Abstract
Deubiquitinating enzymes (DUBs) function in a variety of cellular processes by removing ubiquitin moieties from substrates, but their role in DNA repair has not been elucidated. Yeast Rad4-Rad23 heterodimer is responsible for recognizing DNA damage in nucleotide excision repair (NER). Rad4 binds to UV damage directly while Rad23 stabilizes Rad4 from proteasomal degradation. Here, we show that disruption of yeast deubiquitinase UBP3 leads to enhanced UV resistance, increased repair of UV damage and Rad4 levels in rad23Δ cells, and elevated Rad4 stability. A catalytically inactive Ubp3 (Ubp3-C469A), however, is unable to affect NER or Rad4. Consistent with its role in down-regulating Rad4, Ubp3 physically interacts with Rad4 and the proteasome, both in vivo and in vitro, suggesting that Ubp3 associates with the proteasome to facilitate Rad4 degradation and thus suppresses NER.
Keywords: Deubiquitination, DNA Damage, DNA Nucleotide Excision Repair, Protein Degradation, Yeast, DUBs, Rad23, Rad4
Introduction
Nucleotide excision repair (NER)2 is an important cellular defense mechanism that repairs ultraviolet (UV)-induced cyclobutane pyrimidine dimers (CPDs) and pyrimidine-(6–4)-pyrimidone photoproducts (6–4 PPs), as well as other helix-distorting DNA lesions (1). In humans, defects in NER are associated with the heritable disease xeroderma pigmentosum (XP), which leads to hypersensitivity to sunlight and a high risk of skin cancer (2).
NER is a multi-step process, requiring the coordinated activity of more than 30 proteins responsible for the recognition and removal of DNA lesions (1). Initial damage recognition in yeast is carried out by the Rad4-Rad23 heterodimer (orthologous to the human XPC-HR23B complex). Rad4 functions as the damage sensor protein in the heterodimer (3), and the process of NER is highly dependent upon its availability in cells (4). Though Rad23 alone does not bind to damaged DNA (5), rad23Δ cells are hypersensitive to UV radiation and show marked reduction in repair of UV-induced lesions (6). The contribution of Rad23 to NER appears to be, in part, inhibiting degradation of Rad4 (or XPC) by the ubiquitin proteasome pathway (UPP), as a defect in Rad23 leads to reduced Rad4/XPC protein levels (4, 7), and disruption of the ubiquitin-conjugating enzyme Ubc4 significantly restores the levels of Rad4 and UV tolerance of rad23 cells (4). In addition, it was reported that Rad23 may regulate cellular Rad4 levels by affecting its transcription (8).
UPP is a highly conserved system essential for controlling turnover of numerous proteins in eukaryotic cells (9). Several lines of evidence demonstrate that UPP plays important roles in regulating Rad4 and NER. Initially, it was found that Rad23 physically interacts with the 19 S regulatory particle (RP) of the proteasome (10). Subsequent studies indicate that Rad4 protein levels are stabilized in a variety of proteasome mutants (8, 11). Further analysis points to an antagonistic effect of the RP on NER, since mutations in Rpt6 or Rpt4, two AAA (ATPases associated with a variety of cellular activities) ATPases that are RP subunits and function in unfolding substrates (12), partially rescue the NER defect in rad23Δ cells (13). Mutation in Rpt6 also recovers Rad4 levels in rad23Δ (4), suggesting that deficiency in the RP inhibits proteolysis of Rad4, thus increasing DNA repair. In contrast to the 19 S RP, however, mutations in subunits of the 20S core particle (CP) of the proteasome do not improve NER in rad23Δ, which suggests an unknown non-proteolytic mechanism might also exist (13). Notably, the yeast E3 ligase complex Rad7-Rad16, responsible for attaching ubiquitin to Rad4 has been identified recently and controls its proteolysis in response to UV radiation (8).
Ubiquitination is a reversible process, and the removal of ubiquitin or ubiquitin chains from substrates is catalyzed by the activity of deubiquitinating enzymes (DUBs). Sequence analysis has identified 17 cysteine ubiquitin proteases in the yeast genome, among which 16 are of the ubiquitin-specific processing proteases (UBP) class (14, 15). The UBPs are highly divergent, except for conserved Cys and His boxes that likely form part of a catalytically active site (14, 16). The sequence diversity among the UBPs provides their substrate specificity. Yeast Ubp3, for example, specifically deubiquitinates RNA polymerase II (17). In addition to reversing the ubiquitination process, DUBs also play important roles at the proteasome. The yeast 26 S proteasome is a ∼2.5 MDa protein complex consisting of the RP and CP. Polyubiquitinated substrates are recognized by ubiquitin receptors residing in the RP, and then unfolded before entering the CP for proteolysis (12). DUBs play a key role at the proteasome, catalyzing the removal of polyubiquitin chains from substrates, since proteins with thermodynamically stable ubiqutin chains are too large to pass through the narrow channel into CP (18). Previous studies in yeast have shown that the DUB Rpn11, a subunit of the RP essential for cell viability, is critical for degradation-coupled deubiquitination at the proteasome in vivo and in vitro (19, 20). Other DUBs associated with the proteasome include Ubp4, which serves to facilitate degradation (21), and Ubp6 (22), which delays protein degradation.
Though ubiquitination and degradation of the NER protein Rad4 clearly play critical roles in its regulation, thus far, the role of DUBs in Rad4 activity is not clear. In this study, we screened the yeast DUBs for a potential impact on NER and found that one particular candidate, Ubp3, acts as a negative regulator of this repair pathway. We found that Ubp3 physically interacts with Rad4 and the proteasome, and the ubp3 mutation affects Rad4 stability.
EXPERIMENTAL PROCEDURES
Yeast Strains and Plasmids
Yeast strains and plasmids used in this study are listed in the supplemental Tables S1 and S2, respectively.
UV Sensitivity Measurements
Sensitivity to killing by UV was determined by a colony formation assay. Yeast cells were grown at 30 °C in YPD to an A600 of 0.6. Each culture was diluted, spread on YPD plates, and then irradiated by UV light (254 nm) at the indicated doses. Following UV radiation, the plates were immediately wrapped with aluminum foil and incubated at 30 °C for 48 h, at which time the number of colonies was assessed.
