Abstract
Heme oxygenases (HOs) are the rate-limiting enzymes in the catabolism of heme into biliverdin, free iron, and carbon monoxide. Two genetically distinct isoforms of HO have been characterized: an inducible form, HO-1, and a constitutively expressed form, HO-2. HO-1 is a kind of stress protein, and thus regarded as a sensitive and reliable indicator of cellular oxidative stress. The HO system acts as potent antioxidants, protects endothelial cells from apoptosis, is involved in regulating vascular tone, attenuates inflammatory response in the vessel wall, and participates in angiogenesis and vasculogenesis. Endothelial integrity and activity are thought to occupy the central position in the pathogenesis of cardiovascular diseases. Cardiovascular disease risk conditions converge in the contribution to oxidative stress. The oxidative stress leads to endothelial and vascular smooth muscle cell dysfunction with increases in vessel tone, cell growth, and gene expression that create a pro-thrombotic/pro-inflammatory environment. Subsequent formation, progression, and obstruction of atherosclerotic plaque may result in myocardial infarction, stroke, and cardiovascular death. This background provides the rationale for exploring the potential therapeutic role for HO system in the amelioration of vascular inflammation and prevention of adverse cardiovascular outcomes. Antioxid. Redox Signal. 14, 137–167.
I. Introduction
Heme oxygenase (HO) plays a central role in regulating the levels of intracellular heme by catalyzing the oxidative degradation of heme to biliverdin (BV), free iron and carbon monoxide (CO) (272). BV is subsequently metabolized to bilirubin (BR) by BV reductase (BVR), and the free iron is promptly sequestered by ferritin. The production of CO and BR through heme degradation is protective, but the excessive accumulation of CO and BR is potentially toxic [reviewed in (154)]. To date, three isoforms of HO (e.g., HO-1, HO-2 and HO-3) have been identified (107). The isoforms are produced by different genes and show different tissue distributions and properties. HO-1, a 32-kDa member of the stress protein superfamily, has a broad spectrum of inducers, including metals, cytokines, endotoxin, oxidants, and vaso-active compounds (75, 199). In contrast, HO-2, a 36-kDa protein, is constitutively expressed and present in high levels in the brain and testes (297). HO-3, a lastly cloned 33-kDa protein that closely resembles HO-2 with much lower catalytic activity, is not expressed in humans. Both HO-1 and HO-2 cleave the α-meso-carbon bridge of heme, and this cleavage is inhibited by various metalloporphyrins, including zinc protoporphyrin (ZnPP) or tin protoporphyrin (SnPP) (297). Although interest in HO was originally centered on its ability to degrade the highly oxidative heme, recent findings indicate that heme/HO system and its downstream effector molecules are tightly involved in the regulation of many physiological as well as pathophysiological processes, including fundamental adaptive response to cellular stress, cytoprotection, apoptosis, and inflammation (194). Particularly, the crucial roles of HO-1 in regulating vascular function have been demonstrated (167, 171, 195). Therefore, in this review, we will describe in detail the roles of HO-1 and its enzymatic products not only in vascular diseases (e.g., atherosclerosis, vasculitis, ischemia/reperfusion (I/R) injury, and restenosis), but also in angiogenesis.
II. HO Expression
A. Heme oxygenase-1
HO-1 expression is induced transcriptionally by a large number of pharmacological agents as well as a variety of circumstances, such as heat shock and other forms of extracellular and intracellular stresses (218). HO-1 expression in human cells is repressed under thermal stress or hypoxia, or by the treatment with interferon-γ [reviewed in (233)], which may represent a regulatory mechanism that prevents the noxious induction of HO-1. Because the modulation of HO-1 activity is of potential therapeutic value (142, 241), the detailed understanding of the mechanisms responsible for the transcriptional activation of the ho-1 gene (Hmox-1) is imperative. A number of signaling molecules and transcription factors have been indentified to be involved in regulating HO-1 expression [reviewed in (270)]. These molecules include mitogen-activated protein kinases (MAPKs), nuclear factor E2-related factor 2 (Nrf2), Bach1 (bric-à-brac, tramtrack and broad complex and cap ’n’ collar homology 1), protein kinase C, protein kinase A, phosphatidyl inositol 3-kinase/Akt, activator protein-1 (AP-1), nuclear factor-κB (NF-κB), cyclic adenosine monophosphate-responsive element-binding protein, BVR, and activating transcription factor 2. It is most likely that HO-1 expression may result from the complex cooperative interactions between these molecules (Fig. 1). Because a number of investigations have implicated the involvement of MAPK pathways in the regulation of HO-1 expression and have focused on characterizing the roles of Nrf2/Bach1 system in Hmox-1 activation in diverse cell types in response to various inducing conditions, MAPKs and Nrf2/Bach1 system, together with brief comments on AP-1 (which has previously been proposed to mediate Hmox-1 activation) and BVR (which has recently been recognized as a new transcription factor for Hmox-1 activation), are discussed below.
