cooled CCD camera (Roper Scientific, Tucson, AZ) mounted on an Olympus IX-70 microscope with the appropriate filters and MetaMorph software.
Quantification of the Immunostaining Images
The expression patterns of Mycd derivatives and MRTF-A/B were categorized into three groups: nuclear-specific accumulation (N), even distribution in the nucleus and the cytoplasm (NC), defined as equivalent intensities of immunostaining of the target molecules in the cytoplasm and nucleus, and cytoplasmic localization (C). In each experiment (n = at least three independent experiments), 100–200 cells were analyzed. The proportion of FLAG-positive cells exhibiting the respective expression patterns (mean ± S.E.) is presented.
Knockdown of Mycd Family Members or Importin β1 Using Small Interfering RNA (siRNA)
VSMCs were transfected with each of the indicated siRNAs using Lipofectamine RNAiMAX (Invitrogen) and cultured under the indicated conditions for the respective analyses. The siRNAs against rat Mycd, MRTF-A, MRTF-B, and importin β1 were purchased from Sigma (catalogue numbers Rn_Myocd_8674 for Mycd siRNA, Rn_Mkl1_9379 for MRTF-A siRNA, Rn_RGD15608_0342 for MRTF-B siRNA, Rn_Kpnb1-1 for importin β1 siRNA1, and Rn_Kpnb1-2 for rat importin β1 siRNA2). A scrambled siRNA (Santa Cruz Biotechnology) was used in control experiments.
Quantitative Real-time RT-PCR
Total RNAs were isolated from indicated VSMCs, and respective cDNAs were synthesized using the High Capacity cDNA Archive kit (Applied Biosystems). The expression of the indicated mRNAs in VSMCs was analyzed by real-time PCR using SYBR GreenER qPCR SuperMix (Invitrogen). The levels of Mycd mRNA were normalized to the GAPDH mRNA expression. The primers used in these analyses were as follows: Mycd sense primer, GCAGTGAAACAGCAAATGACTCG; Mycd antisense primer, CATGAATGATCTTCCCTGGCATCC; CaD sense primer, ACAAGTCACCCGCTCCCAAG; CaD antisense primer, CCACGGATTGCTTTTCCCAGAG; MHC-SM2 sense primer, CAGAACAAGGAACTCCGAAGCAA; MHC-SM2 antisense primer, CAACTTGGTGGCTGCCTGTT; SM22α sense primer, AACTTGCTCAGAATCACGCCATT; SM22α antisense primer, GCTCCTCATCATACTTCTTCTCAATCT; smooth muscle α-actin actin sense primer, CAACTGGTATTGTGCTGGACTCTG; smooth muscle α-actin actin antisense primer, ACGGACGATCTCACGCTCAG; GAPDH sense primer, CGTGCCGCCTGGAGAAAC; GAPDH antisense primer, TGGGAGTTGCTGTTGAAGTCG.
Preparation of Cytoplasmic and Nuclear Fractions
The subcellular proteomes were extracted from cultured VSMCs using the ProteoExtract Subcellular Proteome Extraction kit (Calbiochem), according to the manufacturer's instructions. The thus-obtained cytoplasmic and nuclear fractions were analyzed by immunoblotting using the indicated antibodies. In this analysis, histone H2B and α-tubulin were used as markers of the nuclear and cytoplasmic fractions, respectively.
Protein-Protein Interaction Analyses
In vitro translation was performed using the TNT Quick Coupled Transcription/Translation Systems (Promega) according to the manufacturer's instructions. The indicated in vitro-translated proteins were first incubated with control gel for 1 h in immunoprecipitation buffer (20 mm Tris-HCl (pH 7.5), 0.5% Nonidet P-40, 150 mm NaCl, 1 mm EDTA, 50 mm NaF, 10 mm β-glycerophosphate, and proteinase inhibitors (complete Mini (Roche Applied Science)) to clear nonspecific interactions and then incubated with either a control gel or the indicated affinity gel for 6 h at 4 °C. Proteins in the immunoprecipitates were detected by immunoblotting using the indicated antibodies. Target proteins were detected with a SuperSignal chemiluminescence detection kit (Pierce).