Quantification of CPDs and 6,4-PPs
UV-associated DNA lesions were quantified by immuno-slot-blot analysis. Yeast cells were grown to early log phase in YPD, harvested, and washed with sterile water. The washed cells were resuspended in PBS (phosphate-buffered saline) and irradiated with 40 J/m2 UV (254 nm). UV-irradiated cells were allowed to repair by incubating in pre-warmed YPD. Aliquots were taken at the times indicated and genomic DNA was isolated using the glass bead method. All the above steps were carried out under yellow light to avoid photoreactivation. DNA samples were denatured in 0.1 m NaOH and boiled for 5 min before loading. At each time point, equal amounts of DNA (5 μg for 6–4PPs and 100 ng for CPDs) were immobilized on a nitrocellulose membrane (Amersham Biosciences) using the Bio-Dot SF microfiltration apparatus (Bio-Rad). Photolesions within the DNA were detected by Western blotting with antibodies against 6–4 PPs and CPDs. Quantification of the slot blots was performed using a PhosphorImager and IMAGEQUANT software (GE Healthcare). After immunoblotting, the membranes were reprobed with 32P-labeled yeast genomic DNA to determine DNA quantity in each sample as a loading control. Yeast genomic DNA was digested by BamHI and then radiolabeled using [α32P]dATP and Primer-It Random Primer Kit (Stratagene) before the reprobing.
In Vivo Ubiquitination Assay
To characterize endogenous ubiquitination of Rad4, we followed a previously described protocol (23, 24). Yeast cells were transformed with plasmid pUB221 (expressing His6-tagged ubiquitin under the copper-inducible CUP1 promoter), or the control plasmid pUB175 (expressing untagged ubiquitin under the CUP1 promoter). Cells were induced by adding CuSO4 to the growth medium to a final concentration of 0.2 mm, grown for 135 min at 30 °C, and then harvested. Protein was extracted using glass beads in buffer A (6 m guanidine-HCl, 0.1 m Na2HPO4/NaH2PO4, 10 mm imidazole, pH 8.0), which inhibits subsequent ubiquitin protease activity. The lysate was then centrifuged and 1 ml of supernatant was incubated with 100 μl of Ni-NTA resin (Qiagen; pre-equilibrated in buffer A) at 4 °C for 1 h. The beads were collected, washed three times with buffer A, three times with A/TI buffer (1 vol buffer A and 3 vol buffer TI: 25 mm Tri-Cl, pH6.8, 20 mm imidazole), and one time with buffer TI. After removing all the liquid, ubiquitinated proteins were eluted by boiling beads in 66 μl of SDS-PAGE sample buffer for 5 min. Eluates were examined by SDS-PAGE (6% gel) and Western blot analysis. Ubiquitinated Rad4 was detected using TAP-specific antibody. The membrane was reprobed with anti-ubiquitin antibody (Strategene) to assess the loading.
Rad4 Stability Assay
Yeast cells were grown at 30 °C to an A600 of 0.6. Cycloheximide was added at a final concentration of 0.5 mg/ml to block protein synthesis, and a 5-ml aliquot was withdrawn following different incubation times. The total protein in each aliquot was isolated by boiling cell pellets in 100 μl of 1× SDS loading buffer. After centrifugation, 2 μl of the supernatant was analyzed by Western blot.
Purification of Rad4 and 26 S Proteasome
Rad4 and proteasome were purified from Rad4TAP and Rpn11TAP strains, respectively. To purify TAP-tagged protein complexes, we followed a previously described protocol (25). Briefly, 1-liter cultures were grown at 30 °C to an A600 of 1.0. Cells were collected by centrifugation, and the cell pellet was resuspended in 20 ml of cold lysis buffer (10 mm Tris-Cl, pH 7.9, 10 mm KCl, 1.5 mm MgCl2, 0.5 mm DTT, 0.5 mm PMSF, and protease inhibitors), and lysed by bead-beating. After adding 2 ml of 2 m KCl, the cell lysate was centrifuged at 15,000 rpm for 1 h at 4 °C. The supernatant was dialyzed overnight in buffer D (20 mm Tris-Cl pH7.9, 50 mm KCl, 0.2 mm EDTA, 0.5 mm DTT, 0.5 mm PMSF). The dialyzed extract was transferred to a tube, supplemented with 100 μl of 2 m Tris-Cl pH7.9, 400 μl 5 m NaCl, and 200 μl 10% Nonidet P-40, and incubated with 200 μl IgG-agarose bead suspension at 4 °C for 2 h. Beads were collected and washed three times with 20 ml cold wash buffer (10 mm Tris-Cl pH 8.0, 150 mm NaCl, 0.1% Nonidet P-40), one time with 20 ml Tobacco Etch Virus (TEV) protease cleavage buffer (10 mm Tris-Cl pH8.0, 150 mm NaCl, 0.1% Nonidet P-40, 0.5 mm EDTA, 1 mm DTT). IgG beads were transferred to a 1.5-ml microcentrifuge tube and mixed with 1 ml of TEV protease cleavage buffer and 10 μl of TEV protease (Invitrogen, 10 units/μl). Beads were incubated 1 h at room temperature and cleaved proteins were saved for protein interaction analysis.
In Vivo Protein Interaction
UBP3 gene was deleted in the background of Rad4TAP and Rpn11TAP to generate Rad4TAP ubp3Δ and Rpn11TAP ubp3Δ, respectively. The full-length DNA of UBP3 was ligated into the yeast expression vector pYC2/CT (Invitrogen), in which UBP3 is under the control of galactose-inducible GAL1 promoter. The recombinant plasmid, pYC2-UBP3, was transformed into Rad4TAP ubp3Δ and Rpn11TAP ubp3Δ strains to express Ubp3 tagged with V5 and His6 epitopes. Yeast cells harboring pYC2-UBP3 were grown in yeast synthetic complete medium lacking uracil (SC-Ura−, glucose) to an A600 of 1.0. Cells were harvested and washed with sterile water, and then resuspended in galactose medium (SC-Ura−, galactose) to induce Ubp3 expression at 30 °C for 12 h. Rad4 and proteasomes were purified as described above. The association of Ubp3 with Rad4 and the proteasome was analyzed by Western blotting using anti-V5 antibody (Invitrogen). After detecting Ubp3, the membrane was stripped and reprobed with the antibody against the TAP tag.
