Abstract
Estrogen enhances antibody and autoantibody responses through yet to be defined mechanisms. It has been suggested that estrogen up-regulates the expression of activation-induced cytosine deaminase (AID), which is critical for antibody class switch DNA recombination (CSR) and somatic hypermutation (SHM), through direct activation of this gene. AID, as we have shown, is induced by the HoxC4 homeodomain transcription factor, which binds to a conserved HoxC4/Oct site in the AICDA/Aicda promoter. Here we show that estrogen-estrogen receptor (ER) complexes do not directly activate the AID gene promoter in B cells undergoing CSR. Rather, they bind to three evolutionarily conserved and cooperative estrogen response elements (EREs) we identified in the HOXC4/HoxC4 promoter. By binding to these EREs, ERs synergized with CD154 or LPS and IL-4 signaling to up-regulate HoxC4 expression, thereby inducing AID and CSR without affecting B cell proliferation or plasmacytoid differentiation. Estrogen administration in vivo significantly potentiated CSR and SHM in the specific antibody response to the 4-hydroxy-3-nitrophenylacetyl hapten conjugated with chicken γ-globulin. Ablation of HoxC4 (HoxC4−/−) abrogated the estrogen-mediated enhancement of AID gene expression and decreased CSR and SHM. Thus, estrogen enhances AID expression by activating the HOXC4/HoxC4 promoter and inducing the critical AID gene activator, HoxC4.
Keywords: Antibodies, Estrogen, Gene Expression, Gene Regulation, Immunology, AID, HoxC4, Immunoglobulin Class Switch DNA Recombination, Immunoglobulin Somatic Hypermutation
Introduction
Females mount better antibody responses to microbial antigens, as presented in vaccines or as occurring in natural infections, than males (1). For example, neutralizing antibody titers in women in response to seasonal flu vaccination are equivalent to those of men but in response to half the male dose (2, 3). Comparable or greater differences in females versus males are observed in antibody responses to vaccines for smallpox, mumps, rubella, measles, hepatitis A and hepatitis B, and herpes simplex virus in humans and mice (4–12). This has been suggested to be due to the higher level of estrogen in females than in males (8, 11). The higher level of estrogen also probably underlies the stronger response to self-antigens in females than in males (13, 14). Indeed, a female predominance of autoimmunity involving pathogenic autoantibodies, such as anti-double strand DNA autoantibodies in lupus, is well documented (15, 16). In lupus patients, increased estrogen levels underlie increased autoantibody levels, severity of the disease, and pathology (17–19). In lupus-prone mice, increased estrogen levels lead to higher titers of pathogenic autoantibodies and accelerated disease expression (15). Like antibodies to microbial pathogens, pathogenic autoantibodies in autoimmune humans and mice are hypermutated and class-switched, suggesting a role for estrogen in modulating Ig class switch DNA recombination (CSR)4 and somatic hypermutation (SHM).
CSR and SHM are critical for the maturation of antibody response to foreign antigens and self-antigens. CSR recombines DNA of two switch regions, each located upstream of different heavy chain constant (CH) region exon clusters, thereby changing the Ig CH region and endowing antibodies with new biological effector functions. SHM introduces mainly point mutations in Ig variable (diversity) joining (V(D)J) region DNA, thereby providing the structural substrate for selection of higher affinity antibody mutants by antigen. Both CSR and SHM require the intervention of AID, which is expressed mainly in activated B cells in germinal centers (GCs) of peripheral lymphoid organs (20, 21). AID initiates CSR and SHM by deaminating dC residues to yield dU:dG mispairs in DNA (20–26). These dU:dG mispairs trigger DNA repair processes entailing introduction of mismatches (mutations) by error-prone translesion DNA synthesis polymerases in V(D)J regions and double strand DNA breaks in switch regions, leading to CSR (20, 21, 27–36). As we have shown, AID is specifically targeted to switch region DNA by 14-3-3 adaptor proteins, which also enhance AID-mediated dC deamination (37). As we have also shown, in both human and mouse B cells, HOXC4/HoxC4 expression is induced by GC differentiation-inducing stimuli, such as CD154 or LPS and IL-4, that are required for induction of AICDA/Aicda (human/mouse activation-induced cytosine deaminase) expression (38–40). The conserved homeodomain HoxC4 transcription factor is a critical activator of the AID gene promoter (40, 41). HoxC4 binds directly to a highly conserved HoxC4/Oct site in the AICDA/Aicda promoter and activates this promoter in synergy with Oct-1/2, NF-κB, and Sp1/Sp3 (40).
Estrogen freely diffuses through cytoplasmic and nuclear membranes and binds to intracellular estrogen receptors (ERs), including ERα and ERβ. Estrogen-bound ERs function primarily as transcription factors by interacting specifically with estrogen response elements (EREs) in the promoter of estrogen-responsive genes (42). Both ERα and ERβ play a role in modulating B cell development and differentiation, and both induce expression of HOX/Hox genes, including HOXC/HoxC genes, in a variety of cell types (43–45). In human breast cancer MCF-7 cells, estrogen rapidly and effectively induces HOXC4 together with the neighboring HOXC5 and HOXC6 genes (46). After estrogen withdrawal, whereas HOXC5 and HOXC6 expression quickly returns to the prestimulation level, HOXC4 expression persists at a steady high level. HoxC4 expression is probably responsible for the estrogen-induced AID expression reported in MCF-7 cells (47), particularly in light of our demonstration of the critical role of HoxC4 in activating the AICDA/Aicda promoter in B cells (33, 34, 40). This prompted us to reconsider the suggestion that estrogen up-regulates AID gene expression through binding of ERs to the AICDA promoter (47). Further, such a suggestion stemmed from gene reporter assays using a human uterine cervix cancer (SiHa) cell line and the identification of a single, non-conserved putative ERE lying upstream of the AID promoter (47). Like MCF-7 cells, SiHa cells express HoxC4 upon exposure to estrogen (46),5 and the role of a single putative ERE is at odds with the established notion that highly estrogen-responsive genes characteristically rely on the existence of multiple and cooperative EREs in their promoters for effective activation (48–52).
We have used in vitro and in vivo approaches involving HoxC4−/− B cells and HoxC4−/− mice to test the hypothesis that estrogen regulates AID expression (indirectly) through activation of the HOXC4/HoxC4 promoter and expression of this gene. In experiments using B cells, we found the single putative ERE upstream of the AICDA promoter (47) to be non-functional. Instead, consistent with the requirement for multiple EREs for effective activation of estrogen-responsive genes, we identified three evolutionarily conserved and cooperative EREs in the HOXC4/HoxC4 promoter. Estrogen-ER complexes bound to these three EREs and potentiated the activation of the HOXC4/HoxC4 promoter, as induced by CD154 or LPS, thereby leading to AID induction. Accordingly, estrogen significantly enhanced CSR and SHM in the in vivo antibody response to NP-CGG in HoxC4+/+ but not HoxC4−/− mice. Thus, estrogen enhances AID gene expression, CSR, and SHM by directly activating the HOXC4/HoxC4 promoter, thereby potentiating the induction of HoxC4, a critical activator of the AID gene promoter.
EXPERIMENTAL PROCEDURES
Mice; in Vivo CSR and SHM
HoxC4−/− mice were generated as described (40). HoxC4−/− mice and their HoxC4+/+ C57BL/6 littermates were bred under pathogen-free conditions and were used (8–12 weeks of age) to study the effect of estrogen on HoxC4 and Aicda induction as well as CSR and SHM in vivo. Female HoxC4−/− and HoxC4+/+ mice were injected subcutaneously in the back with Oil (100 μl of corn oil) or E2-Oil (0.5 μg of E2 (Sigma-Aldrich) dissolved in 100 μl of corn oil) daily for 11 days; 1 day after the first Oil or E2-Oil injection, these mice were injected intraperitoneally with NP16-CGG (Biosearch Technologies) in alum (Imject® alum, Pierce) (40). Mice were sacrificed 1 day after the last Oil or E2-Oil injection to analyze spleen, lymph node, and/or Peyer's patch B cells. All animal work was approved by the Institutional Animal Care and Use Committee of the University of California, Irvine.
B Cells and CSR
Single cell suspensions were prepared from spleen, Peyer's patches, or pooled (axillary, brachial, mesenteric, and inguinal) lymph nodes of female mice. B cells were stained with phycoerythrin-labeled rat mAb to mouse CD45R (B220) (clone RA3-6B2, eBioscience), Alexa Fluor® 647-labeled peanut agglutinin (PNA) (Invitrogen), fluorescein isothiocyanate (FITC)-labeled rat mAb to mouse IgG1 (clone A85-1, BD Biosciences), FITC-labeled rat mAb to mouse IgG3 (clone R40-82, BD Biosciences), FITC-labeled rat mAb to mouse IgE (clone 23G3, eBiosciences), and/or FITC-labeled rat mAb to mouse CD138 (clone 281-2, BD Biosciences) before fixation with 1% paraformaldehyde in PBS and florescence flow cytometry analysis (53).
The monoclonal human 2E2 B cell line (54) was derived from our CSR- and SHM-inducible human monoclonal IgM+IgD+ CL-01 B cell line (55) by sequential subcloning. 2E2 B cells are IgM+IgD+ and can be induced to express HoxC4 and AID and undergo CSR to IgG, IgE, and IgA upon stimulation by an agonistic anti-huCD40 mAb and appropriate cytokines. 2E2 B cells were precultured in phenol red-free RPMI medium, supplemented with 5% charcoal-stripped FBS (Invitrogen), 2 mm l-glutamine, 100 units/ml penicillin, 100 ng/ml streptomycin (FBS-RPMI) for 72 h. They were induced to undergo CSR to IgG1 by stimulation with anti-huCD40 mAb (IgG1 mAb G28–5, ATCC) and rhIL-4 (37, 39, 40) (Genzyme) in the presence or absence of E2.