RESULTS
Expression Profiles of Mycd Family Members in VSMCs
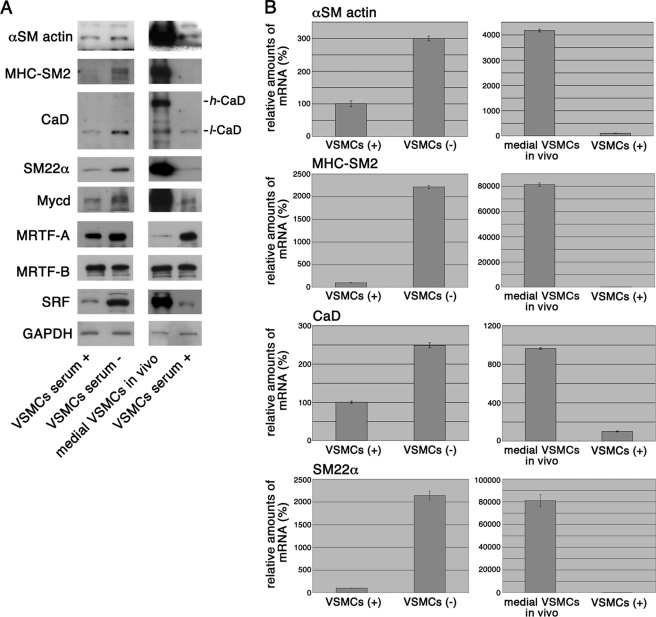
We investigated the expression levels of Mycd family members in cultured VSMCs under serum-stimulated (dedifferentiated) and 100% confluent/serum-starved (partially differentiated) conditions and medial VSMCs in vivo (differentiated VSMCs) (Fig. 1). In cultured VSMCs under 100% confluent/serum-starved conditions, the levels of Mycd, SRF, and the SMC markers myosin heavy chain (MHC)-SM2, low molecular weight caldesmon (l-CaD), smooth muscle α-actin, and SM22α increased significantly compared with the dedifferentiated VSMCs, but the differentiated VSMC-specific high molecular weight CaD (h-CaD) did not. The levels of all these markers, including h-CaD, were even higher in the medial VSMCs. The expression level of Mycd correlated well with the VSMC phenotype-dependent levels of SRF and the SMC markers, but the levels of MRTF-A/B did not necessarily coincide with the VSMC phenotype (Fig. 1A). That is, in cultured VSMCs, the expression levels of MRTF-A/B were not drastically changed by serum stimulation or serum starvation, although the amount of MRTF-A in the medial VSMCs was extremely low compared with that in medial VSMCs, in agreement with the reported non-engagement of MRTF-A upon VSMC differentiation (6). The changes in SMC marker levels were transcriptionally regulated (Fig. 1B), and their levels in cultured VSMCs fully depended on Mycd but not on MRTF-A/B (supplemental Fig. S1). The knockdown of endogenous Mycd severely decreased the expression levels of SMC markers in the VSMCs under serum-starved conditions, but the knockdown of MRTF-A/B did not have this effect. Under these conditions, MRTF-A/B mostly reside in the cytoplasm (4, 10, 11), in agreement with the above finding.
FIGURE 1.
VSMC phenotype-dependent expression of Mycd family members. The expression of Mycd family members, SRF, and the indicated SMC markers were analyzed by immunoblotting (A) and quantitative real-time RT-PCR (B). Whole-cell extracts and total RNAs were isolated from serum-stimulated VSMCs (serum+), 100% confluent VSMCs under serum-starved conditions (12 h) (serum−), and medial VSMCs in vivo. The expression levels of the indicated proteins or mRNAs were normalized to that of GAPDH protein or mRNA, and the levels of the indicated mRNAs in serum-stimulated VSMCs were set as 100% (mean ± S.E. of the results from three independent experiments).
The NH2-terminal Basic Domain (NB) Plays a Critical Role in the Nuclear Accumulation of Mycd
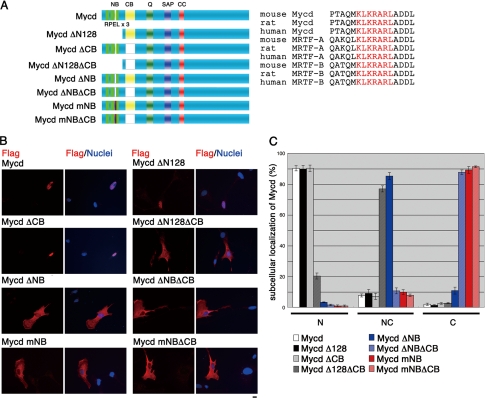
We then investigated the nuclear accumulation of Mycd in VSMCs. A previous study reported that a mutant Mycd (MycdΔN128) lacking the NH2-terminal region (residues 1–128), which contains three RPEL motifs, is predominantly localized to the nucleus and that its basic domain (B1) (13), which corresponds to the central basic domain (CB), plays a critical role in its nuclear accumulation (1). To verify the functional NLS in the Mycd molecule, we expressed a series of Mycd mutants in cultured VSMCs and analyzed their subcellular localization (Fig. 2). In the vast majority of cells expressing full-length Mycd (wild-type Mycd), MycdΔCB, or MycdΔN128, the protein was primarily observed in the nucleus (90.3 ± 1.7, 90.3 ± 2.1, and 89.7 ± 2.4%, respectively). In contrast, the loss of the CB in MycdΔN128 resulted in a small percentage of cells showing a primarily nuclear localization (20.3 ± 1.9%), whereas in most cells, it was evenly distributed in the cytoplasm and nucleus (77.0 ± 2.0%). These results suggest that, although the CB functions as the NLS of MycdΔN128, wild-type Mycd has a more powerful NLS in its NH2-terminal region.
FIGURE 2.
Role of the NB in the subcellular localization of Mycd. A, schematic representation of full-length and mutant Mycds is shown. Sequences of the NB (red letters) in Mycd and MRTF-A/B orthologs from different species are shown (right column). SAP, SAP domain; CC, coiled-coil domain. B, VSMCs were transfected with expression plasmids for the indicated FLAG-tagged Mycd derivatives and cultured under serum-stimulated conditions for 24 h. The cells were stained with an anti-FLAG antibody (red) and Hoechst 33258 (blue). Representative images from at least three independent experiments are shown. Bar = 10 μm. C, the images were quantified as described under “Experimental Procedures.” N, nuclear-specific accumulation; NC, diffuse distribution in the nucleus and the cytoplasm; C, cytoplasmic localization.
