Abstract
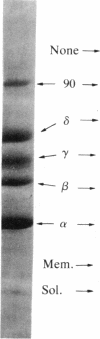
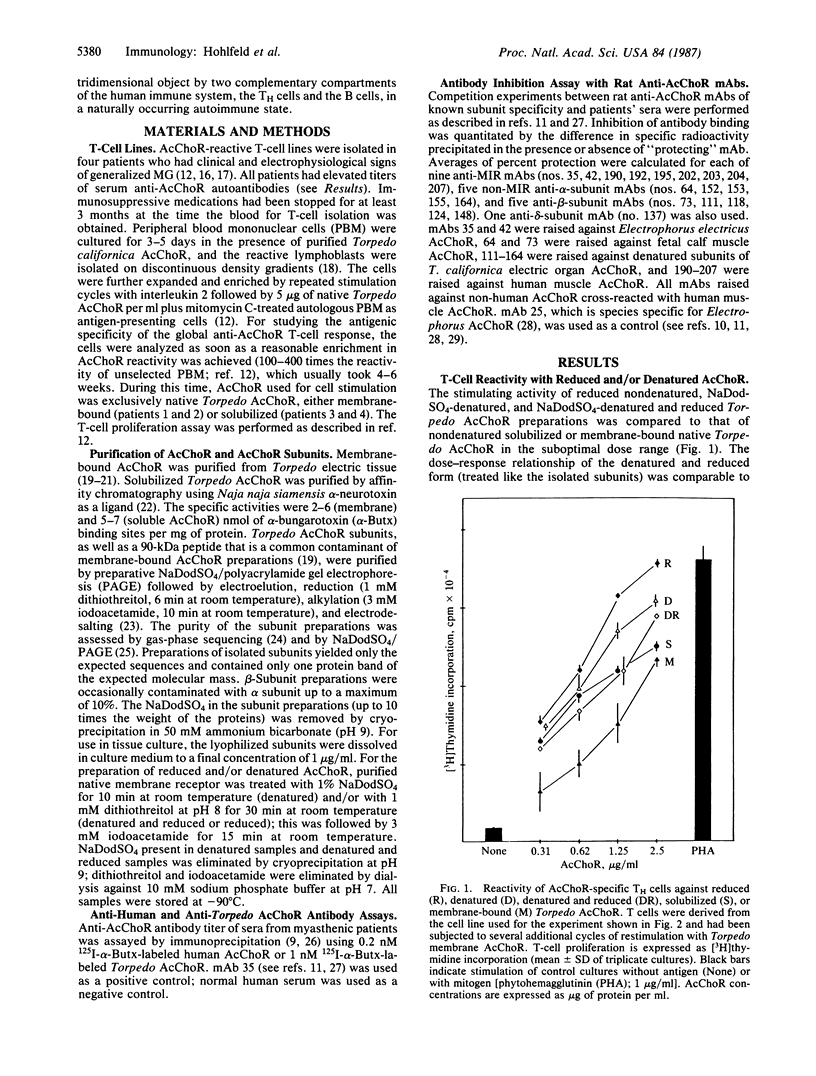
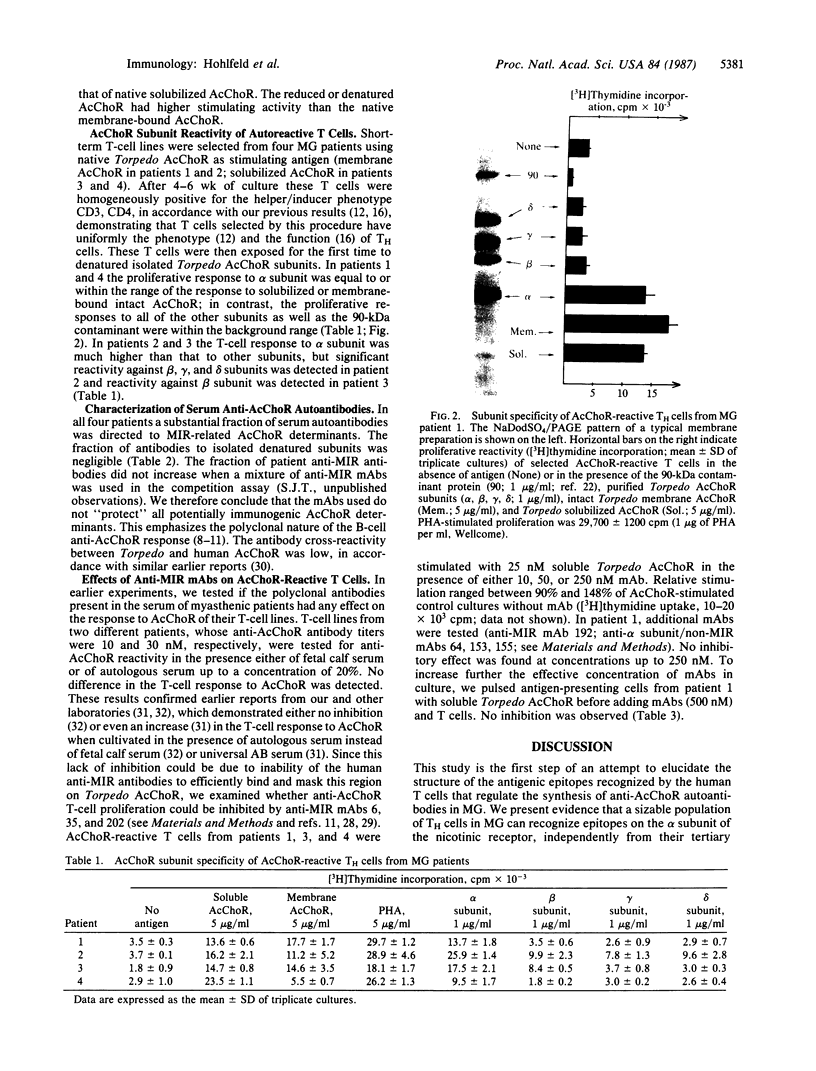
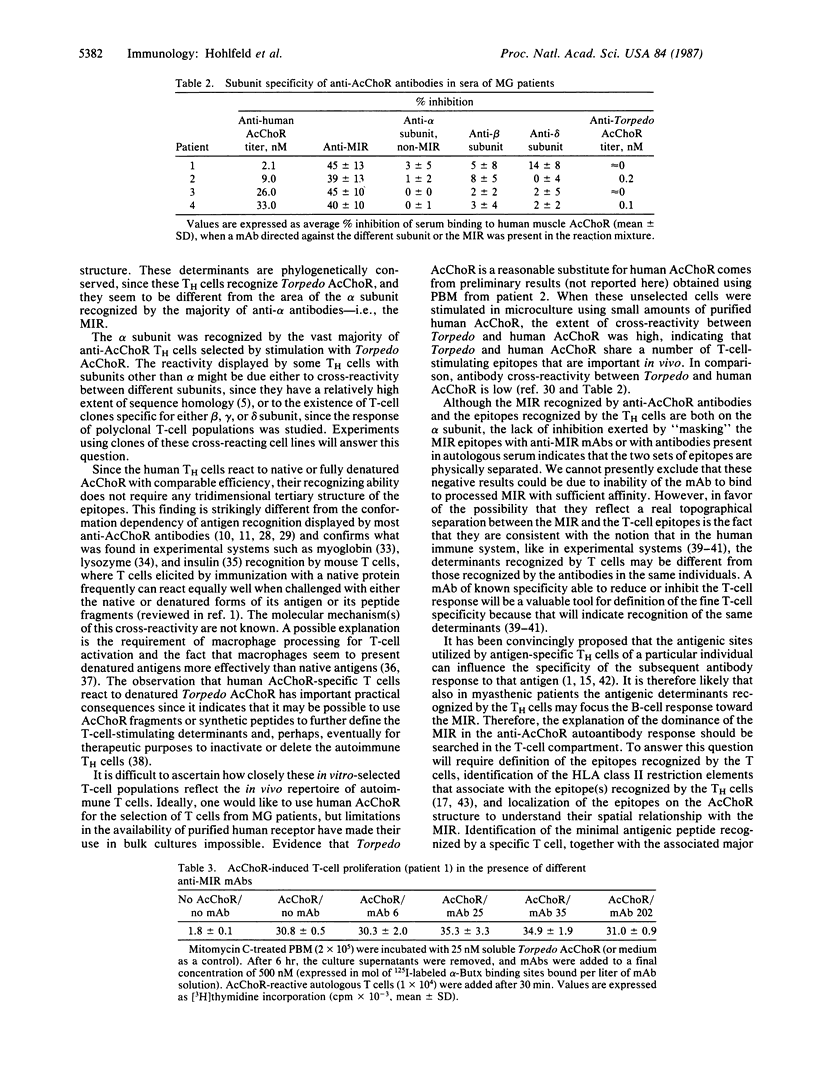
Myasthenia gravis is a human disease caused by an autoimmune response against the nicotinic acetylcholine receptor (AcChoR). Since the molecular structure of AcChoR is well known, myasthenia gravis is an excellent system for studying the recognition of a complex membrane antigen in the human immune system. Human T-helper (TH) cell lines reactive to the AcChoR were isolated from four myasthenic patients by selection with native AcChoR from Torpedo californica. The selected TH cells could efficiently recognize native and fully denatured AcChoR. The vast majority of the TH-stimulating AcChoR epitopes were located on the denatured alpha subunit of AcChoR. Antibody competition experiments using a panel of rat anti-AcChoR monoclonal antibodies showed that 39-45% of the autoantibodies present in the sera of these same patients bound to the conformation-sensitive "main immunogenic region" (MIR), also located on the alpha subunit. However, AcChoR-induced stimulation of the T cells could not be inhibited with up to 20-fold molar excess of different rat anti-MIR monoclonal antibodies. These results suggest that the Torpedo AcChoR alpha subunit contains conformation-insensitive epitopes that play a role in the autosensitization of TH cells and that seem to be physically separated from the MIR. The specificity of the TH cell response may contribute to directing the B-cell response to other alpha-subunit determinants, such as the MIR itself.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barcinski M. A., Rosenthal A. S. Immune response gene control of determinant selection. I. Intramolecular mapping of the immunogenic sites on insulin recognized by guinea pig T and B cells. J Exp Med. 1977 Mar 1;145(3):726–742. doi: 10.1084/jem.145.3.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell J., Rassenti L., Smoot S., Smith K., Newby C., Hohlfeld R., Toyka K., McDevitt H., Steinman L. HLA-DQ beta-chain polymorphism linked to myasthenia gravis. Lancet. 1986 May 10;1(8489):1058–1060. doi: 10.1016/s0140-6736(86)91330-9. [DOI] [PubMed] [Google Scholar]