Primary mouse B cells (red blood cell-depleted splenocytes from non-intentionally immunized female mice) were cultured (5 × 105 cell/ml) in FBS-RPMI containing 50 mm β-mercaptoethanol in 48-well plates and stimulated with either CD154-expressing membrane fragments of baculovirus-infected Sf21 insect cells (referred to as CD154 herein) (37, 39, 40) or LPS (5 μg/ml) from Escherichia coli (serotype 055:B5, Sigma-Aldrich), in the presence of nil for CSR to IgG3 or rmIL-4 (5 ng/ml, R&D Systems) for CSR to IgG1 and IgE, and in the presence or absence of E2. B cell surface IgG1, IgG3 or IgE were analyzed using a FACSCaliburTM flow cytometer (BD Biosciences) (40, 56).
B Cell Proliferation and Apoptosis
Cell proliferation and viability were analyzed by flow cytometry (40, 57). To measure in vivo proliferation, BrdU was injected into mice 16 and 4 h before sacrifice, after which recovered B cells were stained with anti-BrdU antibody. In vitro proliferation was analyzed using the CellTraceTM CFSE cell proliferation kit (Molecular Probes). B cells were washed in serum-free HBSS (Invitrogen) and resuspended at 5 × 105 cells/ml. After adding an equal volume of 2.4 mm CFSE, B cells were incubated at 37 °C for 12 min and then washed in FBS-RPMI before setting them in culture for the induction of CSR. To measure apoptosis, the DNA-binding dye 7-aminoactinomycine (7-AAD) was added to B cells, which were collected for analysis 3 or 4 days later as indicated.
Chromatin Immunoprecipitation (ChIP) Assay
ChIP assays were performed as described (31, 37, 40). Briefly, B cells (5 × 107) were treated with 1% formaldehyde for 10 min at 25 °C to cross-link chromatin. After washing with cold PBS containing protease inhibitors (Roche Applied Science), chromatin was separated using nuclear lysis buffer (10 mm Tris-HCl, 1 mm EDTA, 0.5 m NaCl, 1% Triton X-100, 0.5% sodium deoxycholate, 0.5% Sarkosyl, pH 8.0) and resuspended in IP-1 buffer (20 mm Tris-HCl, 200 mm NaCl, 2 mm EDTA, 0.1% sodium deoxycholate, 0.1% SDS, protease inhibitors). Chromatin was sonicated to yield ∼0.2–1.0-kb DNA fragments, precleared with agarose beads bearing protein G (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and then incubated with rabbit polyclonal Abs to ERα (HC-20, Santa Cruz Biotechnology, Inc.) at 4 °C. After overnight incubation, immune complexes were isolated using agarose beads bearing protein G, eluted with elution buffer (50 mm Tris-HCl, 0.5% SDS, 200 mm NaCl, 100 μg/ml Proteinase K, pH 8.0), and then incubated at 65 °C overnight to reverse formaldehyde cross-links. DNA was extracted by phenol/chloroform and precipitated by ethanol and then resuspended in TE buffer (10 mm Tris-HCl, pH 8.0, 1 mm EDTA). DNA sequences were specified by PCR using the following primers: forward 5′-ACTGGGCTTTTAGAGCAGCA-3′ (huAIDPRF) and reverse 5′-ACAGTGCATTGGCCCTTC-3′ (huAIDPRR) for human AICDA; forward 5′-GGAGGCAGATGTTGGATACC-3′ (moAIDPRF) and reverse 5′-ATATCGGTCTCCAGCGTGAC-3′ (moAIDPRR) for mouse Aicda; forward 5′-TGGACTCTGGTATCCTTGGATG-3′ (huHoxC4PRF1) and reverse 5′-ACTGTCGGGAGGCCAGTGTTCAGC-3′ (huHoxC4PRR2) for human HOXC4; forward 5′-CTGAAATCTGCTTTTGCCAACC-3′ (MoHoxC4PF1) and reverse 5′-CCATTCACGGATCCTCAAGTCCATTTCC-3′ (MoHoxC4PR2) for mouse HoxC4.
Electrophoretic Mobility Shift Assays (EMSAs)
Nuclear extracts from B cells were prepared using a microprocedure involving hypotonic lysis followed by high salt nuclei extraction. The oligonucleotides containing one of the three HoxC4 promoter EREs or disrupted EREs were as follows: ERE-1, 5′-AAATGACGTCAGAATCATTTGCATC-3′; ERE-2, 5′-GGGGCGCTGCCCCGTGACCTACACAGAC-3′; ERE-3, 5′-GGCTGAATCACTGCCTCCCGACAGT-3′; ERE-1mut, 5′-CCTCCAGAAATGACGTCAGAATCATTTGCATCCCGCTGCCTCTACC-3′; ERE-2mut, 5′-CCTCTTCCTGCGAAAGAGGGGCGCTGCCCGTGACCTACACAGACTGAG-3′; ERE-3mut, 5′-GGGGCTGAATCACTGCCTCCCGACAGTCCCC-3′. All reactions were performed as reported (38–40). For supershift EMSA reactions, 5 μg of rabbit IgG or anti-ERα polyclonal antibody (HC-20, Santa Cruz Biotechnology) was preincubated with nuclear extracts for 30 min prior to the addition of probe. All EMSA gels were 7% polyacrylamide and subjected to electrophoresis in 0.25× TBE buffer (pH 7.5). Gels were dried and exposed for autoradiography.
Immunoblotting
B cell extracts were fractionated through 12% SDS-PAGE. Proteins were blotted onto polyvinylidene difluoride membranes (Bio-Rad) overnight (30 V) at 4 °C and then detected using primary (1:2000 to 1:5000) and secondary (1:2500) Abs. After washing with PBS-Tween 20 (0.05%), bound HRP-conjugated Abs or mAbs were detected using Western Lightning® Plus-Enhanced Chemiluminescence reagents (PerkinElmer Life Sciences).
Luciferase Gene Reporter Assays
The reporter constructs consisted of the pGL3-enhancer firefly luciferase gene reporter vector (Promega) containing 1.5- or 1.0-kb DNA sequences upstream of the AICDA transcription initiation site (identical to the corresponding sequences used by Pauklin et al. (47) (supplemental Fig. S2) or the 0.88-kb HOXC4 upstream region (residues −775 to +40 (Fig. 6)). These DNAs were amplified by PCR from human PBMC genomic DNA using the following primers: AICDA unMut (1.5 kb), forward 5′-ACTGACACGCGTGGAAAGACGGCAAGAA-3′ and reverse 5′-CGGACTCTCGAGAGTCTGACAGTGCATTGG-3′; AICDA Mut1 (1.0 kb), forward 5′-ACTGACACGCGTATGGTATCAAAGGCTTGA-3′ and reverse 5′-CGGACTCTCGAGAGTCTGACAGTGCATTGG-3′; HOXC4 unMut (0.88 kb), forward 5′-CGGCGGACGCGTGGGGAAGGCGAGACTTCTGAACTC-3′ and reverse 5′-CGGCGGCTCGAGCTGGAGTGGAAAATAATTTTTCTCG-3′.
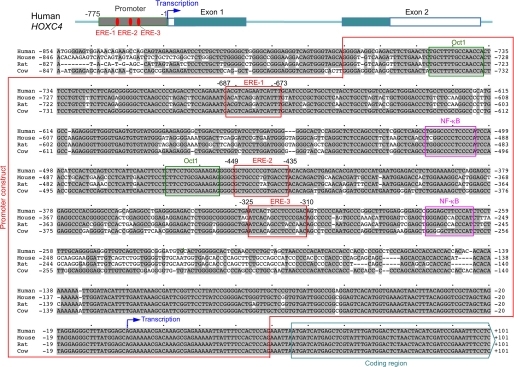
FIGURE 6.
The promoter region of HOXC4/HoxC4 contains three conserved EREs. Alignment of the HOXC4/HoxC4 upstream region, promoter, 5′ non-coding region, and beginning of the coding region sequences in humans, mice, rats, and cows. The three conserved EREs are boxed in red; the conserved Oct-binding sites are boxed in green; the conserved NF-κB-binding sites are boxed in pink. The region utilized for the HOXC4 promoter luciferase gene reporter construct (Promoter construct) is indicated by a red frame. The blue arrow marks the putative initiation of transcription. The beginning of the coding region is indicated. Gray shading marks DNA sequences conserved among the four species.
Various mutant gene reporters were constructed by PCR-based mutagenesis. Sequences of constructs were confirmed by at least two sequencing reactions. Co-transfection of the reporter construct and the constitutively active Renilla reniformis luciferase vector pRL-TK (Promega) was performed by electroporation of human 2E2 B cells (38–40). For transfection of primary mouse spleen B cells, the cells were preactivated with LPS for 24 h to induce blast formation. Preactivated B cells were transfected with reporter construct and control pRL-TK vector using an Amaxa® Mouse B Cell Nucleofector® kit and Nucleofector® device (Lonza Cologne AG). The transfected B cells were cultured in phenol red-free RPMI medium containing 5% charcoal-stripped FBS and immediately treated as indicated in each condition for 24 h. Firefly and Renilla reniformis luciferase activities were measured using the Dual-Luciferase® reporter assay system (Promega) according to the manufacturer's instructions.