Another basic amino acid cluster corresponding to B2 (14) (here, the NB) is completely conserved among the Mycd family members from different species (Fig. 2A). In fact, a mutant Mycd lacking the NB (MycdΔNB) was evenly distributed in the cytoplasm and nucleus of most cells (85.3 ± 2.3%), and the percentage of cells showing a nuclear-specific accumulation was markedly decreased (3.3 ± 0.2%). Furthermore, cells expressing Mycd with a mutated NB (MycdmNB), in which the NB sequence KLKRAR was changed to ALAAAR, predominantly distributed it to the cytoplasm (89.2 ± 2.2%), and few showed a nuclear accumulation. The expression of Mycds with mutations in the NB and CB (MycdΔNBΔCB and MycdmNBΔCB) resulted in an increased proportion of cells with cytoplasmic localization (87.8 ± 1.5 and 91.4 ± 0.5%, respectively) (Figs. 2, B and C). These results strongly suggest that the NB is the critical NLS for the nuclear accumulation of wild-type Mycd and that the CB is a weak but significant NLS.
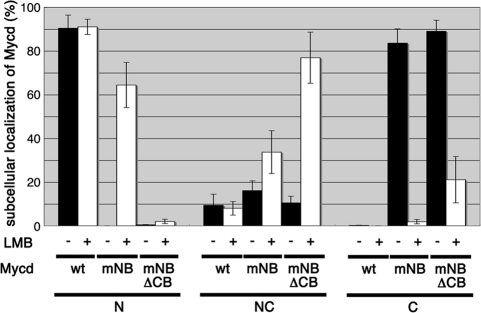
We next examined the effect of LMB, an inhibitor of the exportin Crm1, on the subcellular localization of MycdmNB and MycdmNBΔCB (Fig. 3). LMB had no effect on the nuclear localization of wild-type Mycd, but it markedly decreased the percentage of cells showing the cytoplasmic localization of MycdmNB and increased the percentage exhibiting its nuclear accumulation (from 0 to 64.4 ± 10.3%). The LMB treatment had a similar effect on MycdmNBΔCB presence in the cytoplasm, decreasing the percentage of cells from 89.0 ± 3.0 to 21.1 ± 10.6% and increasing the percentage showing even distribution to the cytoplasm and nucleus from 10.5 ± 3.0 to 76.9 ± 11.7%. However, LMB had little effect on MycdmNBΔCB nuclear accumulation, suggesting that CB has a significant role as an NLS. Taken together, these results suggest that wild-type Mycd escapes nuclear export by Crm1 because of its strong NLSs, whereas the mutant Mycds that lack the major NLSs can be transiently imported into the nucleus but are immediately exported to the cytoplasm by Crm1.
FIGURE 3.
Effect of LMB on the subcellular localization of Mycd derivatives. VSMCs were transfected with expression plasmids for the indicated FLAG-tagged Mycd derivatives and were cultured for 24 h. For the last 2 h the cells were incubated with LMB (5 ng/ml). The subcellular localization of Mycd derivatives was characterized as described under “Experimental Procedures.” mNB, mutated NB; N, nuclear-specific accumulation; NC, diffuse distribution in the nucleus and the cytoplasm; C, cytoplasmic localization.
Importin α/β1 Heterodimer-mediated Nuclear Localization of Mycd
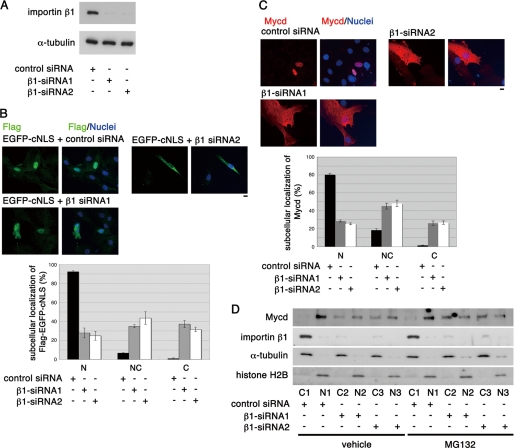
NB function as the critical Mycd NLS, its conservation among Mycd family members, and its similarity with cNLSs (Fig. 2), which consist of a basic amino acid cluster, suggested that the importin α/β1 heterodimer could be the Mycd binding partner because the cNLSs are known to interact with importin α/β1 heterodimers (23, 24). To test this idea, we first examined the effect of siRNA-mediated knockdown of importin β1 (β1-siRNA1 and β1-siRNA2) on the subcellular localization of FLAG-tagged Mycd in VSMCs (both of the siRNAs significantly reduced the expression of endogenous importin β1 in VSMCs (Fig. 4A)). To do this, we examined the effects of knocking down importin β1 on the subcellular localization of a FLAG-tagged EGFP containing a cNLS (EGFP-cNLS) in VSMCs. Of the VSMCs transfected with control siRNA, almost all expressed EGFP-cNLS in the nucleus (92.3 ± 1.0%). By contrast, of the VSMCs transfected with either β1-siRNA1 or β1-siRNA2, the percentage of cells showing the nuclear accumulation of EGFP-cNLS was markedly suppressed (from 92.3 ± 1.0 to 27.9 ± 5.2% or 24.9 ± 4.7%, respectively). Conversely, as expected, the percentages of cells containing EGFP-cNLS in the cytoplasm alone and in both the cytoplasm and the nucleus increased (Fig. 4B). Likewise, the percentages of cells showing the nuclear accumulation of FLAG-Mycd was suppressed by the knockdown of importin β1 (from 80.2 ± 1.6 to 28.6 ± 1.2 and 25.5 ± 1.0%), and the proportions of cells containing FLAG-Mycd in the cytoplasm alone and in both the cytoplasm and nucleus increased (Fig. 4C).
