Abstract
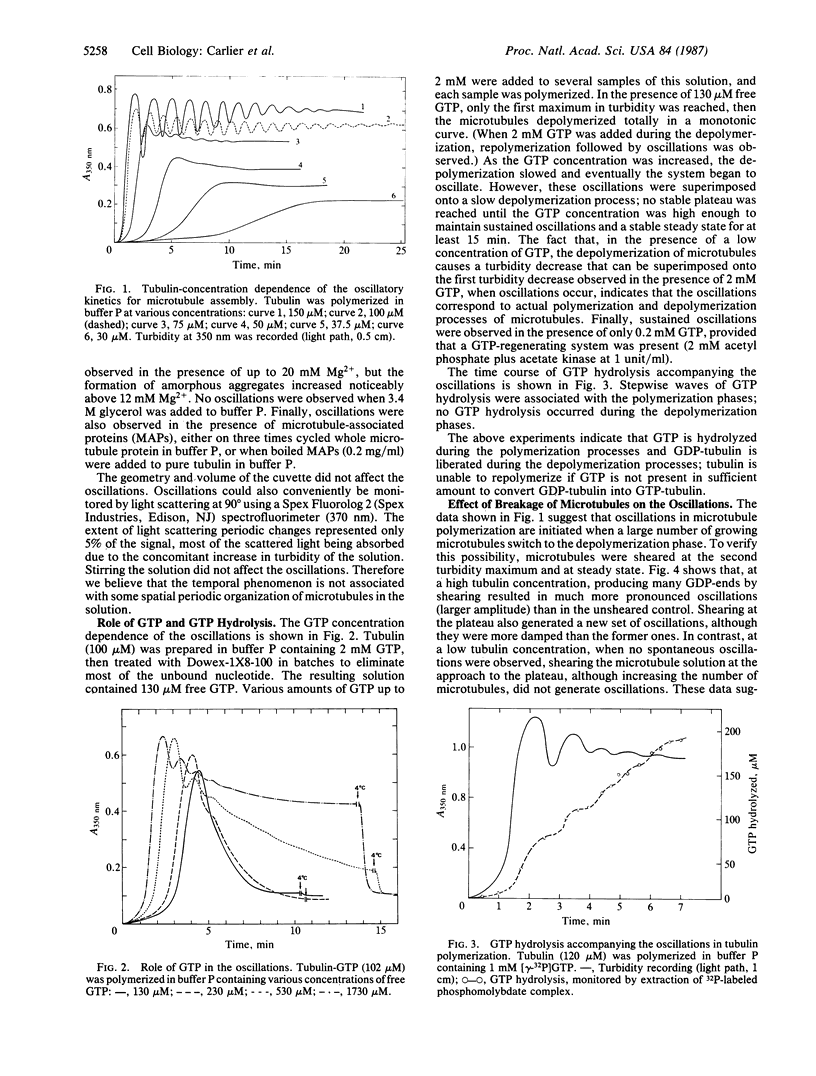
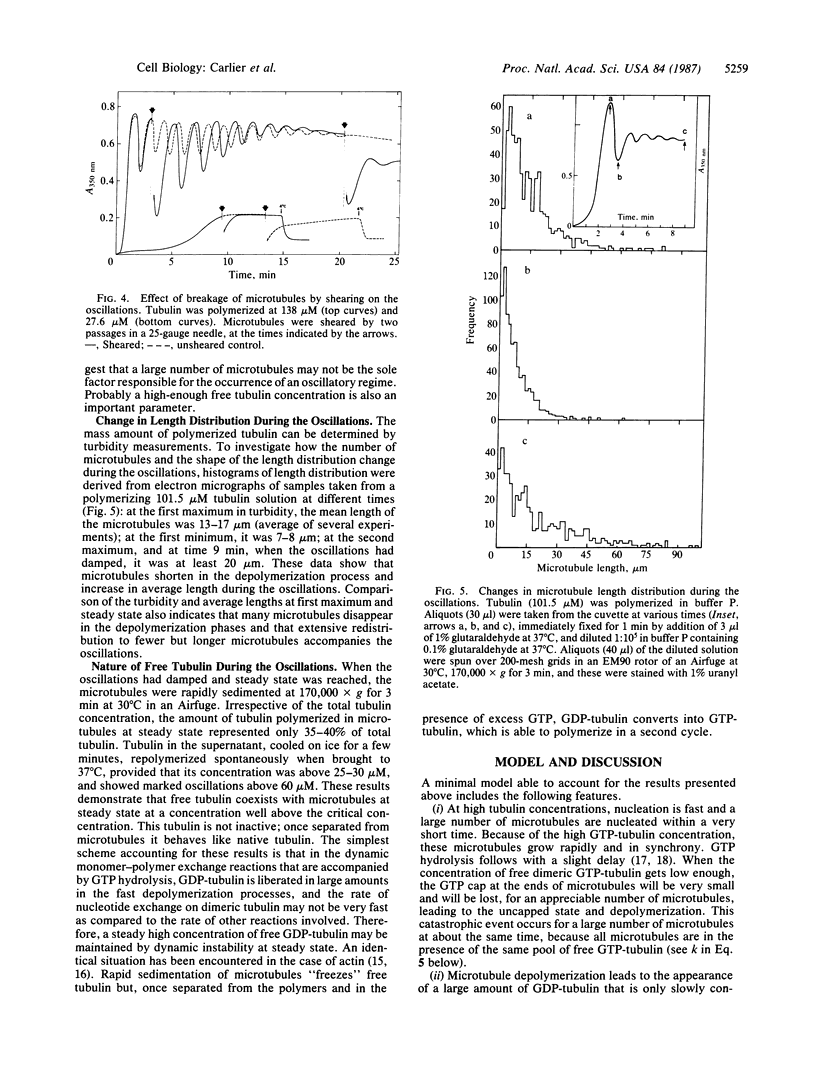
Under conditions where microtubule nucleation and growth are fast (i.e., high magnesium ion and tubulin concentrations and absence of glycerol), microtubule assembly in vitro exhibits an oscillatory regime preceding the establishment of steady state. The amplitude of the oscillations can represent greater than 50% of the maximum turbidity change and oscillations persist for up to 20 periods of 80 s each. Oscillations are accompanied by extensive length redistribution of microtubules. Preliminary work suggests that the oscillatory kinetics can be simulated using a model in which many microtubules undergo synchronous transitions between growing and rapidly depolymerizing phases, complicated by the kinetically limiting rate of nucleotide exchange on free tubulin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bajer A. S. Functional autonomy of monopolar spindle and evidence for oscillatory movement in mitosis. J Cell Biol. 1982 Apr;93(1):33–48. doi: 10.1083/jcb.93.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F., Hill T. L., Chen Y. Interference of GTP hydrolysis in the mechanism of microtubule assembly: an experimental study. Proc Natl Acad Sci U S A. 1984 Feb;81(3):771–775. doi: 10.1073/pnas.81.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlier M. F. Kinetic evidence for a conformation change of tubulin preceding microtubule assembly. J Biol Chem. 1983 Feb 25;258(4):2415–2420. [PubMed] [Google Scholar]
- Carlier M. F., Pantaloni D. Kinetic analysis of guanosine 5'-triphosphate hydrolysis associated with tubulin polymerization. Biochemistry. 1981 Mar 31;20(7):1918–1924. doi: 10.1021/bi00510a030. [DOI] [PubMed] [Google Scholar]