In Vitro Protein Interaction
To express and purify Ubp3 from Escherichia coli, the full-length DNA of UBP3 was cloned into the expression vector pET30a. BL21 cells were transformed with the recombinant plasmid pET30a-UBP3 and plated onto selective agar. A single colony was picked for expansion overnight at 37 °C in 20 ml of LB/Kan media, and then diluted to 200 ml of fresh media and grown to an A600 of 0.6. Expression of Ubp3 was induced by addition of IPTG, to a final concentration of 0.8 mm, at 30 °C for 5 h. Cells were harvested by centrifugation and washed with cold water. The cell pellet was resuspended in 10 ml of cold E. coli lysis buffer (50 mm NaH2PO4, 300 mm NaCl, 10 mm imidazole, pH 8.0), and cells were lysed by sonication (10 s sonication with 8 s breaks, 24 cycles). Cell lysate was centrifuged at 13 K rpm for 20 min at 4 °C and supernatant was transferred to a fresh tube. Ubp3-His6 was purified with Ni-NTA-agarose beads according to the manufacturer's protocol (Qiagen).
Approximately 400 ng of Ubp3-His6 was mixed with 200 ng of purified Rad4 or proteasome in incubation buffer (50 mm HEPES pH 7.4, 100 mm NaCl, 1 mm MgCl2, 0.5 mm DTT) at 37 °C for 30 min. 20 μl Ni-NTA-agarose beads were added and incubated with the mixture at 4 °C for 30 min to precipitate Ubp3-His6. Beads were collected and washed extensively with wash buffer (10 mm Tris-Cl, pH 8.0, 150 mm NaCl, 0.1% Nonidet P-40, 40 mm imidazole). Proteins retained on the beads were eluted with 100 μl of wash buffer plus 250 mm imidazole, and 20 μl aliquots were analyzed by Western blot analysis.
Western Blot Quantification
We quantified Western blots using ImageQuant software for integration of bands with background correction, as previously described (Ref. 26, and manufacturer's manual). Briefly, for well-resolved bands, pixel intensities above and below the band can be used for calculation of a linear background. After subtracting the background, the signal of the band was normalized to the corresponding loading control band (actin or tubulin, in most cases), and this value was used to evaluate protein levels.
RESULTS
Cells Lacking Ubp3 Activity Are More Resistant to UV Irradiation
To identify DUBs that may play a role in NER, we examined the UV sensitivity of yeast strains deficient in individual DUBs. As shown in Fig. 1A, the ubp3 cells were more resistant to UV, whereas several other DUB mutants showed increased UV sensitivity. Cell survival curve also demonstrates that cells lacking Ubp3 gain resistance to UV irradiation (Fig. 1B). We chose to focus on Ubp3 in this study, as it was previously shown to deubiquitinate UV-arrested RNA polymerase II (RNAPII) and prevent RNAPII degradation (17), thus highlighting the likely involvement of Ubp3 in the cellular response to UV damage.
FIGURE 1.
Cells lacking Ubp3 gain resistance to UV irradiation. A, dilution series of yeast wt (MHY501) and 17 dub mutants plated on YPD plates. The plates were irradiated with or without UV light (125 J/m2) and incubated in the dark at 30 °C for 48 h. B, comparison of UV sensitivity between wt (♢) and ubp3 mutant (■). Yeast cells were diluted and plated on YPD plates, and then irradiated with UV light at doses indicated. Colony number was counted after incubating at 30 °C for 48 h. C, UV sensitivity of wt and ubp3 mutant carrying empty vector (pRS315), ubp3 mutant carrying plasmid expressing wild-type Ubp3, and ubp3 mutant carrying plasmid expressing catalytically inactive version Ubp3-C469A. All yeast strains were plated on synthetic complete medium lacking leucine. D, UV sensitivity of wt, ubp3, and bre5 cells.
All UBPs share highly conserved Cys and His boxes (15), and mutation of the conserved Cys residue of Ubp3 (C469A) abolishes its catalytic activity (27). As shown in Fig. 1C, expression of Ubp3 in the ubp3 mutant restored the lower UV resistance observed for wt cells, while plasmid containing Ubp3-C469A failed to reduce the UV resistance of ubp3. The yeast protein Bre5 is a co-factor of Ubp3, necessary for full Ubp3-deubiquitinating activity (27). We found that mutant bre5 cells were resistant to UV at a similar level to that of ubp3 cells (Fig. 1D), consistent with a function for Upb3 in suppressing UV resistance. Taken together, these data indicate that the deubiquitinase Ubp3 is a negative regulator of a cellular response to UV irradiation.
Genetic Interactions between Ubp3 and the Rad4-Rad23 Heterodimer
To test the possible effects of Ubp3 on the Rad4-Rad23 heterodimer, we mutated the UBP3 gene in the genetic background of rad23 and rad4, respectively. The rad23 mutant, in agreement with a previous report (6), showed moderate UV sensitivity (Fig. 2A). Interestingly, disruption of the UBP3 gene partially suppressed the UV sensitivity of rad23 (Fig. 2A). The extreme UV sensitivity caused by deletion of RAD4, however, was not rescued by ubp3 mutation (Fig. 2B).
FIGURE 2.
UBP3 mutation partially rescues UV resistance and NER in rad23Δ cells. A, comparison of UV sensitivity between wt (♢), rad23 null mutant (□), and rad23ubp3 double mutant (△). Yeast cells were diluted and plated on YPD plates. The plates were irradiated with UV light at the doses indicated, and colonies were counted after incubating in the dark at 30 °C for 48 h. B, UV sensitivity in wt (♢), rad4-null mutant (□), and rad4ubp3 double mutant (△). Data represent the mean ± 1 S.D. from three independent experiments. C, left, immuno-slot-blot analysis of 6–4 PPs at different repair times using an antibody specific for 6–4 PPs. “No UV” indicates yeast cells collected before UV radiation. Right, quantification of the results shown on the left, normalized to DNA loading amounts (supplemental Fig. S1). The 6–4 PP signals at time 0 were set to 100, and the remaining signal levels were plotted relative to that value. Data represent the mean ± 1 S.D. of three independent experiments. D, immuno-slot-blots of CPDs at different repair times detected by CPD-specific antibody.