Quantitative Real-time RT-PCR (qRT-PCR)
RNA was extracted from human or mouse B cells using the RNeasy minikit (Qiagen) according to the manufacturer's protocol. Residual DNA was removed by treatment with DNase I (Invitrogen). First strand cDNAs were synthesized from equal amounts of total RNA (2 μg) using the SuperScriptTM preamplification system and oligo(dT) primer (Invitrogen). Germ line IH-CH, circle IH-Cμ, postrecombination Iμ-CH, mature VHDJH-Cγ1, HOXC4/HoxC4, and AICDA/Aicda transcripts were quantified by qRT-PCR using appropriate primers (37, 39, 40) and the DNA Engine Opticon 2 real-time PCR detection system (Bio-Rad) to measure the incorporation of SYBR Green (New England BioLabs) according to the following protocol: 95 °C for 5 min, 40 cycles of 95 °C for 10 s, 60 °C for 20 s, 72 °C for 30 s, 80 °C for 1 s, and data acquisition at 80 °C and 72 °C for 10 min. Melting curve analysis was performed at 72−95 °C, and samples were incubated for another 5 min at 72 °C. The ΔΔCt method was used for data analysis.
Somatic Mutations in V186.2DJH-Cγ1 Transcripts
Spleen B cells were isolated from HoxC4+/+ and HoxC4−/− littermate female mice that were injected with Oil or E2-Oil and immunized with NP16-CGG. Rearranged V186.2DJH-Cγ1 cDNA coding for the anti-NP IgH chain was amplified using a V186.2 leader-specific forward primer (5′-CATGCTCTTCTTGGCAGCAACAGC-3′) and a reverse Cγ1 primer (5′-CCATGGAGTTAGTTTGGGCAG-3′) (57) and Phusion® high fidelity DNA polymerase (New England Biolabs). PCR conditions were 98 °C for 15 s, 58 °C for 45 s, and 72 °C for 45 s for 35 cycles. PCR products were cloned into the pCR-Blunt II-TOPO® vector (Invitrogen) and sequenced. Sequences were analyzed using the MacVectorTM 7.2.3 software (MacVector, Inc.).
Statistical Analysis
Differences in the frequency and spectrum of mutations were analyzed with χ2 tests. Differences in mRNA expression and luciferase activity were analyzed using paired t tests.
RESULTS
Estrogen Enhances CD40- and IL-4-mediated HOXC4, AICDA Expression, and CSR in B Cells
We have shown that stimulation of B cells with CD154 and IL-4 up-regulates HoxC4, which in turn activates the AID gene promoter to induce AID expression (38–40). To determine the effects of estrogen on HoxC4, HoxC4-mediated AID induction, and CSR, we used an agonistic anti-CD40 mAb, which mimics CD154, and recombinant human IL-4 (rhIL-4) to induce human monoclonal IgM+2E2 B cells (54) to undergo CSR to IgG1 in the absence or presence of 2.5, 5, 10, 20, or 30 nm 17β-estradiol (E2) (47), the most abundant estrogen in vivo. E2 alone did not induce appreciable HOXC4 and AICDA expression in 2E2 B cells but powerfully enhanced anti-CD40 mAb and rhIL-4-induced HOXC4 and AICDA expression, as measured by qRT-PCR; the E2 enhancement was dose-dependent and was slightly reduced at the highest E2 dose (30 nm) (Fig. 1, A and B). This enhancement was associated with increased CSR to IgG1, as indicated by increased circle Iγ1-Cμ and mature VHDJH-Cγ1 transcripts (Fig. 1C), and normal B cell viability and proliferation (Fig. 1, D and E). Thus, E2 alone does not significantly induce HoxC4 and AID expression. Rather, it powerfully enhances CD40- and IL-4-mediated HoxC4 induction, AID expression, and CSR.
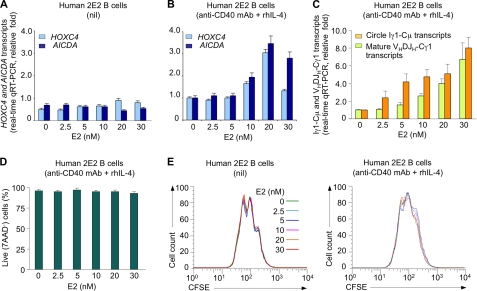
FIGURE 1.
Estrogen enhances CD40 and IL-4-mediated HOXC4, AICDA expression, and CSR. Human 2E2 B cells were treated with E2 at the indicated concentrations in the presence or absence of anti-CD40 mAb and rhIL-4. A, E2 alone does not increase HOXC4 or AICDA mRNA expression. B, E2 enhanced in a dose-dependent manner HOXC4 and AICDA expression in 2E2 B cells stimulated by anti-CD40 mAb and rhIL-4. C, E2 enhanced anti-CD40 mAb and rhIL-4-induced CSR to IgG1, as measured by circle Iγ1-Cμ and mature VHDJH-Cγ1 transcripts. 2E2 B cells were cultured with the indicated concentration of E2 in the presence of nil or anti-CD40 mAb and rhIL-4 for 48 h. HOXC4, AICDA, circle Iγ1-Cμ, and mature VHDJH-Cγ1 transcripts were measured by real-time qRT-PCR using SYBR Green and normalized to GAPDH expression. Their expression level is depicted relative to that in 2E2 B cells cultured with anti-CD40 mAb and rhIL-4 in the absence of E2, set as 1. Data are from three independent experiments (mean ± S.E. (error bars)). D, E2 does not alter B cell viability. 2E2 B cells were cultured with indicated concentration of E2 in the presence of anti-CD40 mAb and rhIL-4 for 4 days and labeled with 7-AAD before flow cytometry analysis; the cells not stained by 7-AAD were viable. Data are from three independent experiments (mean ± S.E.). E, E2 does not alter B cell proliferation. 2E2B cells were labeled with CFSE, cultured with E2 at the indicated concentrations together with nil or anti-CD40 mAb and rhIL-4, and harvested 4 days later for flow cytometry analysis. Data are representative of three independent experiments.
Estrogen-mediated Enhancement of AID Expression and CSR Are Abrogated in HoxC4−/− B Cells
To further address the role of HoxC4 in estrogen enhancement of CD40-mediated AID induction and CSR, we cultured mouse HoxC4+/+ and HoxC4−/− B cells (40) with nil or E2 (10 nm) in the presence of LPS and rmIL-4, CD154 and rmIL-4, or LPS only. E2 significantly enhanced CSR to IgG1, IgE, and IgG3 in HoxC4+/+ but not HoxC4−/− B cells, as shown by increased surface IgG1+, IgE+, and IgG3+ B cells as well as circle Iγ1-Cμ, Iϵ-Cμ, and Iγ3-Cμ and postrecombination Iμ-Cγ1, Iμ-Cϵ, and Iμ-Cγ3 transcripts, without alteration of germ line Iμ-Cμ, Iγ1-Cγ1, Iϵ-Cϵ, or Iγ3-Cγ3 transcripts (Fig. 2, A and B, and supplemental Fig. S1, A and B). In HoxC4+/+ B cells, LPS and mrIL-4-induced HoxC4 and AID expression was time-dependent, with HoxC4 being expressed before AID. Further, in LPS and rmIL-4-, CD154 and rmIL-4-, or LPS only-stimulated B cells, E2 powerfully enhanced the levels of HoxC4 and Aicda transcripts and HoxC4 and AID proteins (Fig. 2, C and D, and supplemental Fig. S1C). Remarkably, the E2-mediated up-regulation of HoxC4 and AID induction was abrogated in HoxC4−/− B cells. Finally, E2 enhancement of induction of HoxC4 and AID and CSR did not result from altered ERα expression, increased B cell survival/proliferation, or altered plasma (B220loCD138hi) cell differentiation (Fig. 2, E–H, and supplemental Fig. S1, D–F). These experiments further demonstrate that estrogen potentiates CD154- or LPS-induced AID expression and CSR; they also show that HoxC4 critically mediates estrogen-enhanced AID induction and CSR.
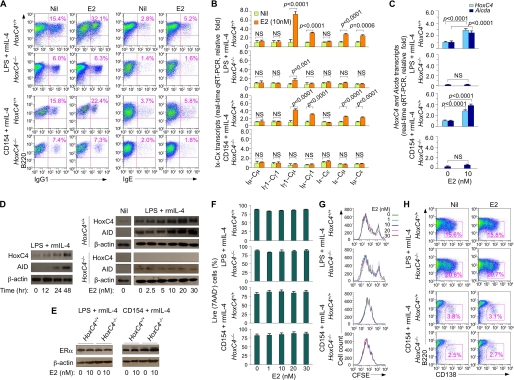
FIGURE 2.
HoxC4 deficiency impairs estrogen-mediated enhancement of AID expression and CSR to IgG1 and IgE, as induced by LPS and IL-4 or CD154 and IL-4. A, HoxC4+/+ and HoxC4−/− B cells were stimulated with LPS and rmIL-4 or with CD154 and rmIL-4 in the presence of nil or E2 (10 nm) for 4 days and then analyzed for surface IgG1+ or IgE+. Data are representative of three independent experiments. B and C, HoxC4 deficiency does not alter the levels of germ line Iμ-Cμ, Iγ1-Cγ1, and Iϵ-Cϵ transcripts but significantly impairs E2-mediated enhancement of circle Iγ1-Cμ and Iϵ-Cμ, postrecombination Iμ-Cγ1 and Iμ-Cϵ transcripts (B), and Aicda expression (C). HoxC4+/+ and HoxC4−/− B cells were stimulated with LPS and rmlL-4 or CD154 and rmlL-4 in the presence or absence of E2 (10 nm) for 48 h. Germline Iμ-Cμ, Iγ1-Cγ1 and Iϵ-Cϵ, circle Iγ1-Cμ and Iϵ-Cμ, post-recombination Iμ-Cγ1 and Iμ-Cϵ transcripts, and HoxC4 and Aicda transcripts were measured by qRT-PCR using SYBR-green; expression is normalized to CD79b levels and is presented as relative folds of enhancement by E2. Data are from three independent experiments (mean ± S.E. (error bars)). D, E2 enhances expression of HoxC4 and AID proteins. Left, HoxC4+/+ B cells stimulated with LPS and rmIL-4 for 0, 12, 24, or 48 h; right, HoxC4+/+ and HoxC4−/− B cells stimulated with nil or with LPS and rmIL-4 in the presence of E2 at the indicated concentrations for 48 h. HoxC4, AID, and β-actin proteins were detected by immunoblotting. Data are representative of three independent experiments. E, E2 does not alter expression of ERα. HoxC4+/+ and HoxC4−/− B cells were stimulated with LPS and rmIL-4 or CD154 and rmIL-4 in the presence or absence of E2 (10 nm) for 48 h; ERα and β-actin proteins were then detected by immunoblotting. Data are representative of three independent experiments. F, E2 does not alter B cell viability. HoxC4+/+ and HoxC4−/− B cells were stimulated with LPS and rmIL-4 or with CD154 and rmIL-4 in the presence of E2 at the indicated concentrations for 3 days and then stained with 7-AAD before flow cytometry analysis; the cells not stained by 7-AAD were viable. Data are from three independent experiments (mean ± S.E.). G, E2 does not alter B cell proliferation. HoxC4+/+ and HoxC4−/− B cells were labeled with CFSE and stimulated with LPS and rmIL-4 or CD154 and rmIL-4 in the presence of E2 at the indicated concentrations for 4 days. Data are representative of three independent experiments. H, E2 does not alter plasma cell differentiation. HoxC4+/+ and HoxC4−/− B cells were stimulated for 4 days with LPS and rmIL-4 or with CD154 and rmIL-4 in the presence or absence of E2 (10 nm). The numbers in outlined areas indicate the percentage of B220loCD138+ (plasma) cells among total cells. Data are representative of three independent experiments. NS, not significant.