- Benjamin D. C., Berzofsky J. A., East I. J., Gurd F. R., Hannum C., Leach S. J., Margoliash E., Michael J. G., Miller A., Prager E. M. The antigenic structure of proteins: a reappraisal. Annu Rev Immunol. 1984;2:67–101. doi: 10.1146/annurev.iy.02.040184.000435. [DOI] [PubMed] [Google Scholar]
- Berzofsky J. A., Richman L. K., Killion D. J. Distinct H-2-linked Ir genes control both antibody and T cell responses to different determinants on the same antigen, myoglobin. Proc Natl Acad Sci U S A. 1979 Aug;76(8):4046–4050. doi: 10.1073/pnas.76.8.4046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berzofsky J. A. T-B reciprocity. An Ia-restricted epitope-specific circuit regulating T cell-B cell interaction and antibody specificity. Surv Immunol Res. 1983;2(3):223–229. doi: 10.1007/BF02918417. [DOI] [PubMed] [Google Scholar]
- Besinger U. A., Toyka K. V., Hömberg M., Heininger K., Hohlfeld R., Fateh-Moghadam A. Myasthenia gravis: long-term correlation of binding and bungarotoxin blocking antibodies against acetylcholine receptors with changes in disease severity. Neurology. 1983 Oct;33(10):1316–1321. doi: 10.1212/wnl.33.10.1316. [DOI] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Hunkapiller M. W., Lindstrom J. M., Raftery M. A. Subunit structure of the acetylcholine receptor from Electrophorus electricus. Proc Natl Acad Sci U S A. 1982 Nov;79(21):6489–6493. doi: 10.1073/pnas.79.21.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti-Tronconi B. M., Morgutti M., Sghirlanzoni A., Clementi F. Cellular immune response against acetylcholine receptor in myasthenia gravis: I. Relevance to clinical course and pathogenesis. Neurology. 1979 Apr;29(4):496–501. doi: 10.1212/wnl.29.4.496. [DOI] [PubMed] [Google Scholar]
- Corradin G., Engers H. D. Inhibition of antigen-induced T-cell clone proliferation by antigen-specific antibodies. Nature. 1984 Apr 5;308(5959):547–548. doi: 10.1038/308547a0. [DOI] [PubMed] [Google Scholar]
- Corradin G., Juillerat M. A., Engers H. D. Differential effects of two anti-apo-cytochrome c-specific monoclonal antibodies on the function of apo-cytochrome c-specific murine T cell clones. J Immunol. 1984 Dec;133(6):2915–2919. [PubMed] [Google Scholar]