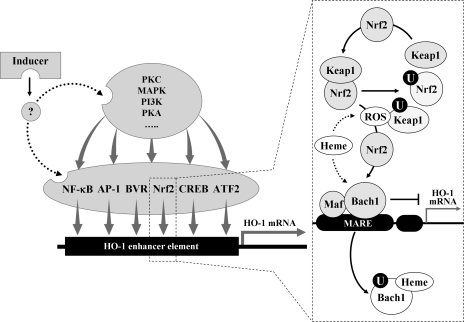
FIG. 1.
Regulation of HO-1 induction by transcription factors and kinases. A HO-1 inducer may activate at least one or more of kinases (e.g., MAPKs, PKC, PKA, and PI3K) and/or one of transcription factors (e.g., NF-κB, activator protein-1, Nrf2, CREB, BVR, and activating transcription factor 2). Under normal conditions, the transcription factors are located in cytosol or nucleus. Upon activation by external stimuli, the active forms of the transcription factors may translocate to the nucleus where they bind to the specific DNA sequence leading to the transcription of ho-1 gene. The dotted box shows a typical example in which Nrf2, Keap1, and Bach1 may interact with each other in response to free heme or ROS. Under normal conditions, Bach1/small Maf complexes bind constitutively MARE in the ho-1 gene promoter and inhibit ho-1 gene transcription. Nrf2 is ubiquitinated by forming a complex with the Keap1 (see text). In response to heme and/or ROS, Bach1 is exported from the nucleus, ubiquitinated (circled U), and degraded, releasing transcriptional repression. ROS caused by free heme may also induce Keap1 ubiquitination and degradation, allowing Nrf2 accumulation in the nucleus. Nrf2/small Maf complexes bind to MARE and promote ho-1 gene transcription. Bach1, bric-à-brac, tramtrack and broad complex and cap ’n’ collar homology 1; BVR, biliverdin reductase; CREB, cyclic adenosine monophosphate-responsive element-binding protein; HO, heme oxygenase; Keap1, Kelch-like ECH-associated protein 1; Maf, Musculo-aponeurotic fibrosarcoma; MAPK, mitogen-activated protein kinase; MARE, Maf protein recognition elements; NF-κB, nuclear factor-κB; Nrf2, nuclear factor E2-related factor 2; PI3K, phosphatidyl inositol 3-kinase; PKA, protein kinase A; PKC, protein kinase C; ROS, reactive oxygen species.
1. Mitogen-activated protein kinases
The MAPKs are a family of serine-threonine protein kinases that regulate many cellular events, including responses to environmental stimuli (199). The MAPK superfamily encompasses three important signaling pathways: the extracellular regulated kinases (ERK) pathway, the c-Jun N-terminal kinases or stress-activated kinases (JNK) pathway, and the p38 MAPKs (p38 pathway). A variety of structurally and functionally diverse agents that induce HO-1 expression also in parallel can activate MAPK cascades in multiple cell types (126, 137, 195), implicating the involvement of MAPK pathway in the regulation of HO-1 expression. Simvastatin, a low-density lipoprotein (LDL) cholesterol-lowering drug, stimulates HO-1 expression in vascular smooth muscle cells (VSMCs) through p38 MAPK pathway (140). Atrial natriuretic peptide, a cardiovascular hormone, induces HO-1 expression in endothelial cells (ECs) through ERK pathway (89). Salicylate, the active metabolite of aspirin, induces expression of HO-1 in ECs via JNK pathway (88). The inhibition of ERK, JNK, or p38 MAPK activation individually is insufficient to completely abolish HO-1 expression by oxidized LDL (Ox-LDL); however, inhibition of these MAPK pathways in combination results in a significantly greater attenuation of HO-1 expression (12), suggesting that these MAPK pathways act in concert to regulate HO-1 expression. The terminal, activated MAPKs phosphorylate their downstream targets. In the case of Hmox-1 transcriptional events, downstream targets of MAPKs may be the transcription factors that directly regulate ho-1 gene activation and/or components that are indirectly involved in activation of the transcription factors.
2. Nrf2 and Bach1
Nrf2, which belongs to the cap ’n’ collar family of transcription factors, controls a critical cellular defense response by coordinated upregulation of many of its target genes, culminating in a cell survival response. Deletion mutagenesis and transfection studies have identified an element designated as the antioxidant response element (ARE) in the promoter regions of Nrf2 downstream genes (215). Molecular cloning and bioinformatics have shown that the promoter of Hmox-1 contains numerous sequence-related cis-elements, a number of which are clustered around −4.0 and −9.0 kb (215). In the mouse, the regions of Hmox-1 promoter around −4.0 and −9.0 kb have been called enhancer 1 and enhancer 2, respectively, and contain multiple stress response elements (StREs) that are likened to Musculo-aponeurotic fibrosarcoma (Maf) protein recognition elements [reviewed in (111)]. The majority of the StREs in Hmox-1 contain the core ARE sequence. Because Hmox-1 has been found to contain functional AREs in the promoter region, HO-1 expression is considered highly likely to be regulated directly by Nrf2.