FIGURE 4.
Effect of knockdown of importin β1 on the subcellular localization of EGFP-cNLS and Mycd in cultured VSMCs. A, VSMCs were transfected with the indicated siRNAs and cultured for 2 days. Whole-cell extracts were then subjected to immunoblotting using the indicated antibodies. The level of α-tubulin served as a loading control. B–D, VSMCs were first transfected with the indicated siRNAs and cultured for 24 h. They were then transfected with the expression plasmid for FLAG-tagged EGFP-cNLS (B) or FLAG-tagged Mycd (C) and cultured for a further 24 h. The cells were stained with an anti-FLAG antibody (green (B) or red (C)) and Hoechst 33258 (blue), and the data in the images were quantified as described under “Experimental Procedures.” Representative images are shown (B and C). Bars = 10 μm. D, VSMCs were transfected with the indicated siRNAs and cultured for 45 h and then further cultured for 3.5 h with vehicle or MG-132 (5 μm). Their cytoplasmic (C) and nuclear (N) fractions were analyzed by immunoblotting using the indicated antibodies. Representative results from at least three independent experiments are shown. NC, diffuse distribution in the nucleus and the cytoplasm.
To address the role of importin β1 in the nuclear import of endogenous Mycd in VSMCs, we quantified the levels of endogenous Mycd in the cytoplasmic and nuclear fractions of VSMCs transfected with a control siRNA or one of the importin β1 siRNAs (Fig. 4D). In VSMCs transfected with control siRNA, Mycd was located exclusively in the nucleus (96.9 ± 1.2%), and importin β1 was detected in the cytoplasmic fraction (25). In VSMCs transfected with either β1-siRNA1 or β1-siRNA2, the proportion of cytoplasmic Mycd increased significantly (41.5 ± 3.3% for β1-siRNA1, 40.5 ± 4.4% for β1-siRNA2, and 3.1 ± 1.2% for control-siRNA) in close correlation with the down-regulation of importin β1. Treatment with a proteasome inhibitor, MG-132, further increased the levels of cytoplasmic Mycd in the importin β1-depleted VSMCs (60.9 ± 1.2% for β1-siRNA1 and 61.0 ± 2.2% for β1-siRNA2) (Fig. 4D). The levels of Mycd mRNA in VSMCs transfected with the control or importin β1-directed siRNAs were comparable (supplemental Fig. S2A). The total Mycd protein was significantly reduced in the importin β1-depleted VSMCs (50.5 ± 4.7% for β1-siRNA1 and 48.1 ± 12.7% for β1-siRNA2), but MG-132 treatment blocked this reduction (supplemental Fig. S2B). These results imply that interactions between Mycd and an importin α/β1 heterodimer are critical for Mycd nuclear import and that cytoplasmic Mycd is unstable, probably because of its proteasome-mediated degradation (26, 27).
Interactions between Mycd Family Members and Importin α/β1 Heterodimers
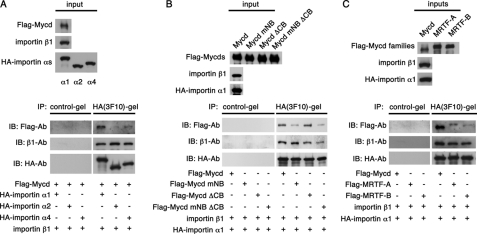
We screened VSMCs by RT-PCR for cDNAs encoding the importin α subtypes (α1, α2, α3, α4, α5, and α6) and amplified the cDNAs for importins α1, α2, and α4 (data not shown), suggesting that these are the major subtypes of importin α expressed in VSMCs. As expected, because importin α is autoinhibited by its binding to importin β1 (23), Mycd did not interact with the importin subunits α1, α2, α4, or β1 expressed alone in vitro (data not shown). We next examined the interactions between Mycd and the importin α1/β1, importin α2/β1, and importin α4/β1 heterodimers. Mycd exhibited the strongest affinity for importin α1/β1. Its affinities for importin α2/β1 and for importin α4/β1 were weak and moderate, respectively (Fig. 5A), suggesting that the nuclear import of Mycd in VSMCs might depend primarily on importin α1/β1.
FIGURE 5.
Interaction between Mycd family members and importin α/β1 heterodimers. A, mixtures of in vitro translated FLAG-Mycd, importin β1, and each of the indicated HA-importin α subtypes were immunoprecipitated with a control gel or anti-HA affinity gel, and the immunoprecipitates (IP) were analyzed by immunoblotting (IB) using the indicated antibodies (Ab). B and C, in vitro translated FLAG-Mycd, its derivatives, FLAG-MRTF-A, or FLAG-MRTF-B was mixed with importin β1 and HA-importin α1, and their interaction was analyzed. The upper and lower panels show input and immunoprecipitation, respectively. Representative results from at least three independent experiments are shown.
To address the relationship between the formation of a Mycd·importin α1/β1 ternary complex and the nuclear import of Mycd, we analyzed the binding of Mycd mutants to importin α1/β1 in vitro (Fig. 5B). Wild-type Mycd and MycdΔCB exhibited comparable levels of binding to the importin α1/β1 heterodimer, whereas much less MycdmNB or MycdmNBΔCB bound to it, indicating their ternary complex with the importin α1/β1 heterodimer was weak. Thus, the NB rather than the CB is probably more critical for Mycd interactions with importin α1/β1.