- DeLisi C., Berzofsky J. A. T-cell antigenic sites tend to be amphipathic structures. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7048–7052. doi: 10.1073/pnas.82.20.7048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Blanchard S. G., Wu W., Miller J., Strader C. D., Hartig P., Moore H. P., Racs J., Raftery M. A. Purification of Torpedo californica post-synaptic membranes and fractionation of their constituent proteins. Biochem J. 1980 Mar 1;185(3):667–677. doi: 10.1042/bj1850667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott J., Dunn S. M., Blanchard S. G., Raftery M. A. Specific binding of perhydrohistrionicotoxin to Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2576–2579. doi: 10.1073/pnas.76.6.2576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel A. G. Myasthenia gravis and myasthenic syndromes. Ann Neurol. 1984 Nov;16(5):519–534. doi: 10.1002/ana.410160502. [DOI] [PubMed] [Google Scholar]
- Hewick R. M., Hunkapiller M. W., Hood L. E., Dreyer W. J. A gas-liquid solid phase peptide and protein sequenator. J Biol Chem. 1981 Aug 10;256(15):7990–7997. [PubMed] [Google Scholar]
- Hohlfeld R., Conti-Tronconi B., Kalies I., Bertrams J., Toyka K. V. Genetic restriction of autoreactive acetylcholine receptor-specific T lymphocytes in myasthenia gravis. J Immunol. 1985 Oct;135(4):2393–2399. [PubMed] [Google Scholar]
- Hohlfeld R., Kalies I., Heinz F., Kalden J. R., Wekerle H. Autoimmune rat T lymphocytes monospecific for acetylcholine receptors: purification and fine specificity. J Immunol. 1981 Apr;126(4):1355–1359. [PubMed] [Google Scholar]
- Hohlfeld R., Kalies I., Kohleisen B., Heininger K., Conti-Tronconi B., Toyka K. V. Myasthenia gravis: stimulation of antireceptor autoantibodies by autoreactive T cell lines. Neurology. 1986 May;36(5):618–621. doi: 10.1212/wnl.36.5.618. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V., Heininger K., Grosse-Wilde H., Kalies I. Autoimmune human T lymphocytes specific for acetylcholine receptor. Nature. 1984 Jul 19;310(5974):244–246. doi: 10.1038/310244a0. [DOI] [PubMed] [Google Scholar]
- Hohlfeld R., Toyka K. V. Strategies for the modulation of neuroimmunological disease at the level of autoreactive T-lymphocytes. J Neuroimmunol. 1985 Aug;9(3-4):193–204. doi: 10.1016/s0165-5728(85)80018-7. [DOI] [PubMed] [Google Scholar]