Under basal conditions, Nrf2 is rapidly ubiquitinated by forming a complex with the Kelch-like ECH-associated protein 1 (Keap1) and degraded by the 26S proteassome [reviewed in (126)]. Keap1 serves as a bridge between Nrf2 and the Cullin-3-dependent E3 ubiquitin ligase complex, leading to ubiquitination of multiple lysine residues located in Nrf2 domain. Keap1 targets Nrf2 for ubiquitin-dependent degradation and, hence, represses Nrf2-dependent gene expression (126). It has been initially proposed that Keap1-dependent degradation of Nrf2 occurs in the cytoplasm (111). Recently, it has also been proposed that Nrf2, even under normal condition, is localized in the nucleus where Nrf2 is targeted for degradation by Keap1 [reviewed in (186)]. It is most likely that oxidative stress may somehow prevent Keap1-dependent degradation of Nrf2 in either the cytoplasm or the nucleus, perhaps resulting in Nrf2 nuclear accumulation. Accumulated or stabilized Nrf2 may bind to ARE sequences to form complexes with the small Maf proteins (111, 126), leading to initiation of Hmox-1 transcription. Thus, Keap1 has been shown to be the main cytosolic inhibitor of Nrf2.
The Bach1 protein is a potent repressor of HO-1 expression. It also binds to StREs (i.e., AREs) in the promoter of Hmox-1 as a heterodimer with small Maf proteins and competes against Nrf2 for binding to the ARE sequences [reviewed in (96)]. In response to oxidative stimuli, Bach1 is exported from the nucleus, ubiquitinated and degraded, allowing the formation of Nrf2/small Maf protein complexes (96). This hypothesis is supported by the findings that Bach1-deficient cells express constitutive high levels of HO-1 mRNA (190), which is not observed in cells lacking both Nrf2 and Bach1. However, it is most likely that the Bach1/Nrf2 transcriptional system may interact functionally with other transcription factors to regulate Hmox-1 transcription.
3. Activator protein-1
AP-1 is a dimeric combination of basic leucine zipper proteins of the Jun and Fos family, Jun dimerization partners, and the closely related activating transcription factor subfamilies [reviewed in (7)]. Due to sequence similarity of the Maf protein recognition elements with the consensus AP-1 binding site, AP-1 factors were proposed to regulate Hmox-1 activation in response to multiple stimuli. A study has demonstrated that the phorbol ester, a classical activator of AP-1 factors, induced HO-1 expression in mice through AP-1 binding to the 5′-flanking region of the target gene (8). Other studies have also demonstrated that expression of HO-1 by sodium arsenite (74) and bacterial lipopolysaccharide (LPS) (42) is mediated by AP-1 activation. Because Maf proteins can form part of the AP-1 complex, it is likely that the dissociation of Bach1 from Maf proteins would enhance the binding of other positive transcriptional regulators of Hmox-1, such as Nrf2.
4. Biliverdin reductase
BVR enables continuous protection of cells against oxidative stress by its conversion of BV to BR, but this is not a sole function of BVR (82, 141, 151). BVR has been shown to have a wide spectrum of its potential functions in cell signaling pathways. BVR has two DNA binding sites known as AP-1 recognition sequences (82). Thus, it may bind to AP-1 recognition sequences of DNA to play a role in the AP-1 pathways of cellular signaling. Because AP-1 binds to multiple copies of consensus sequence in the ho-1 promoter (7), BVR could also bind to DNA of ho-1 promoter to activate Hmox-1 transcription (82). In addition, BVR binds to cyclic adenosine monophosphate-responsive element-binding protein recognition sequences of ho-1 promoter [reviewed in (125)], which may also activate Hmox-1 transcription. In human BVR-infected cells, levels of HO-1 mRNA and protein were increased, while blockage of BVR synthesis by small interfering RNA (siRNA) attenuated chemical-mediated increase in HO-1 expression (164).
B. Heme oxygenase-2
Under physiological conditions, HO-2 expression is found in various tissues, particularly in testis and brain, whereas HO-1 expression is relatively low, with the exception of spleen [reviewed in (218)]. HO-2 expression can be transcriptionally modulated by corticosteroids [reviewed in (154)] but may not by the environmental stress that can remarkably induce HO-1 expression at transcriptional level (218). It is most likely that relatively constant expression levels of HO-2 may be suitable for its regulatory role in heme homeostasis. However, HO-2 activity may be dynamically regulated via posttranslational mechanisms that involve protein phosphorylation (29). Unlike HO-1, HO-2 contains three cysteine residues, each of which is present as a dipeptide of cysteine and proline and may function as the heme-binding site (233). It has been, therefore, postulated that HO-2 may play a regulatory role by sequestering heme to maintain the intracellular heme level (233). It has also been postulated that HO-2 may function as an oxygen sensor (129). In addition, mice deficient in HO-2 have revealed several important functions of this enzyme. For example, HO-2 deletion causes EC activation marked by oxidative stress, inflammation, and angiogenesis (23), which underscores the importance of HO-2 in the regulation of EC homeostasis. HO-2 deficiency disables execution of the acute inflammatory and reparative response after epithelial injury and leads to an exaggerated inflammatory response in antigen-induced peritonitis (231), implicating a role for HO-2 in the regulation of the inflammatory and reparative response to injury. HO-2 deletion has been associated with impaired HO-1 induction (238), which may explain the reason why HO-2-null mice could not compensate for the loss of HO-2 by increasing HO-1 expression. Thus, it is most likely that HO-2 may be critical for HO-1 expression.