Mutation of UBP3 Partially Rescues the NER Defect in rad23 Cells
UV radiation triggers numerous signaling pathways in the cell (28). The suppression of UV sensitivity in a rad23 background by deleting UBP3 could reflect enhanced NER or influence on other aspects of cellular responses to UV radiation. To characterize whether ubp3 mutation can promote NER in rad23, we directly measured the removal of UV-induced photoproducts from yeast genomic DNA at different repair times by immuno-slot-blot using antibodies specific for 6–4 PPs (Fig. 2C, left panel) and CPDs (Fig. 2D, left panel). To evaluate loading of each sample, we radiolabeled yeast genomic DNA using random sequence primers, and reprobed the membranes (supplemental Fig. S1). The quantified immuno-slot-blot antibody signals were normalized to the corresponding DNA loading signals, and the results for each time point are shown in the right hand panels of Fig. 2, C and D. The results show that global repair of 6–4 PPs and CPDs in wt cells was fast, with only about 20% of the lesions remaining 1 h after UV radiation. In agreement with previous results (13), NER in rad23 was much slower than in wt cells. We observed that the removal of 6–4 PPs and CPDs was increased in rad23ubp3 compared with rad23, although it was still less efficient than that of wt (Fig. 2, C and D). These results are consistent with the increased UV resistance, indicating that ubp3 mutation provides enhanced NER in rad23.
Ubp3 Facilitates Rad4 Degradation in Vivo
Because the ubp3 mutation partially restored UV resistance in rad23, but showed no such effect in rad4 (Fig. 2), we tested the possibility that Ubp3 regulates cellular Rad4 abundance. We examined the steady-state levels of Rad4 in a Rad4TAP strain, in which the chromosomal RAD4 was fused with the tandem affinity purification (TAP) epitope. The TAP tag consists of a calmodulin binding peptide (CBP), a tobacco etch virus protease (TEV protease) cleavage site, and two IgG binding domains of protein A (25). The Rad4TAP strain has been used previously to study Rad4 modification and degradation, and the tagged protein proved to be as functional as the unmodified Rad4 (29). Indeed, our UV survival data also indicate that Rad4TAP is fully functional in NER (supplemental Fig. S2).
The cellular levels of Rad4TAP were detected by immunoblotting with anti-TAP antibodies. As shown in Fig. 3, A and B, the abundance of Rad4 was modestly increased in ubp3 compared with wt. Mutation of RAD23 resulted in a significant reduction in Rad4, consistent with previous studies (5, 8). Importantly, the levels of Rad4 were increased by ∼70% in rad23ubp3 compared with rad23 (Fig. 3, A and B, p < 0.05), indicating that loss of Rad4 in the rad23 cells is partially but significantly restored by the ubp3 defect. Overexpression of Ubp3, however, did not show a significant effect on Rad4 levels in either wt or rad23 cells (supplemental Fig. S3), suggesting that the function of Ubp3 in regulating Rad4 may require additional factors.
FIGURE 3.
Ubp3 facilitates Rad4 degradation. A, Western blot analysis of steady-state Rad4TAP levels in wt, ubp3, rad23, and rad23ubp3 cells. The blot was probed with anti-TAP antibody (upper panel) to detect Rad4TAP, and then reprobed with anti-tubulin antibody (bottom panel). B, quantification of the results shown in A. The level of Rad4TAP in wt, normalized to the tubulin signal, was set to 1.0. The levels of normalized Rad4TAP in the mutant cells are shown relative to that of wt. Data represent the mean ± 1 S.D. of three independent experiments. Statistically significant differences were seen for the rad23 and rad23ubp3 samples (*, p < 0.05). C, Western blot analysis of HA-tagged Rad4 in wt, rad23, rad23ubp3 containing empty vector, rad23ubp3 containing plasmid expressing Ubp3, and rad23ubp3 containing plasmid expressing mutant Ubp3-C469A. The blot was probed with anti-HA and tubulin antibodies. D, quantification of the results shown in C. The levels of Rad4HA were normalized to the tubulin signal, and the Rad4HA level in wt was set to 1.0. Data represent the mean ± 1 S.D. of three independent experiments. Statistically significant differences were seen for the rad23 and rad23ubp3, rad23ubp3(Ubp3), and rad23ubp3(Ubp3-C469A) samples (*, p < 0.05). E, Western blot analysis of WT and ubp3 extracts from cells grown in the presence of protein synthesis inhibitor cycloheximide (CHX, 0.5 mg/ml) for different times indicated. The bottom panel shows anti-actin loading control. F, quantification of results shown in E. Rad4 levels were normalized to the loading control. Data represent the mean ± 1 S.D. of three independent experiments.
To test whether Rad4 levels are affected by the TAP tag, we examined cellular Rad4 levels in a Rad4HA strain, in which the chromosomal RAD4 was tagged by the small hemagglutinin (HA) epitope (4). In agreement with our observations for the Rad4TAP strain, reduced Rad4 levels in rad23 were also partially restored by mutation of UBP3 in the Rad4HA strain (Fig. 3, C and D, p < 0.05). Moreover, whereas introduction of the plasmid containing wt UBP3 reduced Rad4 abundance in rad23ubp3 to the level seen in rad23, the plasmid containing UBP3-C469A mutant was unable to affect Rad4 levels (Fig. 3, C and D), indicating that the deubiquitinating activity of Ubp3 is indispensable for down-regulating Rad4 protein levels.
Considering Rad23 is important for stabilizing Rad4 from proteasomal degradation and mutation of UBP3 can significantly rescue the loss of Rad4 caused by rad23Δ, we investigated the effect of Ubp3 on Rad4 stability in vivo. We compared Rad4 stability in both wt and ubp3 by adding the protein synthesis inhibitor cycloheximide (CHX) to actively growing yeast cells. Aliquots of cultures were then withdrawn at different times and proteins were isolated for Western blot analysis. As shown in Fig. 3, E and F, Rad4 levels in wt were decreased after 2 h of protein synthesis inhibition, whereas degradation of Rad4 in ubp3 was significantly slower than in wt. Similarly, the stability of Rad4 was also increased in rad23ubp3 compared with that in rad23 (supplemental Fig. S4), suggesting that Rad4 protein is indeed stabilized by ubp3 mutation. We also performed pulse-chase experiments with [35S]methionine and found the degradation of Rad4 in ubp3 is slower than that in wt (supplemental Fig. S5).