- Kurnick J. T., Ostberg L., Stegagno M., Kimura A. K., Orn A., Sjöberg O. A rapid method for the separation of functional lymphoid cell populations of human and animal origin on PVP-silica (Percoll) density gradients. Scand J Immunol. 1979;10(6):563–573. doi: 10.1111/j.1365-3083.1979.tb01391.x. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lamb J. R., Zanders E. D., Lake P., Webster R. G., Eckels D. D., Woody J. N., Green N., Lerner R. A., Feldmann M. Inhibition of T cell proliferation by antibodies to synthetic peptides. Eur J Immunol. 1984 Feb;14(2):153–157. doi: 10.1002/eji.1830140209. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Campbell M., Nave B. Specificities of antibodies to acetylcholine receptors. Muscle Nerve. 1978 Mar-Apr;1(2):140–145. doi: 10.1002/mus.880010206. [DOI] [PubMed] [Google Scholar]
- Lindstrom J., Einarson B., Tzartos S. Production and assay of antibodies to acetylcholine receptors. Methods Enzymol. 1981;74(Pt 100):432–460. doi: 10.1016/0076-6879(81)74031-x. [DOI] [PubMed] [Google Scholar]
- Lindstrom J. Immunobiology of myasthenia gravis, experimental autoimmune myasthenia gravis, and Lambert-Eaton syndrome. Annu Rev Immunol. 1985;3:109–131. doi: 10.1146/annurev.iy.03.040185.000545. [DOI] [PubMed] [Google Scholar]
- Maizels R. M., Clarke J. A., Harvey M. A., Miller A., Sercarz E. E. Ir-gene control of T cell proliferative responses: two distinct expressions of the genetically nonresponsive state. Eur J Immunol. 1980 Jul;10(7):516–520. doi: 10.1002/eji.1830100706. [DOI] [PubMed] [Google Scholar]
- Manca F., Kunkl A., Fenoglio D., Fowler A., Sercarz E., Celada F. Constraints in T-B cooperation related to epitope topology on E. coli beta-galactosidase. I. The fine specificity of T cells dictates the fine specificity of antibodies directed to conformation-dependent determinants. Eur J Immunol. 1985 Apr;15(4):345–350. doi: 10.1002/eji.1830150408. [DOI] [PubMed] [Google Scholar]
- McCarthy M. P., Earnest J. P., Young E. F., Choe S., Stroud R. M. The molecular neurobiology of the acetylcholine receptor. Annu Rev Neurosci. 1986;9:383–413. doi: 10.1146/annurev.ne.09.030186.002123. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci U S A. 1979 Feb;76(2):690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novotný J., Handschumacher M., Haber E., Bruccoleri R. E., Carlson W. B., Fanning D. W., Smith J. A., Rose G. D. Antigenic determinants in proteins coincide with surface regions accessible to large probes (antibody domains). Proc Natl Acad Sci U S A. 1986 Jan;83(2):226–230. doi: 10.1073/pnas.83.2.