III. HO By-Products
The protective effects of HO-1 have been confirmed in a number of experimental models, but the mechanisms of its multiple actions have not been completely elucidated. Heme, even though the heme is an essential prosthetic group of various enzymes in the biological system, it is inherently dangerous particularly when released from intracellular heme-containing proteins. The extreme hydrophobicity of free heme permits it to intercalate into cell membranes, resulting in increased cellular susceptibility to oxidant-mediated damages as well as generation of reactive oxygen species (ROS) (103). Once heme is in the cell membrane, hydrogen peroxide from sources such as activated leukocytes can split the heme ring and release free redox-active iron, which can catalytically amplify the production of ROS inside the cell (21, 27, 103). Thus, degradation of free heme by HO-1 appears to first aid in tissue protection; however, recent evidence suggests that one or more of the by-products of its heme catabolism (e.g., CO, ferrous iron and BV/BR) mediate the protective effects of HO-1 (Fig. 2).
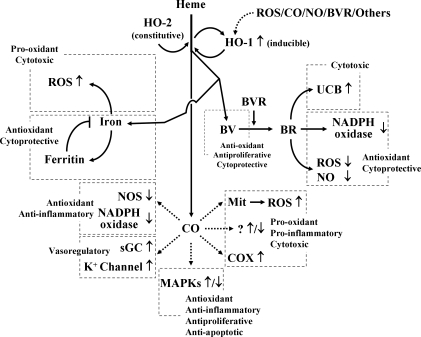
FIG. 2.
Heme degradation products and their effects. HO-1 and HO-2 catalyze the stereospecific degradation of heme to BV, with the concurrent release of Fe2+ and CO. BV is converted to BR by BVR. Iron is sequestered by ferritin, reducing its toxic effects, but it may cause ROS. CO confers cytoprotection by inhibiting apoptosis, proliferation, and inflammation and also by increasing cGMP levels. However, CO may increase the formation of ROS and pro-inflammatory molecules. BV and BR are known to have potent antioxidant effects, but an increase in unconjugated BR may cause BR-induced neurologic damage by deposition of UCB in the central nervous system. HO-1 is induced in response to free heme, ROS, BVR, CO, and NO. BR, bilirubin; BV, biliverdin; cGMP, cyclic guanine monophosphate; CO, carbon monoxide; NO, nitric oxide.
A. Carbon monoxide
CO is classically thought of as a toxic molecule and cellular asphyxiate, but recent studies have revealed that CO has profound effects on the intracellular signaling processes, culminating in antiinflammatory, antiproliferative, and antiapoptotic effects (198, 199). These effects of CO may involve three general mechanisms; (a) CO can activate soluble guanylyl cyclase (sGC), with the resultant production of cyclic guanine monophosphate (cGMP), (b) CO can modulate MAPK pathway, and (c) CO can bind to a range of intracellular heme proteins, thereby resulting in the formation of metal-CO complexes that may generate diverse biological effects, including inhibition or activation of heme-containing enzymes. CO, like nitric oxide (NO), binds to the heme moiety of sGC, leading to the stimulation of sGC and subsequent elevation of cGMP levels that causes protective vascular relaxation (199). Besides binding to sGC, CO may bind to the heme moiety of other hemoproteins thus affecting their enzymatic activity; there is the experimental evidence about the stimulatory role of CO on cyclooxygenase (COX) activity [reviewed in (158)]. Hemin, the substrate of HO stimulated COX to produce prostaglandin E2 in rat hypothalamic explants and in primary cultures of rat hypothalamic astrocytes (156, 159). This effect was inhibited by the HO inhibitor tin-mesoporphyrin-IX and abolished by the CO scavenger hemoglobin (156, 159). Moreover, treatment of rat hypothalamic explants with CO-saturated medium resulted in significant increases in prostaglandin E2 release (156). All these data provided experiment evidence supporting that CO could stimulate COX activity in the rat hypothalamus.
CO induces a general downregulation of pro-inflammatory cytokine production through the MAPK-dependent pathway, leading to antiinflammatory tissue protection (218). Antiapoptotic and antiproliferative actions of CO also involve the modulation of MAPK pathways (169, 218). It has been shown that the effects of CO on MAPK signaling pathway may depend on cell types and stimuli. For examples, the antiapoptotic effect of CO was exerted via the p38 MAPK signaling pathway in human ECs (31), whereas CO-mediated antiapoptotic effect via ERK signaling pathway in rat hepatocytes (56). Whereas CO inhibited the proliferation of pancreatic stellate cell by activating p38 MAPK (226), CO suppressed the proliferation of human airway SMCs by inhibiting ERK activation (240). The mechanisms by which CO can modulate MAPK pathways are not clear. CO slows the rate of electron transport, enabling electrons to accumulate at complex III in mitochondria, and this promotes the formation of ubi-semiquinone from which electrons can be donated to produce superoxide and its conversion to H2O2 by manganese superoxide dismutase (17). It is most likely that the mitochondrium-derived H2O2 may activate the MAPK and Akt signaling pathways [reviewed in (27)].