Physical Interaction between Ubp3 and Rad4
As a deubiquitinase, Ubp3 targets its substrates through protein-protein interaction (17, 30). To test the possibility that Rad4 is a substrate of Ubp3 binding, we verified the interaction between Rad4 and Ubp3. We transformed the Rad4TAPubp3 strain with a CEN plasmid expressing V5His6-tagged Ubp3 (Ubp3-V5His6) and tested whether Ubp3 can be co-purified with Rad4TAP. Plasmid-expressed Ubp3-V5His6 rescued the slow-growing phenotype of ubp3 on yeast media lacking uracil, suggesting that the fusion protein is functional (data not shown). The Rad4TAP protein was captured from cell extracts with IgG beads, and then specifically released using TEV protease. Purified Rad4TAP migrated slightly faster than unpurified protein (Fig. 4A, middle panel), which was caused by the TEV-directed cleavage of two IgG binding domains from the TAP epitope. Ubp3, detected by V5-specific antibody, was co-purified with Rad4TAP (Fig. 4A, upper panel), indicating that Rad4 and Ubp3 interact in vivo. The lower band may reflect partial degradation of Ubp3 during protein isolation, as it was also present in the input samples (Fig. 4A, upper panel). As a control, actin was not found in the purified Rad4TAP sample (Fig. 4A, bottom panel), suggesting that the interaction between Ubp3 and Rad4 is specific.
FIGURE 4.
Interaction between Rad4 and Ubp3. A, in vivo interaction between Rad4 and Ubp3. The plasmid containing Ubp3-V5His6 was transformed into Rad4ubp3 and Rad4TAPubp3 strains, respectively. Rad4TAP was purified as described under “Experimental Procedures.” Purified Rad4TAP was separated by electrophoresis on an 8% polyacrylamide gel and probed with V5, TAP, and actin antibodies. Cell extracts before IgG beads incubation were used as input. B, in vitro interaction between Rad4 and Ubp3. Approximately 400 ng of Ubp3-His6 protein purified from E. coli and 100 ng Rad4TAP protein purified from yeast were incubated at 37 °C. Ubp3-His6 and its associating protein were precipitated by Ni-NTA resin. Eluates were subjected to Western blot analysis with anti-TAP and anti-His antibodies. The input consisted of 10% of the protein mixture before Ni-NTA resin incubation.
In addition, we examined the interaction between Rad4 and Ubp3 in vitro. As expected, Rad4TAP was pulled down with Ubp3-His6 by Ni-NTA resin (Fig. 4B, lane 6), while Rad4TAP alone did not bind to Ni-NTA resin (Fig. 4B, lane 4), indicating that Rad4 and Ubp3 also interact in vitro.
Polyubiquitination of Rad4 Is Impaired in Cells Lacking Ubp3
Although little is known about the endogenous ubiquitination status of Rad4, several lines of evidence suggest that Rad4 ubiquitination occurs in the cell (4, 11). Because our results indicated that Rad4 protein levels are down-regulated by Ubp3, we explored the role for Ubp3 in Rad4 ubiquitination.
To detect ubiquitinated Rad4, we transformed the Rad4TAP strain with a plasmid expressing His6-tagged ubiquitin. After cell lysis in denaturing conditions (see Experimental Procedures), ubiquitinated proteins were specifically purified using nickel-affinity (Ni-NTA) chromatography, and ubiquitinated Rad4 was detected with the TAP specific antibody. Polyubiquitination of Rad4 was observed in yeast cells expressing His6-Ub (Fig. 5A, lanes 3–6), but not in the control cells expressing untagged ubiquitin (Fig. 5A, lane 2). Whole cell extract from wt cells was also loaded to show the position of unmodified Rad4 (Fig. 5A, lane 1). The same membrane was reprobed with anti-ubiquitin antibody to evaluate protein loading (Fig. 5A, bottom panel). Ubiquitinated Rad4 signal was quantified based on loading and normalized to the wt value. The relative Rad4 ubiquitination levels in different strains are shown between the panels in Fig. 5A. The quantification data indicate that Rad4 ubiquitination was reduced by ∼20% in ubp3 compared with wt (Fig. 5A). We also noticed that the polyubiquitination of Rad4 was elevated in the rad23 mutant, where the average length of polyubiquitin chain assembled on Rad4 was extended (Fig. 5A, compare lane 5 to 3). Mutation of UBP3 partially reduced Rad4 ubiquitination in rad23Δ (Fig. 5A, compare lane 6 to 5, with ubiquitination levels of 1.4 and 2.0, respectively), which is in agreement with an increase in the steady-state levels of Rad4 in rad23ubp3.
FIGURE 5.
Disruption of UBP3 leads to impaired polyubiquitination of Rad4. A, cellular Rad4 ubiquitination in WT, ubp3, rad23, and rad23ubp3 strains. Yeast cells were engineered to express His-tagged ubiquitin (+) or untagged ubiquitin (−). Protein isolation and purification of ubiquitinated proteins is described in “Experimental Procedures.” Unmodified Rad4TAP was detected in the total lysate (Input), and ubiquitinated Rad4TAP was found in the His-purified (Ni-NTA) material with antibody against the TAP tag. The asterisk (*) in each panel denotes background binding of unmodified Rad4TAP to the Ni-NTA resin. The same blot was reprobed with anti-ubiquitin antibody to show the protein loading (bottom panel). Ubiquitinated Rad4 signal was quantified and normalized to the loading signal in each lane, and the values are shown between the panels. Migration of protein size markers (in kDa) is indicated on the left. B, ubiquitination of Rad23 is not affected significantly by mutation of UBP3 in vivo. Ubiquitination of Rad23 was measured as described in A.
To characterize whether Ubp3 affects Rad4 ubiquitination via its binding partner, we assessed ubiquitination of Rad23 in wt and ubp3 strains. We found that Rad23 was also polyubiquitinated in intact cells and its ubiquitination levels were comparable between wt and ubp3 (Fig. 5B), suggesting that disruption of UBP3 impairs Rad4 ubiquitination specifically but has little effect on the ubiquitination of Rad23.