In Vivo Estrogen Enhancement of Aicda Expression and CSR Is Dependent on HoxC4
To determine whether increased estrogen levels in vivo lead to increased CSR and the role of HoxC4 in this process, we injected female HoxC4+/+ and HoxC4−/− mice with Oil or E2-Oil (58) daily over a 10-day period of immunization with NP16-CGG (Fig. 3A). E2-Oil injections maintained a blood E2 level consistently 10-fold higher than that in mice injected with Oil (average 0.3 versus 0.03 nm) and resulted in an enhanced antibody response to NP16-CGG in HoxC4+/+ (more than 75% increase in circulating NP-binding IgG1; not shown) but not HoxC4−/− mice. Accordingly, among mice immunized with NP16-CGG and injected with Oil alone, those that were HoxC4+/+ showed an almost 4-fold higher proportion of IgG1+ B cells as compared with their HoxC4−/− counterparts. In HoxC4+/+ mice, E2-Oil injection more than doubled the number of switched IgG1+ GC (B220+PNAhi) B cells as compared with Oil alone (Fig. 3B). Further, E2-Oil-injected HoxC4+/+ mice showed an almost 8-fold higher proportion of switched IgG1+GC (B220+PNAhi) B cells than their HoxC4−/− counterparts and significantly increased levels of postrecombination Iμ-Cγ1 transcripts as well as HoxC4 and Aicda transcripts in spleen, lymph node, and Peyer's patch B cells (Fig. 3, C and D). Importantly, the E2-mediated enhancement of Aicda expression and CSR was abrogated in HoxC4−/− mice and did not result from increased GC (B220+PNAhi) B cell and/or plasma (B220loCD138+) cell differentiation or altered B cell viability and/or proliferation (Fig. 3, E–H). Thus, estrogen increases Aicda expression and potentiates CSR in vivo in a HoxC4-dependent fashion.
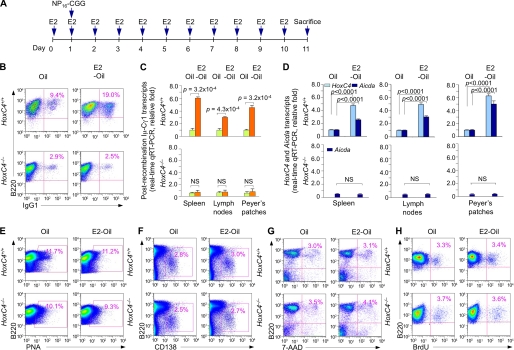
FIGURE 3.
Estrogen enhances HoxC4 and Aicda expression and CSR in HoxC4+/+ but not HoxC4−/− Mice. A, HoxC4+/+ and HoxC4−/− littermate mice were injected with Oil or E2-Oil once a day for 11 days; 1 day after the first Oil or E2-Oil injection, the mice were immunized with NP16-CGG. B, spleen B220+PNAhi GC B cells from these mice were analyzed for surface IgG1 expression. C and D, RNA extracted from spleen, lymph nodes and Peyer's patches was analyzed by real-time qRT-PCR for postrecombination Iμ-Cγ1, HoxC4, and Aicda transcripts; these were normalized to CD79b transcript level and depicted as relative to the expression of Iμ-Cγ1, HoxC4, and Aicda transcripts in Oil-injected HoxC4+/+ mice, set as 1. Data are from three independent experiments (mean ± S.E. (error bars)). E, proportion of spleen B220+PNAhi GC B cells as a percentage of total B220+ cells. F, surface expression of B220 and CD138 on spleen cells. The numbers in outlined areas indicate B220loCD138+ (plasma) cells as a percentage of total B220+ cells. G, the viability of spleen cells was analyzed by 7-AAD staining; the cells not stained by 7-AAD were viable. H, Mice were injected intraperitoneally twice within 16 h with BrdU and sacrificed 4 h after the last injection. The proliferation of spleen B cells was analyzed by the incorporation of BrdU, as stained with APC-anti-BrdU mAb using the BrdU Flow Kit (BD Biosciences). Data are representative of three independent experiments.
Estrogen Enhances SHM in the NP-CGG Antibody Response in a HoxC4-dependent Fashion
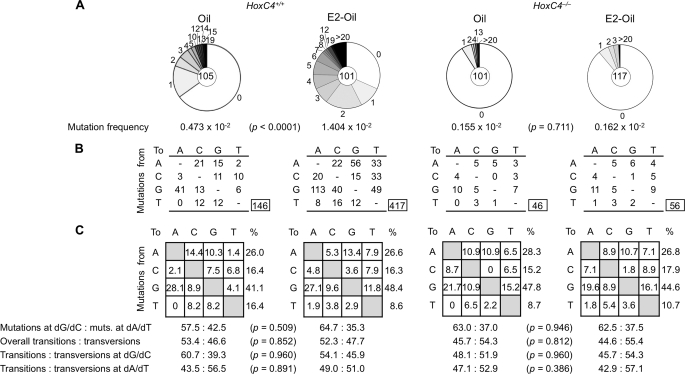
To determine the effect of estrogen on SHM, we sequenced Ig V186.2-DJH transcripts expressed by B cells from the HoxC4+/+ and HoxC4−/− littermate female mice used in the experiments of Fig. 3 (injected daily for 11 days with Oil or E2-Oil and immunized with of NP16-CGG 1 day after the first Oil or E2-Oil injection) because the V186.2 gene dominates the response to NP in these mice (59). We amplified V186.2DJH-Cγ1 cDNAs using an RT-PCR involving nested V186.2 leader DNA-specific forward primers and Cγ1-specific reverse primers (57) and analyzed unique mutations in the 294-bp V186.2 segment. Among mice immunized with NP16-CGG and injected with Oil alone, consistent with our previous findings in IgH JH4-iEμ sequences (40), those that were HoxC4+/+ showed a greater than 3-fold higher frequency of point mutations and a greater than 4-fold frequency of mutated transcripts than their HoxC4−/− counterparts. In HoxC4+/+ mice, injection of E2-Oil increased the overall frequency of mutations by almost 3-fold as compared with Oil alone (1.404 × 10−2 versus 0.473 × 10−2, p < 0.0001; Fig. 4). This increased mutation frequency was associated with an 89% increase in the proportion of mutated transcripts (69 of 101 versus 38 of 105, p < 0.0001) with no significant change in the spectrum of point mutations. It represented a greater than 8.6-fold overall frequency of point mutations (1.404 × 10−2 versus 0.162 × 10−2, p < 0.0001) and a greater than 6-fold frequency of mutated transcripts (69 of 101 versus 13 of 117, p < 0.0001) as compared with their HoxC4−/− counterparts, in which E2-Oil injection did not increase the overall frequency of point mutations or the proportion of mutated transcripts. Thus, estrogen significantly potentiates in a HoxC4-dependent fashion SHM, as specifically induced in a T-dependent antibody response.
FIGURE 4.
Estrogen increases Ig somatic mutations in HoxC4+/+ but Not HoxC4−/− Mice. V186.2DJH-Cγ1 transcripts were amplified from the B cells of the three sets of HoxC4+/+ and HoxC4−/− littermate female mice used in the experiments of Fig. 3 (two HoxC4+/+ and two HoxC4−/− littermates for each set, injected daily for 11 days with Oil or E2-Oil and immunized with NP16-CGG 1 day after the first Oil or E2-Oil injection). A, pie charts depict the proportion of sequences that carry 1, 2, 3, etc. mutations over the 294-bp V186.2 cDNA of V186.2DJH-Cγ1 transcripts analyzed. The numbers of sequences analyzed are at the centers of the pie charts. B, numbers and nature of independent mutational events scored. C, compilations, with the numbers indicating percentages of all mutations scored in the pool of the target sequences from different mice. Below the compilations, the ratio of mutations at dG/dC to those at dA/dT is indicated, as is the ratio of transition/transversion substitutions at dG/dC and dA/dT.