Besides its general protective mechanisms, CO may have an ability to activate other protective pathways. CO expose induced expression of heat shock protein (HSP) 70, which plays vital roles in its protective effects against cytokine-induced EC apoptosis (131). Treatment of PC12 cells with a CO donor upregulated expression of the catalytic subunit of glutamate-cysteine ligase, the rate-limiting enzyme in glutathione biosynthesis, and this was associated with the protective effects of CO against NO-induced cell death (54).
CO does not always exert cytoprotective activity; CO also produces noxious effects in certain organs (e.g., the brain) (233). CO increases the formation of pro-inflammatory prostaglandins by activating COX in rat hypothalamic explants and in primary culture of rat hypothalamic astrocytes (see references in 153, 154, 233), suggesting that CO stimulates pro-inflammatory responses at least in the brain. Moreover, CO reduces cellular levels of antioxidants (e.g., glutathione) by increasing mitochondrial ROS formation through the binding of CO to cytochromes residing in complex IV (i.e., cytochrome a3) [reviewed in (27)], indicating that CO may cause oxidative tissue damages. Whether the effects of CO would be protective or not, in all probability, may depend on several factors, including the type of cells, the concentration of CO produced or administered, and the tissue-specific signaling transduction pathway(s) that may be involved in its biological activity, and this has been well reviewed by Mancuso and Barone (154).
B. Ferrous iron and ferritin
Ferrous iron, an extremely pro-oxidative molecule, is released during the breakdown of free heme by HO-1, but this molecule is rapidly removed by ferritin, a ubiquitously existing intracellular protein that is able to effectively sequester intracellular iron and, hence, limits the pro-oxidant capacity of ferrous iron. It is most likely that increased ferritin expression in conjunction with HO-1 expression may contribute to additional protection afforded by HO-1 (16, 199, 218).
C. BV and BR
BV and BR have been shown to provide vascular protection by preserving EC integrity, preventing EC death, enhancing vascular reactivity, and inhibiting restenosis (218, 252). In the cellular system, BV resulting from the HO-1 catabolism of free heme is rapidly converted into BR. BV and BR are now recognized as being potent antioxidants manufactured by the body (147). Indeed, BV and BR can directly scavenge ROS and interact with the free radical NO and the oxidant peroxinitrite (155, 157, 159). BR also exerts a potent suppression of the activity of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase that generates the free radical superoxide (62, 86). The mechanisms by which BR can inhibit NADPH oxidase activity are not clear, but independent of its ROS-scavenging effect (62). Moreover, general antiinflammatory effects of BV and BR based on their antioxidant potentials have been described (94, 199).
IV. Effect of HO-1 on Vascular Inflammation
HO-1 serves as a protective gene by virtue of its antiinflammatory, antiapoptotic, and antiproliferative actions, as being variously manifested in endothelial, epithelial, smooth muscle, and other cell types (31, 138, 207, 263). HO-1 is also involved in blood vessel relaxation regulating vascular tone and participates in blood vessel formation by means of angiogenesis and vasculogenesis. Current accumulating data have highlighted the critical importance of HO-1 in ameliorating vascular inflammation in various diseases (11, 22, 116, 177, 178, 246, 257). The scope of this section will be to address the recent discoveries on the role of HO-1 in vascular inflammation, with special emphasis on its antiinflammatory mechanisms implicated in various mediators and cells, especially macrophages and ECs.
A. Cytokines, chemokines, and mediators
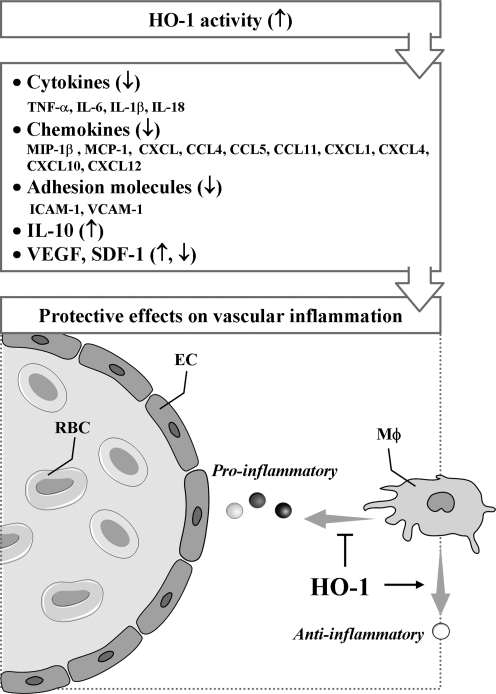
Various cytokines are implicated in vascular inflammation (259). HO-1 and its metabolites are well known to attenuate the secretion of pro-inflammatory cytokines and to augment the production of antiinflammatory cytokines (Fig. 3). Actually, HO-1 reduces the production of tumor necrosis factor (TNF)-α, interleukin (IL)-1β, and IL-6, whereas it enhances IL-10 expression in vitro (183, 197). In addition, HO-1 upregulation blocks IL-18 signaling and reduces IL-18-dependent vascular injury and inflammation (297). IL-18 contributes to both systemic and acute inflammation by inducing expression of TNF-α and other pro-inflammatory cytokines and activation of NF-κB. A study has demonstrated that HO-1-dependent blockade of NF-κB, which can be induced by IL-18, is associated with reduction in EC death (297).