Vartiainen et al. (14) recently reported that the B2 region of MRTF-A (i.e. the NB of Mycd) is required for its nuclear accumulation. However, the nature of the nuclear import machinery remained unclear. It was possible that the nuclear import of MRTF-A/B was also mediated by the importin α1/β1 heterodimer. To test this we compared the interactions between the different Mycd family members and importin α1/β1 in vitro (Fig. 5C). Compared with Mycd, MRTF-A/B showed low but significant binding to the importin α1/β1 heterodimer. The order of affinity for importin α1/β1 binding was Mycd ≫ MRTF-A > MRTF-B. We confirmed this hierarchy using the same assay with different dosages of the respective Mycd family members (supplemental Fig. S3). To assess the binding of MRTF-A/B to the other importin heterodimers, we examined the ternary complex formation between them and importin α1/β1, importin α2/β1, and importin α4/β1 (supplemental Fig. S4A). Unlike Mycd, MRTF-A/B did not show significantly different affinities for the different heterodimers, although MRTF-A bound with a higher affinity than MRTF-B in every case. Moreover, MRTF-A/B constructs bearing an NB mutation failed to bind importin α1/β1, indicating that NB was the MRTF-A/B binding site for importin α1/β1 as well (supplemental Fig. S4B). These results suggest that the nuclear import of MRTF-A/B may be regulated similarly to that of Mycd, i.e. by their interactions with importin α/β1 heterodimers.
To examine the functional involvement of the importin α/β1 heterodimers in the nuclear import of MRTF-A/B, we analyzed the subcellular localization of FLAG-labeled MRTF-A/B in VSMCs with siRNA-mediated knockdown of endogenous importin β1 that were or were not treated with LMB to inhibit Crm1 (supplemental Fig. S5). Of the VSMCs transfected with the control siRNA and without LMB treatment, most showed MRTF-A/B localized to the cytoplasm (79.1 ± 2.4% for MRTF-A and 88.5 ± 2.7% for MRTF-B). Treatment with LMB strongly enhanced the proportion of cells showing a nuclear accumulation of MRTF-A (from 8.3 ± 1.2 to 65.6 ± 2.2%) and reduced the proportion showing cytoplasmic localization (from 79.1 ± 2.4 to 9.9 ± 3.0%). The proportion of cells showing an LMB-induced nuclear accumulation of MRTF-B was, however, comparatively moderate in the control siRNA-transfected VSMCs as was the reduction in the percentage of cells showing its cytoplasmic distribution (from 88.5 ± 2.7 to 58.3 ± 1.5%) (supplemental Figs. S5, A and B, left columns). These results indicated that MRTF-A has a stronger tendency to accumulate in the nucleus than MRTF-B. The knockdown of importin β1 had no effect on the subcellular localization of MRTF-A in the absence of LMB, but it significantly reduced the proportion of cells showing an LMB-induced nuclear accumulation of MRTF-A (24.0 ± 1.0% for β1-siRNA, 19.6 ± 1.4% for β1-siRNA2, and 65.6 ± 2.2% for control-siRNA) (supplemental Fig. S5A). For MRTF-B, the moderate proportion of cells showing its nuclear translocation in response to LMB (24.4 ± 2.3% for control siRNA) was lowered to the basal level by the siRNA-mediated knockdown of importin β1 (4.9 ± 2.1% for β1-siRNA1 with LMB, 7.6 ± 1.2% for β1-siRNA2 with LMB, and 2.5 ± 1.3% for control-siRNA without LMB) (supplemental Fig. S5B). These results indicated that the nuclear import of MRTF-A/B also depends at least in part on the importin α/β1 heterodimer system.
Effect of Actin Dynamics on the Importin α1/β1-mediated Nuclear Import of Mycd Family Members
We next examined the effects of G-actin on the ternary complex formation between Mycd family members and the importin α1/β1 heterodimer. Mycd family members bound to β-actin in vitro with the following hierarchy of affinities: MRTF-B > MRTF-A ≫ Mycd (supplemental Fig. S6A). Because MRTF-B showed no significant difference in its affinity for wild-type β-actin or a nonpolymerizable mutant β-actin, R62D (28) (supplemental Fig. S6B), the above hierarchy indicates the Mycd family members' relative affinities for G-actin specifically. Interestingly, β-actin had no effect on the interaction between Mycd and importin α1/β1, but it suppressed the MRTF-A/B interaction with importin α1/β1 in vitro (supplemental Fig. S7) moderately (for MRTF-A) or severely (for MRTF-B) in accordance with each protein's relative affinity for β-actin.
We also examined the serum-induced nuclear import of MRTF-A/B in VSMCs (supplemental Fig. S8). Short term serum stimulation (10 min) reduced the proportion of cells showing the cytoplasmic localization of MRTF-A/B (from 81.1 ± 1.3% (serum−) to 39.6 ± 0.7% (serum+) for MRTF-A and from 88.5 ± 1.3% (serum−) to 50.7 ± 4.6% (serum+) for MRTF-B) and increased the proportion showing a diffuse distribution of MRTF-A/B in the cytoplasm and nucleus (from 14.3 ± 1.1% (serum−) to 31.1 ± 2.6% (serum+) for MRTF-A and from 9.0 ± 2.0% (serum−) to 38.8 ± 5.6% (serum+) for MRTF-B). The serum-induced nuclear accumulation of MRTF-A was, however, moderate (from 3.6 ± 0.3% (serum−) to 28.6 ± 3.0% (serum+)) (supplemental Fig. S8A), suggesting that the nuclear accumulation of MRTF-A in response to serum stimulation was weaker than that caused by LMB treatment (supplemental Fig. S5A, left column). Furthermore, the serum-induced nuclear accumulation of MRTF-B (from 2.5 ± 1.3% (serum−) to 10.5 ± 1.0% (serum+)) was also lower than that induced by LMB treatment (supplemental Figs. S8B and S5B, left columns). The knockdown of importin β1 also inhibited the serum-triggered nuclear accumulation of MRTF-A/B, and the nuclear accumulation of Mycd was not affected by the presence or absence of serum (data not shown). Taken together, actin-specific effects on the nuclear import of MRTF-A/B, but not Mycd, were due to the inhibitory role of G-actin on the formation of a ternary complex between MRTF-A/B and importin α/β1.