Estrogen-mediated Enhancement of AICDA Promoter Activation Is Independent of a Putative (Non-conserved) ERE in the AICDA Promoter but Is Dependent on HoxC4
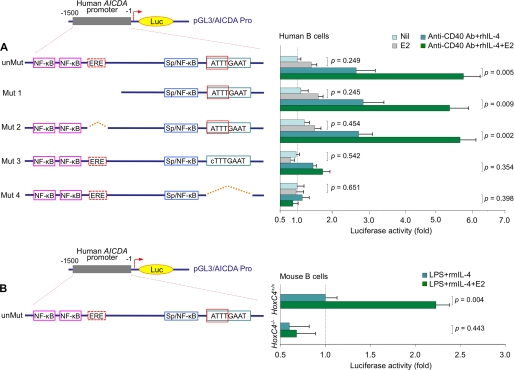
We have shown that HoxC4 activates the AICDA/Aicda promoter by binding to a conserved HoxC4/Oct-binding 5′-ATTTGAAT-3′ motif (residues −29 to −22) (40). It has recently been suggested that estrogen directly activates AICDA transcription by binding to a putative ERE lying upstream (residues −1089 to −1075) of this gene transcription initiation site (47). We confirmed the presence of this putative ERE (score = 0.84, threshold score = 0.80, MatchTM, BIOBASE Corp., Beverly, MA) in the human AICDA gene locus (47). This motif, however, unlike the AICDA/Aicda promoter HoxC4/Oct-binding 5′-ATTTGAAT-3′ motif (residues −29 to −22) (40), is not conserved, because it is not present in other species, such as mice, rats, or cows (supplemental Fig. S2). Interestingly, the conserved 5′-ATTTGAAT-3′ motif also overlaps with a putative ERE (residues −36 to −22), but this did not appear to be functional in direct estrogen-mediated AICDA activation (47).
We sought to determine whether estrogen enhances AICDA induction through direct binding of ERs to the AICDA promoter or, possibly, through an ER-induced up-regulation of HoxC4 and binding of this transcription factor to the AICDA promoter (40). To this end, we constructed luciferase gene reporter vectors containing the 1.5-kb region upstream of the transcription initiation site of human AICDA; this 1.5-kb DNA was identical to the corresponding sequence used by Pauklin et al. (47), as such (unMut) or with deletion or mutation of the putative non-conserved ERE (residues −1089 to −1075) (Mut 2) or the conserved HoxC4/Oct-binding 5′-ATTTGAAT-3′ motif (residues −29 to −22) (Mut 3 and Mut 4). In addition, we created a truncated 1.0-kb AICDA upstream region construct (Mut 1); this 1.0-kb DNA was identical to the corresponding truncated sequence used by Pauklin et al. (47), in which the region containing the single putative ERE as well as two putative NF-κB sites was deleted. In human B cells, neither deletion of the single putative ERE nor truncation of the upstream region dampened the E2-mediated enhancement of the AICDA promoter activation (Fig. 5A). By contrast, deletion of the conserved HoxC4/Oct-binding 5′-ATTTGAAT-3′ motif or mutation of this motif to 5′-cTTTGAAT-3′ (the dA to dC mutation abolished HoxC4-binding without affecting Oct-binding (40) and would not abolish the putative ER binding activity of the ERE that overlaps with this site) significantly dampened the promoter activation induced by anti-CD40 mAb and rhIL-4 and abolished the E2-induced enhancement. These findings together with the failure of the unmutated 1.5-kb sequence upstream of the transcription initiation site of the AICDA gene to mediate activation pGL3-enhancer luciferase reporter vector in transfected HoxC4−/− B cells treated with E2 (10 nm) (Fig. 5B) demonstrated that E2-induced enhancement of AICDA promoter activation is independent of the single putative ERE in the AICDA promoter. Rather, it is dependent on HoxC4 expression and the presence of the HoxC4 transcription factor-binding site in the AID gene promoter.
FIGURE 5.
The conserved HoxC4-binding site (residues −29 to −22) but not the non-conserved putative ERE site (residues −1089 to −1075) is essential for E2-mediated enhancement of AICDA promoter activation. A, 2E2 B cells were transfected with pGL3-enhancer luciferase gene reporter constructs containing unmutated (unMut) or mutated (Mut 1, Mut 2, Mut 3, and Mut 4) AICDA upstream region. The transfected B cells were stimulated with nil, E2 (10 nm) alone, or anti-CD40 mAb and rhIL-4 in the presence or absence of E2 (10 nm), and luciferase activity was measured 24 h thereafter. Data are from three independent experiments (mean ± S.D. (error bars)). B, spleen B cells from HoxC4+/+ and HoxC4−/− female mice were preactivated with LPS and transfected with the pGL3-enhancer luciferase gene reporter construct containing the AICDA promoter using an Amaxa® mouse B cell Nucleofector® kit and a Nucleofector® device. Transfected B cells were stimulated with LPS and rmIL-4 in the presence or absence of E2 (10 nm), and luciferase activity was measured 24 h thereafter. Data are from three independent experiments (mean ± S.D.).
Identification of Three Conserved EREs in the HoxC4 Promoter and Their Recruitment of ERs
HoxC4 is known to be up-regulated in breast cancer cells upon exposure to estrogen (46). Our failure to identify a role for the single putative non-conserved ERE in the AICDA promoter (47) in AID expression, and the known requirement for multiple EREs for the efficient activation of estrogen-responsive genes (50–52) prompted us to hypothesize that estrogen-ER complexes modulate AICDA/Aicda expression through induction of HoxC4 by binding to multiple EREs in the HOXC4/HoxC4 promoter.
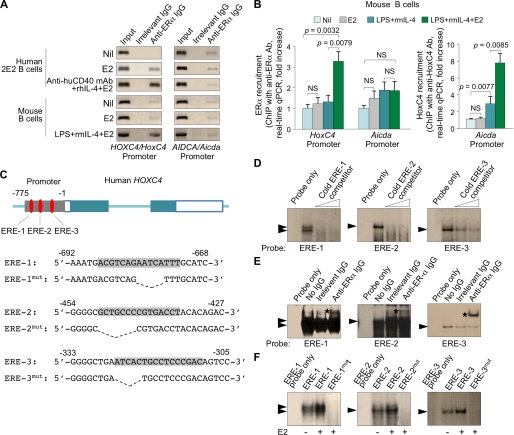
Indeed, we identified three ERE motifs (score = 0.85, 1.0, and 0.83, respectively; threshold score = 0.80; MatchTM) upstream of the putative transcriptional initiation site of HOXC4/HoxC4 and showed that these three EREs are conserved in humans, mice, rats, and cows (Fig. 6). To determine whether these three HOXC4/HoxC4 promoter EREs recruited ERs, we immunoprecipitated chromatin from human 2E2 B cells and primary mouse B cells using an Ab to ERα. These B cells were either unstimulated or treated with E2 alone or E2 together with an agonistic anti-CD40 mAb (human B cells) or LPS (mouse B cells) and IL-4 for 3 days. We readily specified the HOXC4/HoxC4 but not the AICDA/Aicda promoter sequence in the DNA precipitated by Ab to ERα from human 2E2 and mouse B cells stimulated by anti-CD40 mAb and rhIL-4 or LPS and rmIL-4 in the presence of E2 (Fig. 7A). This prompted us to quantify the difference in recruitment of ERs to the HOXC4/HoxC4 and AICDA/Aicda promoters by quantitative PCR. An Ab to ERα precipitated a significantly greater amount of HoxC4 promoter DNA, but not Aicda promoter DNA, in primary mouse B cells induced to express AID and undergo CSR by LPS and rmIL-4 in the presence of E2, as compared with B cells cultured under similar conditions but in the absence of E2 or E2 alone or nil (Fig. 7B). In addition, consistent with our finding that estrogen alone did not significantly induce HoxC4 expression but powerfully potentiated CD154- or LPS-induced HoxC4 expression and HoxC4-mediated AID induction, an Ab to HoxC4 precipitated a significantly greater amount of Aicda promoter DNA in primary mouse B cells induced by LPS and rmIL-4 in the presence of E2 as compared with B cells cultured under similar conditions but in the absence of E2 or B cells cultured with E2 alone or nil.
FIGURE 7.
ERs are recruited to the HOXC4/HoxC4 promoter and specifically bind to the three conserved EREs in HOXC4 promoter sequence. A, genomic DNA was precipitated from cross-linked chromatin of human 2E2 B cells or primary mouse B cells treated with nil, E2 (10 nm) alone, or E2 (10 nm) together with an agonistic anti-CD40 mAb (human B cells) or LPS (mouse B cells) and IL-4 for 3 days using a rabbit Ab specific for ERα or preimmune control rabbit IgG. The precipitated DNA was specified by PCR for HOXC4/HoxC4 or AICDA/Aicda promoter using specific primers. Data are representative of three independent experiments. B, genomic DNA was precipitated from cross-linked chromatin of primary mouse B cells treated with nil, E2 (10 nm) alone, LPS and rmIL-4, or E2 (10 nm) together with LPS and rmIL-4 for 3 days using rabbit IgG Abs specific for ERα or HoxC4 or preimmune control rabbit IgG. Shown is the enrichment of HoxC4 and Aicda promoter DNA relative to nil samples, as analyzed by real-time quantitative PCR. Data are from three independent experiments (mean ± S.E. (error bars)). C, oligonucleotide probes (ERE-1, ERE-2, and ERE-3) containing one of the three EREs in the HOXC4 promoter, respectively, as well as mutant oligonucleotides (ERE-1mut, ERE-2mut, and ERE-3mut), in which the EREs were deleted, were used as probes for EMSA. D–F, nuclear proteins from anti-CD40 mAb and rhIL-4-stimulated human 2E2 B cells treated with E2 (10 nm) specifically bound to oligonucleotide probes containing one of the EREs. D, efficient competition of the ERE oligonucleotide probes for the formation of the protein-DNA complexes with nuclear proteins of E2-treated anti-CD40 mAb and rhIL-4-stimulated 2E2 B cells was achieved by a 25- or 50-fold molar excess of unlabeled unmutated probe for each specific ERE. Data are representative of three independent experiments. E, the formation of the protein-DNA complexes was shifted by anti-ERα Ab. Rabbit IgG with irrelevant binding activity served as a negative control. F, nuclear proteins from anti-CD40 mAb and rhIL-4-stimulated human 2E2 B cells treated with E2 did not bind to the deletion mutant ERE oligonucleotide probes. Data are representative of three independent experiments. NS, not significant.