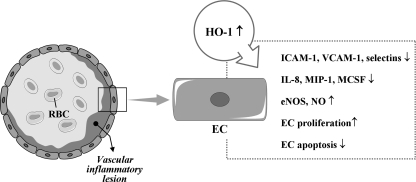
FIG. 3.
Protective effects of HO-1 on vascular inflammation through modulation of cytokines, chemokines, and mediators. EC, endothelial cell; RBC, red blood cell; MΦ, macrophage;↑, increased;↓, decreased; ⊣, attenuated.
CO has been shown to be responsible for the inhibition of pro-inflammatory cytokine production, including TNF-α, IL-1β, IL-6, and macrophage inflammatory protein (MIP)-1β, in macrophages stimulated with LPS (193). The effects of CO on its regulation of pro-inflammatory and antiinflammatory cytokine production, at least, in macrophages are exerted mainly via the p38 MAPK signal pathway [reviewed in (130)]. In addition, upregulation of IL-10 is a further mechanism responsible for the antiinflammatory actions of CO (193). IL-10 can downregulate the pro-inflammatory response only when HO-1 expression or activity is upregulated in response to IL-10 (139). When HO activity is blocked pharmacologically, the antiinflammatory effect of IL-10 is lost (139). The ability of IL-10 to prevent lethality from endotoxic shock (139) and to attenuate inflammation in aortic allografts (49) is also abolished when HO activity is inhibited pharmacologically. After HO-1 is activated by IL-10, HO-1 itself, along with the products of heme degradation (e.g., CO), can mediate the IL-10 effect (49, 139). Hence, these observations indicate that IL-10 can upregulate HO-1 expression and its activity but also is augmented by HO-1, thereby exerting its antiinflammatory properties. In addition, several studies have suggested that expression of HO-1 is essential for the function of a variety of endogenous molecules and pharmacologic agents used therapeutically to suppress inflammation (1, 15).
CCL2, also known as a monocyte chemotatic protein 1 (MCP-1), was the first chemokine shown to affect atherosclerosis. CCL2 and its receptor are most prominently involved in monocyte recruitment from bone marrow into the arterial wall (230, 266, 285). Overexpression of HO-1 or administration of CO suppresses the production of the chemokines such as MCP-1 or MIP-1β (CCL3). Another HO-1 product, BR has been shown to inhibit MCP-1 expression in human ECs (127), but the underlying mechanism(s) remains to be established. As for vascular pathologies, a study has shown that elevation of IL-8 level in plasma can be a biomarker predicting early recurrence of ischemia, myocardial infarction, and sudden cardiac death after percutaneous coronary interventional procedures (211). Blocking of IL-8 signaling substantially attenuates polymorphonuclear neutrophil infiltration and vascular and tissue injury in the postischemic heart (30). Induction of HO-1 expression/activity has been reported to be associated with reduction in IL-8 production in microvascular endothelium in vitro and in vivo (188), but it is not clear that HO-1 expression could modulate IL-8 production. Other chemokines, such as CCL4, CCL5, and CCL11 are implicated in vascular inflammation; however, their relationship with HO-1 remains to be clarified.
It has been shown that HO-1-mediated resolution of inflammation may originate from the modulation of adhesion molecule expression. Studies have shown that overexpression of HO-1 attenuates expression of adhesion molecules, such as intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin, in vitro and in vivo, whereas selective inhibition of HO-1 activity aggravates expression of those adhesion molecules (104, 268, 272, 273). Confirming these observations, Rucker et al. (217) have shown the decrease in ICAM-1 expression in an animal model of arterial injury when HO-1 was upregulated before injury. Sun et al. (248) have also reported in vitro data showing that induction of HO-1 inhibits TNF-α-induced monocyte adhesion to human ECs by suppressing expression of ICAM-1. In addition, HO activity decreases expression of VCAM-1 and ICAM-1 by depleting heme and generating antioxidants (i.e., BV/BR) and another gaseous mediator (i.e., CO), which shares many properties with NO (24, 63).