- Cassimeris L. U., Wadsworth P., Salmon E. D. Dynamics of microtubule depolymerization in monocytes. J Cell Biol. 1986 Jun;102(6):2023–2032. doi: 10.1083/jcb.102.6.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Hill T. L. Theoretical treatment of microtubules disappearing in solution. Proc Natl Acad Sci U S A. 1985 Jun;82(12):4127–4131. doi: 10.1073/pnas.82.12.4127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrone F. A., Hofrichter J., Eaton W. A. Kinetics of sickle hemoglobin polymerization. II. A double nucleation mechanism. J Mol Biol. 1985 Jun 25;183(4):611–631. doi: 10.1016/0022-2836(85)90175-5. [DOI] [PubMed] [Google Scholar]
- Hill T. L., Carlier M. F. Steady-state theory of the interference of GTP hydrolysis in the mechanism of microtubule assembly. Proc Natl Acad Sci U S A. 1983 Dec;80(23):7234–7238. doi: 10.1073/pnas.80.23.7234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L., Chen Y. Phase changes at the end of a microtubule with a GTP cap. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5772–5776. doi: 10.1073/pnas.81.18.5772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill T. L. Introductory analysis of the GTP-cap phase-change kinetics at the end of a microtubule. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6728–6732. doi: 10.1073/pnas.81.21.6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horio T., Hotani H. Visualization of the dynamic instability of individual microtubules by dark-field microscopy. Nature. 1986 Jun 5;321(6070):605–607. doi: 10.1038/321605a0. [DOI] [PubMed] [Google Scholar]
- Kirschner M., Mitchison T. Beyond self-assembly: from microtubules to morphogenesis. Cell. 1986 May 9;45(3):329–342. doi: 10.1016/0092-8674(86)90318-1. [DOI] [PubMed] [Google Scholar]
- Kristofferson D., Mitchison T., Kirschner M. Direct observation of steady-state microtubule dynamics. J Cell Biol. 1986 Mar;102(3):1007–1019. doi: 10.1083/jcb.102.3.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchison T., Kirschner M. Dynamic instability of microtubule growth. Nature. 1984 Nov 15;312(5991):237–242. doi: 10.1038/312237a0. [DOI] [PubMed] [Google Scholar]