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streicher H. Z., Berkower I. J., Busch M., Gurd F. R., Berzofsky J. A. Antigen conformation determines processing requirements for T-cell activation. Proc Natl Acad Sci U S A. 1984 Nov;81(21):6831–6835. doi: 10.1073/pnas.81.21.6831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Lindstrom J. M. Monoclonal antibodies used to probe acetylcholine receptor structure: localization of the main immunogenic region and detection of similarities between subunits. Proc Natl Acad Sci U S A. 1980 Feb;77(2):755–759. doi: 10.1073/pnas.77.2.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Rand D. E., Einarson B. L., Lindstrom J. M. Mapping of surface structures of electrophorus acetylcholine receptor using monoclonal antibodies. J Biol Chem. 1981 Aug 25;256(16):8635–8645. [PubMed] [Google Scholar]
- Tzartos S. J., Seybold M. E., Lindstrom J. M. Specificities of antibodies to acetylcholine receptors in sera from myasthenia gravis patients measured by monoclonal antibodies. Proc Natl Acad Sci U S A. 1982 Jan;79(1):188–192. doi: 10.1073/pnas.79.1.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzartos S. J., Sophianos D., Efthimiadis A. Role of the main immunogenic region of acetylcholine receptor in myasthenia gravis. An Fab monoclonal antibody protects against antigenic modulation by human sera. J Immunol. 1985 Apr;134(4):2343–2349. [PubMed] [Google Scholar]
- Tzartos S., Langeberg L., Hochschwender S., Lindstrom J. Demonstration of a main immunogenic region on acetylcholine receptors from human muscle using monoclonal antibodies to human receptor. FEBS Lett. 1983 Jul 11;158(1):116–118. doi: 10.1016/0014-5793(83)80688-7. [DOI] [PubMed] [Google Scholar]
- Unanue E. R. Antigen-presenting function of the macrophage. Annu Rev Immunol. 1984;2:395–428. doi: 10.1146/annurev.iy.02.040184.002143. [DOI] [PubMed] [Google Scholar]
- Vincent A., Newsom-Davis J. Acetylcholine receptor antibody characteristics in myasthenia gravis. I. Patients with generalized myasthenia or disease restricted to ocular muscles. Clin Exp Immunol. 1982 Aug;49(2):257–265. [PMC free article] [PubMed] [Google Scholar]