Relationship between the VSMC Phenotype and the Expression of Importins α1 and β1
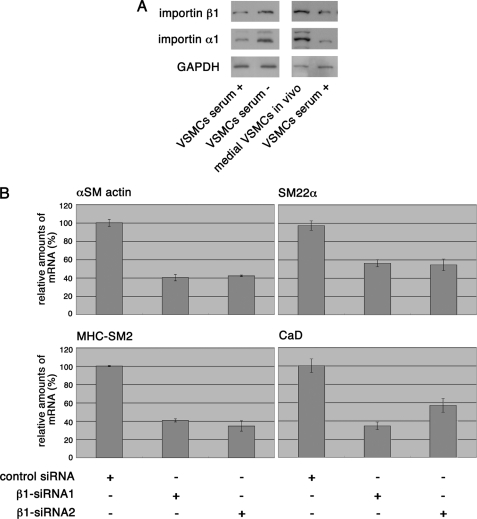
To address the biological significance of Mycd high affinity for the importin α1/β1 heterodimer, we investigated the expression levels of importins α1 and β1 in differentiated and dedifferentiated VSMCs. The levels of importins α1 and β1 corresponded well with the VSMC phenotype-dependent up- or down-regulation of Mycd, SRF, and SMC markers (Figs. 6A and 1). We then examined the transcript levels of the SMC markers in importin β1-depleted VSMCs. Under these conditions, the amount of nuclear Mycd decreased (Fig. 4D), and the transcription levels of SMC markers were down-regulated concomitantly (Fig. 6B). These effects on transcription probably do not fully depend on the inhibition of Mycd nuclear accumulation, because the importin α/β1-mediated nuclear import system is probably a component of other transcriptional machineries. Nonetheless, these results suggest that the expression of the nuclear import machineries for Mycd plays at least a partial role in the VSMC phenotype-dependent transcription of SMC markers.
FIGURE 6.
Relationship between VSMC phenotype and the expression of importins α1 and β1 and the significance of the nuclear accumulation of Mycd. A, expression levels of importins α1 and β1 in VSMCs under different conditions were analyzed by immunoblotting as described in the legend for Fig. 1. B, VSMCs were transfected with the indicated siRNAs, cultured for 36 h, and further cultured for 12 h without serum. The transcript levels of the indicated SMC markers were analyzed by quantitative real-time RT-PCR as described in the legend for supplemental Fig. S1.
DISCUSSION
Here, we demonstrated for the first time that the nuclear import of Mycd family members depends on the importin α/β1 heterodimer-mediated system in VSMCs and that the expression of their nuclear import machineries is linked to the VSMC phenotype. Mycd forms a ternary complex with the heterodimers such as importins α1/β1, α2/β1, and α4/β1 (these importin α subtypes are mainly expressed in VSMCs) via its NB, and has the strongest binding to the importin α1/β1 heterodimer. Thus, the interaction between Mycd and the importin α1/β1 heterodimer plays a pivotal role in the nuclear import of Mycd. In addition, we found that MRTF-A/B also interacts with importin α/β1 heterodimers. The binding of Mycd to the importin α1/β1 heterodimer, which is resistant to G-actin, was stronger than that of MRTF-A/B to any of the importin α/β1 heterodimers, which is sensitive to G-actin. This hierarchy in the binding to importin α/β1 heterodimers was evident even under conditions where G-actin was absent. These findings suggest that differences in the nuclear import of Mycd family members are critically determined by differences in their binding affinities for importin α/β1 heterodimer and that actin dynamics play a role for the nuclear import of MRTF-A/B, but its role is adjunctive. The expression profiles of importins α1 and β1 were associated with those of Mycd and SRF and the SMC markers but not with those of MRTF-A/B in differentiated and dedifferentiated VSMCs. Thus, in this study we revealed the nuclear import mechanism for Mycd and the differences in its mechanism among Mycd family members and the relationship between the expression of nuclear import machineries of Mycd and the VSMC phenotype.
NLSs in Mycd Family Members
Previous studies (1, 14) report that basic amino acid clusters are functionally involved in the nuclear import of Mycd family members, which suggested that importin α/β1 heterodimers might mediate their translocation, but this is the first study to test this hypothesis. Our present study revealed that the NB acts as an NLS for Mycd and that the role of the CB as an NLS is weak but significant. Furthermore, the results of our analysis using LMB suggest that one or more as-yet unidentified minor NLS(s) may be present in Mycd. Although the NB is a critical site for the interactions with the importin α1/β1 heterodimer, the MycdmNBΔCB mutant formed a faint but significant ternary complex with importin α1/β1, which supports the idea that an additional site is involved in Mycd nuclear import.