Ubp3 Associates with the Proteasome
Several studies have shown that a subset of DUBs associate with the proteasome and promote substrate proteolysis (19–21), though the interaction between Ubp3 and the proteasome has not been determined. Our results showing the down-regulation of Rad4 by Ubp3 prompted us to investigate whether Ubp3 is associated with the proteasome.
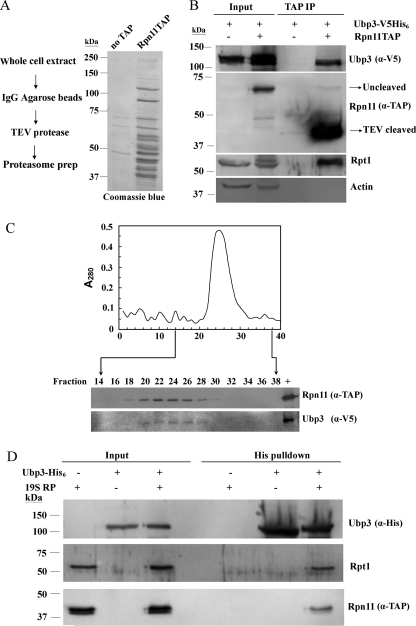
To study this potential interaction in intact cells, we transformed the plasmid expressing Ubp3-V5His6 into an Rpn11TAPubp3 strain, in which the 19 S proteasome subunit Rpn11 was tagged by the TAP epitope. The subsequent TAP-mediated purification of proteasomes was performed as described in a previous study, which found higher proteasome purity and yields than conventional methods (22). The integrity of our purified proteasomes was visualized by Coomassie Blue staining after polyacrylamide gel electrophoresis (Fig. 6A). In addition, Rpn11 and Rpt1, two subunits residing at the lid and base of the 19 S RP, respectively (12), were detected by Western blotting using TAP and Rpt1 antibodies. Both subunits were abundant in our purified material but not in the control sample which was isolated from an untagged Rpn11 yeast strain (Fig. 6B). We note that Rpn11TAP cleaved by TEV protease migrated faster than the uncleaved protein due to the loss of the IgG binding domains of protein A. Ubp3was detected in purified proteasomes but not in the control sample (Fig. 6B, upper panel). Quantification of the IP and Input fractions indicates that about 5% of the total cellular Ubp3 protein is associated with the proteasome in these pull-down experiments. Actin protein was used as a negative control and was not found in the purified sample (Fig. 6B, bottom panel). To confirm the association of Ubp3 with the proteasome in vivo, purified proteasomes were fractionated by gel filtration chromatography. Fractions were collected for Western blot analysis. Ubp3 cofractionated with the proteasomal subunit Rpn11 as well as protein content (Fig. 6C), consistent with an association of Ubp3 with the proteasome.
FIGURE 6.
Association of Ubp3 with the proteasome. A, purification of the proteasome from Rpn11TAP strain. Components of the purified proteasome were separated on an 8% gel and visualized with Coomassie Blue. B, in vivo interaction between Ubp3 and the proteasome. Yeast Rpn11ubp3 and Rpn11TAPubp3 were transformed with plasmid expressing Ubp3-V5His6. The strain in which Rpn11 was not tagged with TAP was used as the negative control. Purified proteasomes were subjected to Western blot analysis using V5, TAP, Rpt1, and Actin antibodies. C, co-fractionation of Ubp3 with the proteasome. Proteasomes were bound to IgG resin and specifically released with TEV protease. The eluate was fractionated by Sephacryl S-500 gel filtration chromatography. Fractions were collected for analysis of protein content (upper panel) and Western blot analysis (bottom panel). “+” represents ∼10% of total purified proteasomes. D, interaction between Ubp3 and the proteasome in vitro. Purified Ubp3-His6 (∼400 ng) and purified proteasome (100 ng) were co-incubated and Ni-NTA resin was added to precipitate Ubp3-His6. The presence of proteasome in the eluates was detected by Western blot with anti-TAP (to detect 19S subunit Rpn11) and Rpt1 antibodies.
The interaction between Ubp3 and the proteasome was further studied by a His-tag pull-down assay in vitro. His-tagged Ubp3 was purified from E. coli and incubated with purified proteasomes. Samples were then precipitated on a Ni-NTA resin to capture Ubp3-His6, whereas proteasomes alone cannot bind to Ni-NTA resin and was not detected in the eluates (Fig. 6D). Consistent with the in vivo data, subunits of proteasomes co-eluted with Ubp3-His6 from Ni-NTA resin (Fig. 6D, middle and bottom panel), indicating that Ubp3 interacts with the proteasome in vitro.
DISCUSSION
Protein ubiquitination plays significant roles in a variety of DNA repair processes (31). For example, human DDB2 and XPC, two damage recognition factors in NER, are polyubiquitinated (32). Ubiquitinated DDB2 is targeted for degradation by the proteasome (33), while XPC is not (34). On the other hand, Rad4, the yeast homolog of XPC, is degraded by the proteasome (8, 11). Protein ubiquitination and degradation are regulated by a large number of enzymes. Among them, E1, E2, and E3 form enzymatic cascades that attach ubiquitin moieties to substrates, while DUBs are generally thought to reverse the ubiquitination process (9, 15). Given that NER plays a key role in preventing carcinogenesis and human diseases such as XP, characterizing the ubiquitin enzymes regulating NER activity has recently attracted substantial attention.
In this study, we provide evidence that the yeast deubiquitinating enzyme Ubp3 negatively regulates NER by promoting degradation of Rad4. Among the 17 dub mutants in yeast Saccharomyces cerevisiae, only ubp3 showed increased resistance to UV irradiation (Fig. 1, A and B). In addition, mutation of UBP3 enhanced UV survival in rad23, but not in rad4 cells (Fig. 2, A and B), and repair of UV-induced photolesions was increased when UBP3 was mutated in a rad23 background (Fig. 2, C and D). Since Rad23 forms a heterodimer with Rad4 and prevents its degradation by the proteasome (4), and we found disruption of UBP3 resulted in increased steady-state levels of Rad4, especially in the absence of Rad23 (Fig. 3, A and C), we conclude that mutation of UBP3 reduces degradation of Rad4, leading to increased availability of Rad4 in cells to function in the repair of UV lesions. Indeed, our experiments using protein synthesis inhibitor cycloheximide reveal that the stability of Rad4 protein is increased when Ubp3 is absent (Fig. 3, E and F, and supplemental Fig. S4).