To further demonstrate that HoxC4 binds to the conserved HOXC4/HoxC4 promoter EREs, we performed EMSAs, using oligonucleotides encompassing the three individual EREs of the human HOXC4 promoter sequence or oligonucleotides with disrupted ERE sequences as probes (Fig. 7C). Incubation of these probes with nuclear extracts from anti-CD40 mAb and rhIL-4-stimulated 2E2 B cells gave rise to major protein-DNA complexes, the formation of which was enhanced by E2 and abrogated by the respective cold ERE-1, ERE-2, or ERE-3 probe (Fig. 7D). Moreover, similar nuclear protein-DNA complexes were supershifted by specific Ab to ERα or did not form when using probes with deletion mutations in any of the three EREs (Fig. 7, E and F). These experiments show that ERs bind specifically to the three conserved ERE sites we identified in the HOXC4/HoxC4 promoter.
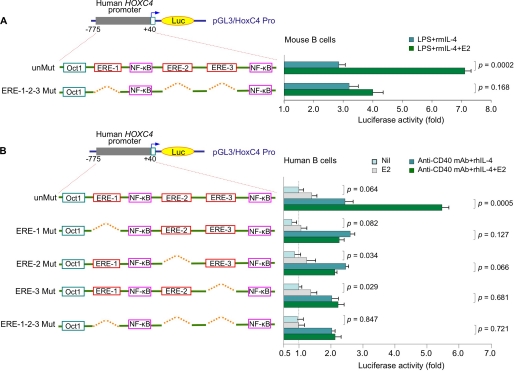
The Three Conserved EREs Are Critical and Cooperate in Estrogen-mediated HOXC4 Promoter Activation
To determine the contribution the three conserved EREs to HOXC4 promoter activation, we constructed luciferase gene reporter pGL3-enhancer vectors containing the human HOXC4 promoter sequence (residues −775 to +40) as such (unMut) or with deletion of ERE-1, ERE-2, or ERE-3 (ERE-1 Mut, ERE-2 Mut, or ERE-3 Mut) (Fig. 8); we also constructed a vector containing a mutant HOXC4 promoter, in which all three EREs were deleted (ERE-1-2-3 Mut). In primary mouse B cells transfected with those constructs and cultured with LPS and rmIL-4, E2 (10 nm) significantly enhanced activation of the unmutated HOXC4 promoter (p = 0.0002) but not the triple ERE deletion mutant (p = 0.168; Fig. 8A). We then used these two vectors together with the three different single ERE deletion mutants to transfect human 2E2 B cells and induced them to undergo CSR. In these B cells, E2 alone did not significantly induce HOXC4 promoter activation. However, it further enhanced HOXC4 promoter activation, as induced by anti-CD40 mAb and rhIL-4 (p = 0.0005; Fig. 8B). Consistent with the notion that multiple and clustered EREs are required and cooperate to effectively activate the promoter of highly estrogen-responsive genes (48–52), deletion of any of the three EREs abrogated the E2-mediated enhancement of HOXC4 promoter activation. Thus, the three conserved EREs in the HOXC4/HoxC4 promoter cooperate to mediate full activation of this gene, as induced by CD40 and IL-4 signaling.
FIGURE 8.
The three conserved EREs are essential and cooperative for full HOXC4 promoter activation. A, spleen B cells from HoxC4+/+ female mice were transfected with pGL3-enhancer luciferase gene reporter constructs containing unmutated (unMut) or mutated HOXC4 promoter in which all three of the EREs were deleted (ERE-1-2-3 Mut). The transfected B cells were stimulated with LPS and rmIL-4 in the presence or absence of E2 (10 nm), and luciferase activity was measured 24 h thereafter. Data are from three independent experiments (mean ± S.D. (error bars)). B, human 2E2 B cells were transfected with pGL3-enhancer luciferase gene reporter constructs containing unmutated or mutated HOXC4 promoter in which one (ERE-1 Mut, ERE-2 Mut, or ERE-3 Mut) or three (ERE-1-2-3 Mut) of the EREs were deleted. The transfected B cells were stimulated with nil, E2 (10 nm) alone, or anti-CD40 mAb and rhIL-4 in the presence or absence of E2 (10 nm), and luciferase activity was measured 24 h thereafter. Data are from three independent experiments (mean ± S.D.).
DISCUSSION
We show here that, in B cells, estrogen effectively potentiates AID induction, CSR, and SHM through activation of HOXC4/HoxC4 promoter and up-regulation of the HoxC4 transcription factor, which critically mediates AID expression by binding to and activating the AICDA/Aicda promoter (40, 41). Our findings outline a mechanistic basis for the stronger antibody response to vaccines and infections in females (4–12) and, possibly, for the epidemiologic evidence supporting a role of estrogen in potentiating autoantibody responses and aggravating autoimmunity (1, 13, 14). Estrogen contribution to the female bias in autoantibody responses and autoimmunity has been suggested for decades (60, 61). Women are disparately affected by autoimmunity, with the prevalence of autoimmune diseases, such as rheumatoid arthritis, systemic lupus erythematosus, and Sjogren's syndrome, being 3–9 times higher in women than in men (13, 14, 16). Further, in men with Klinefelter's syndrome who have an extra X chromosome, a relatively common disorder characterized by excess estrogen and deficient androgen production, the incidence of lupus is 14-fold greater than in normal men (62). Finally, estrogen induces higher autoantibody titers and accelerates disease development in lupus-prone MRL/faslpr/lpr and NZBxNZW F1 mice (63). In MRL/faslpr/lpr mice, the emergence of class-switched and hypermutated autoantibodies is associated with up-regulated expression of HoxC4 and AID (53).5
Findings from gene reporter assays in human uterine cervix cancer SiHa cells were interpreted to suggest that ERs are recruited to a single putative ERE (residues −1089 to −1075) in the human AICDA locus to activate transcription (47). We show here that this ERE is not present in other species, such as the mouse and rat, and its deletion did not alter estrogen-mediated AICDA promoter activation in B cells, indicating that this ERE plays no role in the overall estrogen-enhanced AID induction in switching B cells. Rather, estrogen-mediated activation of the AID gene was abrogated by deletion of the conserved AICDA promoter HoxC4-binding site (residues −29 to −22). Interestingly, estrogen induced HOXC4 expression in SiHa cells,5 suggesting that the estrogen-enhanced AICDA promoter activation detected in these cells by Pauklin et al. in gene reporter assays (47) resulted from the estrogen-induced up-regulation of HoxC4. Accordingly, in our experiments, the estrogen-induced enhancement of AICDA promoter activation and AID expression was virtually abolished in HoxC4−/− B cells and HoxC4−/− mice. Thus, HoxC4 is a critical link in the chain of events that leads to estrogen-induced enhancement of AID expression, CSR, and SHM and, therefore, maturation of the antibody response.
Genes that are highly responsive to estrogen characteristically contain multiple EREs within their regulatory regions, such as promoters and enhancers (50–52, 64). This is particularly true of mammalian estrogen-responsive genes, whose EREs are typically “imperfect” (48–51) and must be cooperatively occupied by multiple ERs for efficient promoter activation. Accordingly, we have identified here three highly conserved and cooperative EREs clustered in the HOXC4/HoxC4 promoter (within residues −687 to −310 in human HOXC4) and have shown that direct binding of ERs to these conserved EREs mediates estrogen enhancement of CD154- or LPS-induced HOXC4/HoxC4 expression; deletion of any of these three EREs virtually abrogated E2-mediated activation of the HOXC4 promoter. That estrogen alone did not induce activation of the HOXC4/HoxC4 promoter but effectively potentiated the activation of this promoter upon stimulation with CD154 or LPS is reminiscent of the NKG2E gene, whose estrogen-mediated promoter activation requires cooperation between ER and multiple transcription factors (65). Other transcription factors, including NF-κB, c-Myc, Smad3, Hsf-2, and c/EBPβ, binding to DNA near EREs can cooperate with ERs to activate an estrogen-responsive gene (65–67). Indeed, we have identified within the HOXC4/HoxC4 promoter two conserved putative NF-κB-binding sites, which could be targeted by NF-κB, as induced by CD154, LPS (68), or perhaps BAFF (69). Thus, NF-κB and possibly other CD154- or LPS-induced transcription factors would cooperate with ERs to mediate full HoxC4 expression.
Our experiments using HoxC4−/− B cells and HoxC4−/− mice have provided proof that estrogen enhances the intrinsic function of the CSR/SHM machinery through HoxC4-mediated activation of the Aicda promoter. They, however, do not rule out the possibility that estrogen enhances the binding of other transcription factors, such as NF-κB, to the Aicda promoter (47), further contributing to an overall estrogen-mediated enhancement of AID expression. Estrogen may play multiple roles in antibody and autoantibody responses (15, 43, 70–73), implying that the increase of CSR and SHM in E2-injected mice might reflect, at least in part, E2-regulated cell functions independent of HoxC4, such as survival or proliferation. Although estrogen might or might not promote B cell survival (74, 75), at the (physiologic) concentrations used in our in vivo and in vitro experiments, estrogen did not promote B cell proliferation; nor did it alter the viability of normal B cells, as it does in some neoplastic (breast cancer) cells (76) or plasmacytoid differentiation.