The role of HO-1 in angiogenesis seems to vary depending on the underlying conditions. Bussolati and coworkers (34, 35) have demonstrated that vascular endothelial growth factor (VEGF)-induced angiogenesis requires HO-1 activity, whereas inflammation-induced blood vessel formation is attenuated by HO-1 overexpression. Thus, a concept that during chronic inflammation, HO-1 may inhibit leukocytic infiltration and facilitate tissue repair by promoting VEGF-driven noninflammatory angiogenesis has been accepted (35). Recently, Deshane et al. (67) have also demonstrated that stromal cell-derived factor-1 (SDF-1) promotes angiogenesis and the function of endothelial precursor cells (EPCs) through a mechanism dependent on HO-1 expression. Although the molecular mechanisms underlying the induction of VEGF and SDF-1 by HO-1 or vice versa remain unclear, it is envisioned that HO-1 and angiogenic factors can activate a positive-feedback circuit to amplify neovascularization in adult tissues. Consistent with these observations, HO-1-mediated cardioprotection after ischemic injury can be offered by promoting neovascularization through inducing expression of VEGF and SDF-1 and the recruitment of circulating progenitor/stem cells (145).
B. Macrophages
Macrophages seem not only to promote inflammation, but also to downregulate it (77, 283, 288) (Fig. 4). Macrophages produce pro-inflammatory cytokines, participate in lipid retention and vascular cell remodeling, and express pattern-recognition receptors (PPRs) during atherosclerosis (90). During apoptosis, however, macrophages can suppress inflammatory responses through phagocytosis of apoptotic debris (214, 216). In addition, macrophages act as the first-line defense against invading pathogens through undergoing immediate oxidative burst to overproduce superoxide anion radicals, delaying overproduction of NO, and thereby leading to generation of peroxynitrite. While the peroxynitrite can kill invading pathogens, this oxidant can also kill macrophages and surrounding host tissues (243). However, macrophages and host cells can protect themselves from the toxicity of various assailants, including peroxynitrite, by enhancing expression of HO-1 and other protective enzymes. Under vascular inflammatory conditions, HO-1 is highly expressed in all the main cell types, including ECs, macrophages, and SMCs, but HO-1 is virtually absent in neighboring unaffected vascular tissue (113, 274).
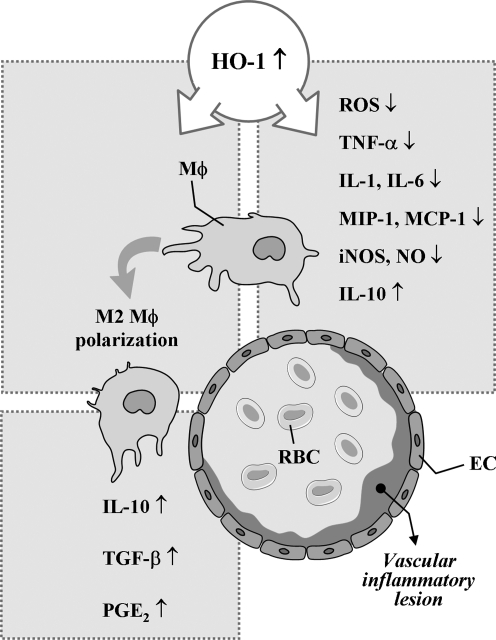
FIG. 4.
HO-1 in macrophage at vascular inflammation. ↑, increased; ↓, decreased.
HO-1 can inhibit NO production through suppression of inducible NO synthase (iNOS) expression and HO-1 by-product, BR, has been shown to effectively counteract NO-induced stress through its ability to bind and inactivate NO (37, 39), indicating the possible presence of functional interaction between NO/iNOS and CO/HO-1 systems in regulating inflammation. There is an excellent study supporting the negative feedback regulation of the pro-inflammatory NO/iNOS system by the antiinflammatory CO/HO-1 system (13). Using iNOS-deficient macrophages, Ashino et al. (13) showed that LPS failed to induce HO-1 expression, suggesting that NO/iNOS is implicated in HO-1 expression. In other experimental sets, they investigated iNOS and HO-1 expression by LPS using Nrf2-deficient macrophages. Whereas LPS failed to induce HO-1 expression, LPS strongly induced iNOS expression in Nrf2-deficient macrophages, supporting that CO/HO-1 system can inhibit iNOS expression.
Recent studies have demonstrated that the major site of HO-1 expression is mainly monocytes/macrophages in the inflammatory and ischemic injured areas (48, 99, 140, 149). As expected, HO-1 expression in macrophages is one of the antiinflammatory mechanisms of macrophage. Orozco et al. (192) have shown that HO-1 expression in macrophages constitutes an important component of its antiatherogenic effects. Supporting these in vivo data, macrophages stimulated with LPS produce several pro-inflammatory cytokines, including TNF-α (26). If macrophages overexpress HO-1 or are exposed to CO in vitro before stimulation with LPS, the pro-inflammatory response (i.e., TNF-α production) is markedly inhibited, whereas the antiinflammatory response (i.e., IL-10 production) is enhanced (163). In addition, chalcone potently induces HO-1 expression in murine macrophages, leading to reduced LPS-mediated NO and TNF-α production (5).