The affinities of the Mycd family members to the importin α/β1 heterodimers differ even though their NB sequences are identical. The NH2-terminal regions near the NBs have divergent sequences and varying conformations among the Mycd family members, which could cause such differences. The reason for Mycd high affinity for the importin α1/β1 heterodimer is an interesting issue for future study. Most cells expressing the MycdmNBΔCB and MycdΔNBΔCB mutants showed them localized to the cytoplasm, whereas the majority of cells expressing MycdΔN128ΔCB exhibited a diffuse distribution in the cytoplasm and nucleus. These findings suggest that the NH2-terminal region of Mycd (residues 1–128), except for the NB, may promote its Crm1-mediated nuclear export. The partial loss of this region could explain why MycdΔ128 was mostly located in the nucleus even though the NB was absent.
Relationship between the Nuclear Import Machinery of Mycd and the VSMC Phenotype
Almost no VSMCs that were transfected with the importin β1-directed siRNAs showed a cytoplasm-restricted localization of Mycd or EGFP-cNLS. This may have been due to an incomplete blockade of the importin α/β1 heterodimer-mediated nuclear import system or to the function of other nuclear import systems. The different binding properties of the Mycd family members for the importin α subtypes and the VSMC phenotype-dependent up- and down-regulation of importins α1 and β1, which was associated with the expression profiles of Mycd, SRF, and the SMC markers, suggest that the expression of the nuclear import machineries plays a vital role in cell type-specific gene expression programs. Consistent with this idea, Yasuhara et al. (29) reported that the forced change of importin α subtype expression has a major impact on the neural differentiation of mouse embryonic stem cells. Because ternary complexes composed of MRTF-A or MRTF-B and importin α/β1 heterodimers were detected in vitro and the siRNA-mediated knockdown of endogenous importin β1 increased the cytoplasmic localization of MRTF-A/B when Crm1 was inhibited by LMB, the mechanism mediated by the importin α/β1 heterodimers also appears to regulate the nuclear import of MRTF-A/B. However, the expression levels of the SMC markers in cultured VSMCs fully depended on Mycd but not on MRTF-A/B (supplemental Fig. 1). Therefore, the down-regulation of the SMC marker expression in importin β1-depleted VSMCs could be partially due to the inhibition of Mycd nuclear import.
Regulation of the Subcellular Localization of Mycd Family Members
LMB treatment differentially induced the nuclear accumulation of MRTF-A/B (supplemental Fig. S5), and these differences were in good agreement with the molecule respective affinities for the importin α/β1 heterodimers (Fig. 5 and supplemental Fig. S4), indicating both that the nuclear import of Mycd family members are not identically regulated and that their relative binding affinity for the importin α/β1 heterodimers determines the degrees of their nuclear import. Although the suppressive effect of G-actin on the ternary complex between the MRTF-A/B and importin α1/β1 heterodimer (supplemental Fig. S7) showed good agreement with the model proposed by Guettler et al. (12), their model did not take into account the possibility of differences in the Mycd family member nuclear import even under the G-actin-depleted conditions, and their study did not identify a critical machinery responsible for their nuclear import. The negative regulation by G-actin in the nuclear import of MRTF-A/B must be adjunctive, because their serum-induced nuclear import also correlates with their binding affinities for the importin α/β1 heterodimers (supplemental Figs. S8 and S4).
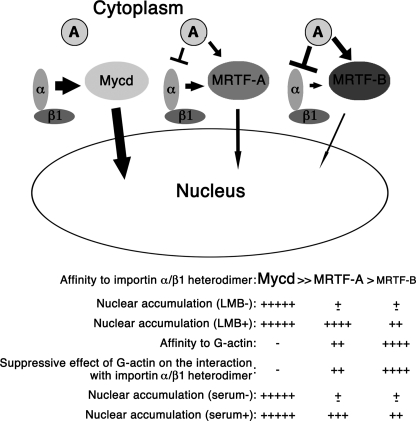
Taking our findings together, we conclude that the constitutive nuclear accumulation of Mycd is due to its strong affinity for the importin α1/β1 heterodimer, and the low nuclear import abilities of MRTF-A/B is attributable to their weak binding affinities for importin α/β1 heterodimers, in which actin dynamics play a adjunctive role (Fig. 7), indicating that the Mycd family member binding to importin α/β1 plays a critical role in determining their nuclear import.
FIGURE 7.
Schematic summarizing the results of this study. The nuclear import of Mycd family members depends on the hierarchy of their binding affinities for the importin α/β1 heterodimers. Actin dynamics adjunctively regulate the nuclear import of MRTF-A/B but not that of Mycd. A, G-actin; α, importin α; β1, importin β1.
This work was supported by Grants-in-aid for Scientific Research from the Ministry of Education, Science, Sports, and Culture of Japan 20590279 (to K. H.) and 15GS0312 (to K. S.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Fig. S1–S8.
- Mycd
- myocardin
- VSMC
- vascular smooth muscle cell (SMC)
- SRF
- serum response factor
- CaD
- caldesmon
- CB
- central basic domain
- NLS
- nuclear localization signal
- cNLS
- classical NLS
- EGFP
- enhanced green fluorescent ptotein
- LMB
- leptomycin B
- MHC
- myosin heavy chain
- MRTF-A/B
- myocardin-related transcription factor A and B
- NB
- NH2-terminal basic domain.