Ubp3 has multiple substrates and, in most cases, promotes the removal of ubiquitin from substrates, thus protecting them from degradation (17, 27). In contrast, our study demonstrates that Rad4 is destabilized by Ubp3 (Fig. 3, E and F), and Rad4 ubiquitination is decreased when Ubp3 is absent (Fig. 5A). Interestingly, Rad4 is not the sole substrate destabilized by Ubp3, as degradation of the yeast protein kinase C homolog (Pkc1), is also promoted by Ubp3 (30). These data suggest that there is an (as of yet) unidentified mechanism of Ubp3 that facilitates substrate degradation. We found that a fraction of cellular Ubp3 is associated with the proteasome, both in vivo and in vitro (Fig. 6), which provides an explanation for this “abnormal” function of Ubp3. Previous studies have demonstrated that other proteasome-associated DUBs, such as Rpn11, play essential roles in facilitating degradation through the deubiquitination of substrates at the proteasome (19, 20). Though Rpn11 appears to play a generic role without substrate specificity, it remains to be determined whether some substrates require specific DUBs to aid in proteosome-associated degradation. Our study supports a model in which Ubp3 acts as a specific deubiquitinase for Rad4 to facilitate its proteosome-associated degradation (Fig. 7). Degradation of Rad4 requires the cooperation between Ubp3 and other factors (e.g. proteasome components), as overexpresson of Ubp3 alone has little effect on Rad4 degradation (supplemental Fig. S3). Ubp3 substrate recognition is likely specific for the substrates themselves rather than their polyubiquitin chain modifications, as Ubp3 physically interacts directly with both Rad4 (Fig. 4, A and B) and Pkc1 (30). On the other hand, Rad23 plays an essential role in inhibiting proteasomal degradation of Rad4. In the absence of Rad23, the polyubiquitin chain assembled on Rad4 is elongated (Fig. 5A), which presumably enhances the affinity of Rad4-Ub for the proteasome and results in a reduction in the steady-state levels of Rad4.
FIGURE 7.
A model for the regulation of Rad4 by Ubp3. Ubiquitination of Rad4 is catalyzed by a ubiquitin enzymatic cascade. Rad23 forms a heterodimer with Rad4 and inhibits polyubiquitin chain assembly on Rad4, thus preventing its degradation. Polyubiquitinated Rad4 is targeted for proteasomal degradation. Ubp3 associated with the proteasome recognizes Rad4 and removes its polyubiquitin chain, facilitating the entry of Rad4 into the CP for proteolysis.
Previous studies have led to different conclusions regarding the regulation of Rad4 by Rad23. It was reported that Rad23 prevents proteasomal degradation of Rad4 (4), but it was also reported that transcription of Rad4 is down-regulated in rad23 cells (8). In the present report, we find that Rad23 inhibits polyubiquitin chain assembly on Rad4, indicating that Rad23 inhibits Rad4 degradation by preventing Rad4 ubiquitination. A defect in Ubp3 may affect deubiquitination of Rad4 at the proteasome and thus slow down Rad4 turnover, leading to recovery of Rad4 levels in rad23 cells. Consistent with this hypothesis, the function of Ubp3 in regulating Rad4 is highly dependent on its deubiquitinating activity (Figs. 1C and 3, C and D).
In contrast to the function of Ubp3 as a “normal” deubiquitinase in reducing ubiquitination of substrates, mutation of UBP3 results in decreased ubiquitination of Rad4 (Fig. 5A). In addition, Ubp3 appears to affect Rad4 ubiquitination specifically, as polyubiquitination of Rad23 is not affected by this mutation (Fig. 5B). Also notable, is that it appears that disruption of UBP3 leads to a more significant enhancement of Rad4 stability than is accounted for by simply decreased Rad4 ubiquitination, as UBP3 mutation causes an ∼70% increase in Rad4 levels whereas there is only an ∼30% decrease in Rad4 polyubiquitination in rad23 cells (Figs. 3A and 5A). This difference may reflect that Ubp3, by associating with the proteasome, primarily facilitates Rad4 degradation. The defect in proteosome-mediated degradation of Rad4 in ubp3 cells may have some unknown feedback effect on Rad4 ubiquitination (Fig. 7). This effect appears to also exist in mammalian cells, as a recent study shows that disrupting Usp14 or Uch37, two proteasome-associating DUBs in human cells, leads to impaired accumulation of cellular polyubiquitinated proteins (35). Alternatively, nonproteasomal Ubp3 may prevent degradation of ubiquitination enzymes and thus indirectly enhance Rad4 ubiquitination. Indeed, the ubiquitin conjugating enzyme Ubc4 has been shown to down-regulate Rad4 abundance (4). We also found a decrease in Rad4 ubiquitination in ubc4 cells (supplemental Fig. S6A). However, we did not observe decreased Ubc4 protein levels in either ubp3 or bre5 cells compared with wt (supplemental Fig. S6B). These results indicate that Ubp3-targeted degradation of Ubc4 is not a mechanism for promoting Rad4 ubiquitination. It will be interesting to determine the levels of other ubiquitination enzymes that affect Rad4 ubiquitination (e.g. Rad7-Rad16 E3 complex) (8) in the ubp3 background.
Finally, genomic analysis indicates that the likely human homolog of yeast Ubp3 is Usp10, a protein with unknown function in DNA repair. Clearly, it will also be of interest to investigate the potential for Usp10-dependent regulation of XPC levels and ubiquitination, as well as NER capacity, in human cells.
Acknowledgments
We thank Drs. Mark Hochstrasser (Yale University) and Kiran Madura (Robert Wood Johnson Medical School) for yeast strains, Daniel Finley (Harvard Medical School) and Matthias Peter (ETH Zürich, Switzerland) for plasmids, and Kiran Madura for antibodies used in this study. We also thank Drs. John Hinz and Mingrui Duan (Washington State University) for critical reading of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant ES002614 from NIEHS.