The importance of estrogen as a positive regulator of HoxC4 and thus of AID is reflected in the high conservation of the three EREs in the HOXC4/HoxC4 promoter among different mammalian species. The evolutionary benefit of this conservation can only be conjectured but suggests that females during their high estrogen reproductive years are afforded more vigorous and more diverse antibody responses to infections. This would be important for the protection of the fetus and the newborn. An endogenous and effective humoral immune response is not present at birth, and the newborn is dependent on antibodies passively transferred from the mother across the placenta in late pregnancy (IgG) and antibodies passed through breast milk (IgA). The fact that estrogen alone does not increase AID induction and CSR/SHM in the absence of CD154 or LPS and IL-4 stimulation would ensure that estrogen potentiates AID expression only or mainly in the course of specific antibody responses, such as those triggered by microbial pathogens. Thus, the estrogen-induced broadening and potentiation of the antibody repertoire against pathogens that have infected the mother would be a highly regulated process that also benefits the offspring.
Estrogen has been shown to up-regulate HoxC4 expression in human breast cancer cells (46), in which AID expression was also up-regulated (47), indicating that estrogen-enhanced HoxC4 expression can result in up-regulation of AID and, possibly, tumorigenesis in non-B cells. Indeed, aberrations in the expression of Hox genes, including HoxC4, have been reported in abnormal development and malignancy (77). AID is a robust DNA mutator, and aberrant AID expression would result in widespread DNA damage and, possibly, mutagenesis and neoplastic transformation in non-B cells (78, 79). Because ERs are widely expressed in different tissues, estrogen-mediated AID up-regulation must be tightly and specifically controlled to avoid a threat to genome integrity. The inability of estrogen to directly activate the AICDA/Aicda promoter and its dependence on HoxC4, as a mediator of AICDA/Aicda promoter activation, probably provides an additional level of control of AID expression. Once activated, AID gene expression could be directly dampened by progesterone. In fact, in contrast to the indirect estrogen up-regulation of AID, progesterone has been suggested to down-regulate AID expression possibly by binding to the AID gene promoter (80). Also, in contrast with the immunopotentiating and, possibly, proinflammatory activity of estrogen, progesterone possesses immunosuppressive and anti-inflammatory properties. Progesterone probably plays an important role in maternal-fetal tolerance (81) and has been shown to effectively reduce total serum IgG and anti-double strand DNA IgG2a autoantibody levels in lupus patients and lupus-prone mice (17, 82).
Hormones, including estrogen, have been shown to regulate Hox gene expression and thereby mediate development in the embryo (44). Like in B cells undergoing CSR/SHM, HoxC4 is highly expressed in embryonic stem cells (83). Given the importance of Hox genes in development and organogenesis, it has been postulated that they continue to remain integral to tissue-specific stem cells in the adult, particularly for quiescence and self-renewal (77). Epigenetic reprogramming, which includes DNA demethylation, occurs in mammalian primordial germ cells and in early embryos and is important for return to pluripotency (84, 85). In stem cells and germ cells, a higher level of estrogen would enhance HoxC4 expression, potentially inducing AID, which might be one of the factors playing a role in DNA demethylation and epigenetic homeostasis. Thus, our findings provide evidence for the HoxC4-mediated regulation of AID expression, as enhanced by estrogen, and suggest a possible role of this homeodomain transcription factor in mediating immunopotentiation in gestation and neonatal and adult life.
Acknowledgments
We thank Moon Kang, Derrick Lee, Solomon Yao, Lynne Le, and Sona Ardeshna for excellent technical assistance.
This work was supported, in whole or in part, by National Institutes of Health Grants AI 045011, AI 060573, and AI 079705 (to P. C.).

The on-line version of this article (available at http://www.jbc.org) contains supplemental Figs. S1 and S2.
T. Mai, H. Zan, J. Zhang, J. S. Hawkins, Z. Xu, and P. Casali, unpublished data.
- CSR
- class switch DNA recombination
- AID
- activation-induced cytosine deaminase
- CH
- heavy chain constant region
- D
- J, and V, immunoglobulin diversity, joining, and variable region, respectively
- GC
- germinal center
- E2
- 17β-estradiol
- E2-Oil
- E2 dissolved in corn oil
- ER
- estrogen receptor
- ERE
- estrogen response element
- Iμ
- Iγ1, Iγ3, and Iϵ, intervening regions of Cμ, Cγ1, Cγ3, and Cϵ genes
- Mut
- mutated
- unMut
- unmutated
- NP-CGG
- 4-hydroxy-3-nitrophenylacetyl hapten conjugated with chicken γ-globulin
- PNA
- peanut agglutinin
- qRT-PCR
- quantitative real-time RT-PCR
- rhIL-4
- recombinant human IL-4
- rmIL-4
- recombinant mouse IL-4
- SHM
- somatic hypermutation
- 7-AAD
- 7-aminoactinomycine.
REFERENCES
- 1.Fish E. N. (2008) Nat. Rev. Immunol. 8, 737–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engler R. J., Nelson M. R., Klote M. M., VanRaden M. J., Huang C. Y., Cox N. J., Klimov A., Keitel W. A., Nichol K. L., Carr W. W., Treanor J. J. (2008) Arch. Intern. Med. 168, 2405–2414 [DOI] [PubMed] [Google Scholar]
- 3.Klein S. L., Greenberger P. (October28, 2009) The New York Times, A33 [Google Scholar]
- 4.Green M. S., Shohat T., Lerman Y., Cohen D., Slepon R., Duvdevani P., Varsano N., Dagan R., Mendelson E. (1994) Int. J. Epidemiol. 23, 1078–1081 [DOI] [PubMed] [Google Scholar]
- 5.Stanberry L. R., Spruance S. L., Cunningham A. L., Bernstein D. I., Mindel A., Sacks S., Tyring S., Aoki F. Y., Slaoui M., Denis M., Vandepapeliere P., Dubin G. (2002) New Engl. J. Med. 347, 1652–1661 [DOI] [PubMed] [Google Scholar]
- 6.Ovsyannikova I. G., Jacobson R. M., Vierkant R. A., Jacobsen S. J., Pankratz V. S., Poland G. A. (2004) Hum. Immunol. 65, 1506–1515 [DOI] [PubMed] [Google Scholar]
- 7.Weissman S., Feucht C., Moore B. A. (2006) J. Viral Hepat. 13, 81–86 [DOI] [PubMed] [Google Scholar]
- 8.Bhavanam S., Snider D. P., Kaushic C. (2008) Vaccine 26, 6165–6172 [DOI] [PubMed] [Google Scholar]
- 9.Dhiman N., Ovsyannikova I. G., Vierkant R. A., Pankratz V. S., Jacobson R. M., Poland G. A. (2008) Tissue Antigens 72, 211–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kennedy R. B., Ovsyannikova I. G., Pankratz V. S., Vierkant R. A., Jacobson R. M., Ryan M. A., Poland G. A. (2009) Vaccine 27, 3319–3323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pennock J. W., Stegall R., Bell B., Vargas G., Motamedi M., Milligan G., Bourne N. (2009) Vaccine 27, 5830–5836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas C., Moridani M. (2009) Toxicology, in press [DOI] [PubMed] [Google Scholar]
- 13.Whitacre C. C. (2001) Nat. Immunol. 2, 777–780 [DOI] [PubMed] [Google Scholar]
- 14.Lockshin M. D. (2006) Lupus 15, 753–756 [DOI] [PubMed] [Google Scholar]
- 15.Cohen-Solal J. F., Jeganathan V., Hill L., Kawabata D., Rodriguez-Pinto D., Grimaldi C., Diamond B. (2008) Lupus 17, 528–532 [DOI] [PubMed] [Google Scholar]
- 16.Fairweather D., Frisancho-Kiss S., Rose N. R. (2008) Am. J. Pathol. 173, 600–609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buyon J. P., Petri M. A., Kim M. Y., Kalunian K. C., Grossman J., Hahn B. H., Merrill J. T., Sammaritano L., Lockshin M., Alarcón G. S., Manzi S., Belmont H. M., Askanase A. D., Sigler L., Dooley M. A., Von Feldt J., McCune W. J., Friedman A., Wachs J., Cronin M., Hearth-Holmes M., Tan M., Licciardi F. (2005) Ann. Intern. Med. 142, 953–962 [DOI] [PubMed] [Google Scholar]
- 18.Cutolo M., Capellino S., Sulli A., Serioli B., Secchi M. E., Villaggio B., Straub R. H. (2006) Ann. N.Y. Acad. Sci. 1089, 538–547 [DOI] [PubMed] [Google Scholar]
- 19.Cutolo M., Brizzolara R., Atzeni F., Capellino S., Straub R. H., Puttini P. C. (2010) Ann. N.Y. Acad. Sci. 1193, 36–42 [DOI] [PubMed] [Google Scholar]
- 20.Di Noia J. M., Neuberger M. S. (2007) Annu. Rev. Biochem. 76, 1–22 [DOI] [PubMed] [Google Scholar]
- 21.Stavnezer J., Guikema J. E., Schrader C. E. (2008) Annu. Rev. Immunol. 26, 261–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maizels N. (2005) Annu. Rev. Genet. 39, 23–46 [DOI] [PubMed] [Google Scholar]