- Neidl C., Engel J. Exchange of ADP, ATP and 1: N6-ethenoadenosine 5'-triphosphate at G-actin. Equilibrium and kinetics. Eur J Biochem. 1979 Nov 1;101(1):163–169. doi: 10.1111/j.1432-1033.1979.tb04228.x. [DOI] [PubMed] [Google Scholar]
- Pantaloni D., Carlier M. F., Coué M., Lal A. A., Brenner S. L., Korn E. D. The critical concentration of actin in the presence of ATP increases with the number concentration of filaments and approaches the critical concentration of actin.ADP. J Biol Chem. 1984 May 25;259(10):6274–6283. [PubMed] [Google Scholar]
- Salmon E. D., Leslie R. J., Saxton W. M., Karow M. L., McIntosh J. R. Spindle microtubule dynamics in sea urchin embryos: analysis using a fluorescein-labeled tubulin and measurements of fluorescence redistribution after laser photobleaching. J Cell Biol. 1984 Dec;99(6):2165–2174. doi: 10.1083/jcb.99.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sammak P. J., Gorbsky G. J., Borisy G. G. Microtubule dynamics in vivo: a test of mechanisms of turnover. J Cell Biol. 1987 Mar;104(3):395–405. doi: 10.1083/jcb.104.3.395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. Dynamic and stable populations of microtubules in cells. J Cell Biol. 1987 Feb;104(2):277–288. doi: 10.1083/jcb.104.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelanski M. L., Gaskin F., Cantor C. R. Microtubule assembly in the absence of added nucleotides. Proc Natl Acad Sci U S A. 1973 Mar;70(3):765–768. doi: 10.1073/pnas.70.3.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voter W. A., Erickson H. P. The kinetics of microtubule assembly. Evidence for a two-stage nucleation mechanism. J Biol Chem. 1984 Aug 25;259(16):10430–10438. [PubMed] [Google Scholar]
- Wegner A., Savko P. Fragmentation of actin filaments. Biochemistry. 1982 Apr 13;21(8):1909–1913. doi: 10.1021/bi00537a032. [DOI] [PubMed] [Google Scholar]
- Weingarten M. D., Lockwood A. H., Hwo S. Y., Kirschner M. W. A protein factor essential for microtubule assembly. Proc Natl Acad Sci U S A. 1975 May;72(5):1858–1862. doi: 10.1073/pnas.72.5.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]