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1–S6 and Table S1 and S2.
- NER
- nucleotide excision repair
- DUB
- deubiquitinase
- Ub
- ubiquitin
- CPD
- cyclobutane pyrimidine dimer
- 6-4PPs
- pyrimidine-6-4-pyrimidone photoproducts
- NTA
- nitrilotriacetic acid
- UPP
- ubiquitin proteasome pathway.
REFERENCES
- 1.Friedberg E. C., Walker G. C., Siede W., Wood R. D., Schultz R. A., Ellenberger T. (2006) DNA Repair and Mutagenesis, 2nd Ed., pp. 317–343, ASM Press, Washington, D. C. [Google Scholar]
- 2.Hoeijmakers J. H. (2001) Nature 411, 366–374 [DOI] [PubMed] [Google Scholar]
- 3.Min J. H., Pavletich N. P. (2007) Nature 449, 570–575 [DOI] [PubMed] [Google Scholar]
- 4.Ortolan T. G., Chen L., Tongaonkar P., Madura K. (2004) Nucleic Acids Res. 32, 6490–6500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xie Z., Liu S., Zhang Y., Wang Z. (2004) Nucleic Acids Res. 32, 5981–5990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mueller J. P., Smerdon M. J. (1996) Mol. Cell. Biol. 16, 2361–2368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ng J. M., Vermeulen W., van der Horst G. T., Bergink S., Sugasawa K., Vrieling H., Hoeijmakers J. H. (2003) Genes Dev. 17, 1630–1645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gillette T. G., Yu S., Zhou Z., Waters R., Johnston S. A., Reed S. H. (2006) EMBO J. 25, 2529–2538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kerscher O., Felberbaum R., Hochstrasser M. (2006) Annu. Rev. Cell Dev. Biol. 22, 159–180 [DOI] [PubMed] [Google Scholar]
- 10.Schauber C., Chen L., Tongaonkar P., Vega I., Lambertson D., Potts W., Madura K. (1998) Nature 391, 715–718 [DOI] [PubMed] [Google Scholar]
- 11.Lommel L., Ortolan T., Chen L., Madura K., Sweder K. S. (2002) Curr. Genetics 42, 9–20 [DOI] [PubMed] [Google Scholar]
- 12.Hanna J., Finley D. (2007) FEBS Lett. 581, 2854–2861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gillette T. G., Huang W., Russell S. J., Reed S. H., Johnston S. A., Friedberg E. C. (2001) Genes Dev. 15, 1528–1539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Amerik A. Y., Li S. J., Hochstrasser M. (2000) Biol. Chem. 381, 981–992 [DOI] [PubMed] [Google Scholar]
- 15.Hochstrasser M. (1996) Annu. Rev. Genetics 30, 405–439 [DOI] [PubMed] [Google Scholar]
- 16.Baker R. T., Tobias J. W., Varshavsky A. (1992) J. Biol. Chem. 267, 23364–23375 [PubMed] [Google Scholar]
- 17.Kvint K., Uhler J. P., Taschner M. J., Sigurdsson S., Erdjument-Bromage H., Tempst P., Svejstrup J. Q. (2008) Mol. Cell 30, 498–506 [DOI] [PubMed] [Google Scholar]
- 18.Finley D. (2009) Annu. Rev. Biochem. 78, 477–513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao T., Cohen R. E. (2002) Nature 419, 403–407 [DOI] [PubMed] [Google Scholar]
- 20.Verma R., Aravind L., Oania R., McDonald W. H., Yates J. R., 3rd, Koonin E. V., Deshaies R. J. (2002) Science 298, 611–615 [DOI] [PubMed] [Google Scholar]
- 21.Papa F. R., Amerik A. Y., Hochstrasser M. (1999) Mol. Biol. Cell 10, 741–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Leggett D. S., Hanna J., Borodovsky A., Crosas B., Schmidt M., Baker R. T., Walz T., Ploegh H., Finley D. (2002) Mol. Cell 10, 495–507 [DOI] [PubMed] [Google Scholar]
- 23.Kaiser P., Tagwerker C. (2005) Methods Enzymol. 399, 243–248 [DOI] [PubMed] [Google Scholar]
- 24.Geng F., Tansey W. P. (2008) Mol. Biol. Cell 19, 3616–3624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rigaut G., Shevchenko A., Rutz B., Wilm M., Mann M., Séraphin B. (1999) Nat. Biotechnol. 17, 1030–1032 [DOI] [PubMed] [Google Scholar]
- 26.Li S., Waters R., Smerdon M. J. (2000) Methods 22, 170–179 [DOI] [PubMed] [Google Scholar]
- 27.Cohen M., Stutz F., Belgareh N., Haguenauer-Tsapis R., Dargemont C. (2003) Nat. Cell Biol. 5, 661–667 [DOI] [PubMed] [Google Scholar]
- 28.Herrlich P., Karin M., Weiss C. (2008) Mol. Cell 29, 279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.den Dulk B., van Eijk P., de Ruijter M., Brandsma J. A., Brouwer J. (2008) DNA Repair 7, 858–868 [DOI] [PubMed] [Google Scholar]
- 30.Wang Y., Zhu M., Ayalew M., Ruff J. A. (2008) J. Biol. Chem. 283, 1954–1961 [DOI] [PubMed] [Google Scholar]
- 31.Bergink S., Jentsch S. (2009) Nature 458, 461–467 [DOI] [PubMed] [Google Scholar]
- 32.Sugasawa K., Okuda Y., Saijo M., Nishi R., Matsuda N., Chu G., Mori T., Iwai S., Tanaka K., Tanaka K., Hanaoka F. (2005) Cell 121, 387–400 [DOI] [PubMed] [Google Scholar]
- 33.Chen X., Zhang Y., Douglas L., Zhou P. (2001) J. Biol. Chem. 276, 48175–48182 [DOI] [PubMed] [Google Scholar]
- 34.Sugasawa K. (2006) J. Mol. Histol. 37, 189–202 [DOI] [PubMed] [Google Scholar]
- 35.Koulich E., Li X., DeMartino G. N. (2008) Mol. Biol. Cell 19, 1072–1082 [DOI] [PMC free article] [PubMed] [Google Scholar]