- 23.Teng G., Papavasiliou F. N. (2007) Annu. Rev. Genet. 41, 107–120 [DOI] [PubMed] [Google Scholar]
- 24.Peled J. U., Kuang F. L., Iglesias-Ussel M. D., Roa S., Kalis S. L., Goodman M. F., Scharff M. D. (2008) Annu. Rev. Immunol. 26, 481–511 [DOI] [PubMed] [Google Scholar]
- 25.Odegard V. H., Schatz D. G. (2006) Nat. Rev. Immunol. 6, 573–583 [DOI] [PubMed] [Google Scholar]
- 26.Yu K., Huang F. T., Lieber M. R. (2004) J. Biol. Chem. 279, 6496–6500 [DOI] [PubMed] [Google Scholar]
- 27.Zan H., Komori A., Li Z., Cerutti A., Schaffer A., Flajnik M. F., Diaz M., Casali P. (2001) Immunity 14, 643–653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Diaz M., Casali P. (2002) Curr. Opin. Immunol. 14, 235–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Papavasiliou F. N., Schatz D. G. (2002) Cell 109, S35–S44 [DOI] [PubMed] [Google Scholar]
- 30.Wu X., Feng J., Komori A., Kim E. C., Zan H., Casali P. (2003) J. Clin. Immunol. 23, 235–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zan H., Wu X., Komori A., Holloman W. K., Casali P. (2003) Immunity 18, 727–738 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xu Z., Fulop Z., Zhong Y., Evinger A. J., 3rd, Zan H., Casali P. (2005) Ann. N.Y. Acad. Sci. 1050, 146–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu Z., Pone E. J., Al-Qahtani A., Park S. R., Zan H., Casali P. (2007) Crit. Rev. Immunol. 27, 367–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu Z., Zan H., Pal Z., Casali P. (2007) Adv. Exp. Med. Biol. 596, 111–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M., Schatz D. G. (2009) Trends Immunol. 30, 173–181 [DOI] [PubMed] [Google Scholar]
- 36.Lieber M. R. (2010) Annu. Rev. Biochem. 79, 181–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xu Z., Fulop Z., Wu G., Pone E. J., Zhang J., Mai T., Thomas L. M., Al-Qahtani A., White C. A., Park S. R., Steinacker P., Li Z., Yates J., 3rd, Herron B., Otto M., Zan H., Fu H., Casali P. (2010) Nat. Struct. Mol. Biol. 17, 1124–1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schaffer A., Kim E. C., Wu X., Zan H., Testoni L., Salamon S., Cerutti A., Casali P. (2003) J. Biol. Chem. 278, 23141–23150 [DOI] [PubMed] [Google Scholar]
- 39.Kim E. C., Edmonston C. R., Wu X., Schaffer A., Casali P. (2004) J. Biol. Chem. 279, 42258–42269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park S. R., Zan H., Pal Z., Zhang J., Al-Qahtani A., Pone E. J., Xu Z., Mai T., Casali P. (2009) Nat. Immunol. 10, 540–550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Delker R. K., Fugmann S. D., Papavasiliou F. N. (2009) Nat. Immunol. 10, 1147–1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Heldring N., Pike A., Andersson S., Matthews J., Cheng G., Hartman J., Tujague M., Ström A., Treuter E., Warner M., Gustafsson J. A. (2007) Physiol. Rev. 87, 905–931 [DOI] [PubMed] [Google Scholar]
- 43.Grimaldi C. M., Hill L., Xu X., Peeva E., Diamond B. (2005) Mol. Immunol. 42, 811–820 [DOI] [PubMed] [Google Scholar]
- 44.Daftary G. S., Taylor H. S. (2006) Endocr. Rev. 27, 331–355 [DOI] [PubMed] [Google Scholar]
- 45.Dai R., Phillips R. A., Ahmed S. A. (2007) J. Immunol. 179, 1776–1783 [DOI] [PubMed] [Google Scholar]
- 46.Frasor J., Danes J. M., Komm B., Chang K. C., Lyttle C. R., Katzenellenbogen B. S. (2003) Endocrinology 144, 4562–4574 [DOI] [PubMed] [Google Scholar]
- 47.Pauklin S., Sernández I. V., Bachmann G., Ramiro A. R., Petersen-Mahrt S. K. (2009) J. Exp. Med. 206, 99–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinez E., Wahli W. (1989) EMBO J. 8, 3781–3791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ponglikitmongkol M., White J. H., Chambon P. (1990) EMBO J. 9, 2221–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sathya G., Li W., Klinge C. M., Anolik J. H., Hilf R., Bambara R. A. (1997) Mol. Endocrinol. 11, 1994–2003 [DOI] [PubMed] [Google Scholar]
- 51.Tyulmenkov V. V., Jernigan S. C., Klinge C. M. (2000) Mol. Cell. Endocrinol. 165, 151–161 [DOI] [PubMed] [Google Scholar]
- 52.Ramsey T. L., Klinge C. M. (2001) J. Mol. Endocrinol. 27, 275–292 [DOI] [PubMed] [Google Scholar]
- 53.Zan H., Zhang J., Ardeshna S., Xu Z., Park S. R., Casali P. (2009) Autoimmunity 42, 89–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zan H., Casali P. (2008) Mol. Immunol. 46, 45–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zan H., Cerutti A., Dramitinos P., Schaffer A., Casali P. (1998) J. Immunol. 161, 5217–5225 [PMC free article] [PubMed] [Google Scholar]
- 56.Wu X., Tsai C. Y., Patam M. B., Zan H., Chen J. P., Lipkin S. M., Casali P. (2006) J. Immunol. 176, 5426–5437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zan H., Shima N., Xu Z., Al-Qahtani A., Evinger A. J., 3rd, Zhong Y., Schimenti J. C., Casali P. (2005) EMBO J. 24, 3757–3769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tai P., Wang J., Jin H., Song X., Yan J., Kang Y., Zhao L., An X., Du X., Chen X., Wang S., Xia G., Wang B. (2008) J. Cell. Physiol. 214, 456–464 [DOI] [PubMed] [Google Scholar]
- 59.Takahashi Y., Dutta P. R., Cerasoli D. M., Kelsoe G. (1998) J. Exp. Med. 187, 885–895 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Whitacre C. C., Reingold S. C., O'Looney P. A. (1999) Science 283, 1277–1278 [DOI] [PubMed] [Google Scholar]
- 61.Rubtsov A. V., Rubtsova K., Kappler J. W., Marrack P. (2010) Autoimmun. Rev. 9, 494–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sawalha A. H., Harley J. B., Scofield R. H. (2009) J. Autoimmun. 33, 31–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Greenstein B., Roa R., Dhaher Y., Nunn E., Greenstein A., Khamashta M., Hughes G. R. (2001) Int. Immunopharmacol. 1, 1025–1035 [DOI] [PubMed] [Google Scholar]
- 64.Kraus W. L., Montano M. M., Katzenellenbogen B. S. (1994) Mol. Endocrinol. 8, 952–969 [DOI] [PubMed] [Google Scholar]
- 65.Levy N., Zhao X., Tang H., Jaffe R. B., Speed T. P., Leitman D. C. (2007) Endocrinology 148, 3449–3458 [DOI] [PubMed] [Google Scholar]
- 66.Cheng A. S., Jin V. X., Fan M., Smith L. T., Liyanarachchi S., Yan P. S., Leu Y. W., Chan M. W., Plass C., Nephew K. P., Davuluri R. V., Huang T. H. (2006) Mol. Cell 21, 393–404 [DOI] [PubMed] [Google Scholar]
- 67.Gionet N., Jansson D., Mader S., Pratt M. A. (2009) J. Cell. Biochem. 107, 448–459 [DOI] [PubMed] [Google Scholar]
- 68.Pone E. J., Zan H., Zhang J., Al-Qahtani A., Xu Z., Casali P. (2010) Crit. Rev. Immunol. 30, 1–29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mackay F., Schneider P. (2009) Nat. Rev. Immunol. 9, 491–502 [DOI] [PubMed] [Google Scholar]
- 70.Bynoe M. S., Grimaldi C. M., Diamond B. (2000) Proc. Natl. Acad. Sci. U.S.A. 97, 2703–2708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Peeva E., Venkatesh J., Diamond B. (2005) J. Immunol. 175, 1415–1423 [DOI] [PubMed] [Google Scholar]
- 72.Cohen-Solal J. F., Jeganathan V., Grimaldi C. M., Peeva E., Diamond B. (2006) Curr. Top. Microbiol. Immunol. 305, 67–88 [DOI] [PubMed] [Google Scholar]
- 73.Straub R. H. (2007) Endocr. Rev. 28, 521–574 [DOI] [PubMed] [Google Scholar]
- 74.Grimaldi C. M., Cleary J., Dagtas A. S., Moussai D., Diamond B. (2002) J. Clin. Invest. 109, 1625–1633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Grimaldi C. M. (2006) Curr. Opin. Rheumatol. 18, 456–461 [DOI] [PubMed] [Google Scholar]
- 76.Musgrove E. A., Sutherland R. L. (2009) Nat. Rev. Cancer 9, 631–643 [DOI] [PubMed] [Google Scholar]
- 77.Shah N., Sukumar S. (2010) Nat. Rev. Cancer 10, 361–371 [DOI] [PubMed] [Google Scholar]
- 78.Okazaki I. M., Hiai H., Kakazu N., Yamada S., Muramatsu M., Kinoshita K., Honjo T. (2003) J. Exp. Med. 197, 1173–1181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chiba T., Marusawa H. (2009) J. Mol. Med. 87, 1023–1027 [DOI] [PubMed] [Google Scholar]
- 80.Pauklin S., Petersen-Mahrt S. K. (2009) J. Immunol. 183, 1238–1244 [DOI] [PubMed] [Google Scholar]
- 81.Bouman A., Heineman M. J., Faas M. M. (2005) Hum. Reprod. Update 11, 411–423 [DOI] [PubMed] [Google Scholar]
- 82.Hughes G. C., Martin D., Zhang K., Hudkins K. L., Alpers C. E., Clark E. A., Elkon K. B. (2009) Arthritis Rheum. 60, 1775–1784 [DOI] [PubMed] [Google Scholar]
- 83.Phinney D. G., Gray A. J., Hill K., Pandey A. (2005) Biochem. Biophys. Res. Commun. 338, 1759–1765 [DOI] [PubMed] [Google Scholar]
- 84.Sasaki H., Matsui Y. (2008) Nat. Rev. Genet 9, 129–140 [DOI] [PubMed] [Google Scholar]
- 85.Hajkova P., Jeffries S. J., Lee C., Miller N., Jackson S. P., Surani M. A. (2010) Science 329, 78–82 [DOI] [PMC free article] [PubMed] [Google Scholar]