REFERENCES
- 1.Wang D., Chang P. S., Wang Z., Sutherland L., Richardson J. A., Small E., Krieg P. A., Olson E. N. (2001) Cell 105, 851–862 [DOI] [PubMed] [Google Scholar]
- 2.Ma Z., Morris S. W., Valentine V., Li M., Herbrick J. A., Cui X., Bouman D., Li Y., Mehta P. K., Nizetic D., Kaneko Y., Chan G. C., Chan L. C., Squire J., Scherer S. W., Hitzler J. K. (2001) Nat. Genet. 28, 220–221 [DOI] [PubMed] [Google Scholar]
- 3.Mercher T., Coniat M. B., Monni R., Mauchauffe M., Nguyen Khac F., Gressin L., Mugneret F., Leblanc T., Dastugue N., Berger R., Bernard O. A. (2001) Proc. Natl. Acad. Sci. U.S.A. 98, 5776–5779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miralles F., Posern G., Zaromytidou A. I., Treisman R. (2003) Cell 113, 329–342 [DOI] [PubMed] [Google Scholar]
- 5.Wang D. Z., Li S., Hockemeyer D., Sutherland L., Wang Z., Schratt G., Richardson J. A., Nordheim A., Olson E. N. (2002) Proc. Natl. Acad. Sci. U.S.A. 99, 14855–14860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu N., Olson E. N. (2006) Curr. Opin. Cell Biol. 18, 715–722 [DOI] [PubMed] [Google Scholar]
- 7.Wang Z., Wang D. Z., Pipes G. C., Olson E. N. (2003) Proc. Natl. Acad. Sci. U.S.A. 100, 7129–7134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi K., Nakamura S., Nishida W., Sobue K. (2006) Mol. Cell. Biol. 26, 9456–9470 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang Z., Wang D. Z., Hockemeyer D., McAnally J., Nordheim A., Olson E. N. (2004) Nature 428, 185–189 [DOI] [PubMed] [Google Scholar]
- 10.Du K. L., Chen M., Li J., Lepore J. J., Mericko P., Parmacek M. S. (2004) J. Biol. Chem. 279, 17578–17586 [DOI] [PubMed] [Google Scholar]
- 11.Hinson J. S., Medlin M. D., Lockman K., Taylor J. M., Mack C. P. (2007) Am. J. Physiol. Heart Circ. Physiol. 292, H1170–H1180 [DOI] [PubMed] [Google Scholar]
- 12.Guettler S., Vartiainen M. K., Miralles F., Larijani B., Treisman R. (2008) Mol. Cell. Biol. 28, 732–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Posern G., Treisman R. (2006) Trends Cell Biol. 16, 588–596 [DOI] [PubMed] [Google Scholar]
- 14.Vartiainen M. K., Guettler S., Larijani B., Treisman R. (2007) Science 316, 1749–1752 [DOI] [PubMed] [Google Scholar]
- 15.Muehlich S., Wang R., Lee S. M., Lewis T. C., Dai C., Prywes R. (2008) Mol. Cell. Biol. 28, 6302–6313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hayashi K., Saga H., Chimori Y., Kimura K., Yamanaka Y., Sobue K. (1998) J. Biol. Chem. 273, 28860–28867 [DOI] [PubMed] [Google Scholar]
- 17.Kimura Y., Morita T., Hayashi K., Miki T., Sobue K. (2010) Cancer Res. 70, 501–511 [DOI] [PubMed] [Google Scholar]
- 18.Morita T., Mayanagi T., Sobue K. (2007) Exp. Cell Res. 313, 3432–3445 [DOI] [PubMed] [Google Scholar]
- 19.Thierbach G., Kalinowski J., Bachmann B., Pühler A. (1990) Appl. Microbiol. Biotechnol. 32, 443–448 [DOI] [PubMed] [Google Scholar]
- 20.Yoneda Y. (1997) J. Biochem. 121, 811–817 [DOI] [PubMed] [Google Scholar]
- 21.Hayashi K., Takahashi M., Nishida W., Yoshida K., Ohkawa Y., Kitabatake A., Aoki J., Arai H., Sobue K. (2001) Circ. Res. 89, 251–258 [DOI] [PubMed] [Google Scholar]
- 22.Hayashi K., Shibata K., Morita T., Iwasaki K., Watanabe M., Sobue K. (2004) J. Biol. Chem. 279, 40807–40818 [DOI] [PubMed] [Google Scholar]
- 23.Goldfarb D. S., Corbett A. H., Mason D. A., Harreman M. T., Adam S. A. (2004) Trends Cell Biol. 14, 505–514 [DOI] [PubMed] [Google Scholar]
- 24.Lange A., Mills R. E., Lange C. J., Stewart M., Devine S. E., Corbett A. H. (2007) J. Biol. Chem. 282, 5101–5105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Moroianu J., Hijikata M., Blobel G., Radu A. (1995) Proc. Natl. Acad. Sci. U.S.A. 92, 6532–6536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hu G., Wang X., Saunders D. N., Henderson M., Russell A. J., Herring B. P., Zhou J. (2010) J. Biol. Chem. 285, 11800–11809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Xie P., Fan Y., Zhang H., Zhang Y., She M., Gu D., Patterson C., Li H. (2009) Mol. Cell. Biol. 29, 2398–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Posern G., Sotiropoulos A., Treisman R. (2002) Mol. Biol. Cell. 13, 4167–4178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yasuhara N., Shibazaki N., Tanaka S., Nagai M., Kamikawa Y., Oe S., Asally M., Kamachi Y., Kondoh H., Yoneda Y. (2007) Nat. Cell Biol. 9, 72–79 [DOI] [PubMed] [Google Scholar]