A study using a CO-releasing molecule (CORM) has demonstrated that overproduction of CO allows the survival of LPS-stimulated macrophages (a) by eliminating the free heme to prevent Fenton reaction, (b) by limiting the availability of free heme required for induction of NO producing heme enzyme (i.e., iNOS), and (c) by limiting additional production of superoxide and NO via CO-derived inhibition of the activities of heme enzymes like NADPH oxidase and iNOS, allowing the LPS-activated macrophages to return back to the normal quiet state (243). Further, in LPS-stimulated murine macrophages, hydrogen sulfide (H2S), a putative vasodilator, can inhibit NO production and NF-κB activation through a mechanism that involves the action of HO-1/CO, displaying antiinflammatory effects (189). However, H2S can reduce NO reactivity by interacting with it and forming an S-nitrosothiol compound (282).
Macrophages are classically activated by microbial cell wall components and/or interferon-γ. The resulting phenotype is known as M1 macrophage, which is characterized by the production of pro-inflammatory mediators such as NO, superoxide, TNF-α, IL-1β, and IL-6 (93, 95). Polarization toward the alternatively activated phenotype (i.e., M2 macrophage) is achieved by, for example, glucocorticoids, IL-4, IL-13, or IL-10 (94, 160). M1 and M2 macrophages play opposite roles during inflammation, but both are present in vascular inflammatory diseases, such as atherosclerosis. M2 macrophages suppress the release of pro-inflammatory mediators and provoke the formation of antiinflammatory mediators, such as IL-10, transforming growth factor-β, or prostaglandin E2 (79, 87, 271). In addition, the antiinflammatory mechanisms of macrophages are at least in part attributed to defective LPS-induced NF-κB activation (61). A recent work by Weis and colleagues (281) has demonstrated that the establishment of this antiinflammatory phenotype of macrophages is in part dependent on the induction of HO-1 by apoptotic cell-derived sphingosine-1-phosphate. Taken together, these findings suggest that HO-1 expression in macrophage and the downstream effectors could be a promising target for the therapeutic approach for various vascular pathologies.
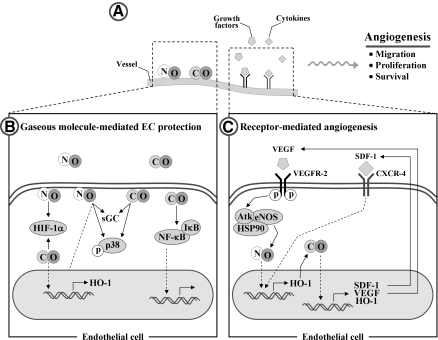
C. Endothelial cells
Although the molecular basis of the antiinflammatory effects of HO-1 in ECs remains to be fully elucidated, it has been reported that HO-1 overexpression in human ECs reduces TNF-α-induced E-selectin and VCAM-1 expression but not ICAM-1 expression and inhibits monocyte chemotaxis (34, 237) (Fig. 5). In addition, substantial expression of HO-1 induced by hypoxia inducible factor (HIF)-1α attenuates IL-8 secretion in microvascular endothelium in vitro and in vivo (104). The products of HO, such as BV, BR, and CO, have also cytoprotective effects for ECs against various stresses (210, 287). BR inhibits activation of ECs by suppressing expression of E-selectin, VCAM-1, MIP-1, and monocyte/macrophage-colony stimulation factor (127, 202, 237). Notably, CORMs have been shown to have antiinflammatory effects on vascular ECs. CORMs inhibit EC death via suppression of IL-18-mediated NF-κB activation and phosphatase and tensin homolog induction and reversion of IL-18-mediated suppression of Akt activity (265), suggesting that CO donors have the therapeutic potential to block IL-18 signaling and reduce IL-18-dependent vascular injury and inflammation. CORM-3 modulates polymorphonuclear leukocyte migration across the vascular endothelium by reducing levels of cell surface-bound elastase (165), and also inhibits TNF-α-induced expression of VCAM-1 and E-selectin in human umbilical vein ECs (239). CORM-2 attenuates ICAM-1 expression induced by high glucose in ECs (187).
FIG. 5.
HO-1 and ECs in vascular inflammation. ↑, increased; ↓, decreased.
Kim et al. (133) have demonstrated that HO-1 expression by exogenous CO administration in ECs. HO-1 activity, in turn, protected the cells from endoplasmic reticulum (ER) stressors. The novelty of this study is that protein kinase R-like ER kinase-dependent activation of Nrf2 by CO is critical for cell survival signal during ER stress. They suggested a positive feedback loop of the signaling pathway in HO-1/CO axis, in which protein kinase R-like ER kinase-Nrf2 signaling played a critical role in endothelium.
Recently, Kawamura et al. (127) have revealed that BR restores endothelial NO synthase (eNOS) expression and improves endothelium-dependent vascular relaxation responses in atherosclerosis. On the other hand, in diabetic mice, HO-1 induction decreases EC sloughing and fragmentation, partly by a mechanism involving increased eNOS and decreased iNOS expression (137). Conversely, it has been evidenced that NO-producing cells possess regulatory pathways where protective mechanisms can operate to control pro-inflammatory responses via its induction of cytoprotective enzymes, such as HO-1, and thus limit the destructive potential (200). Durante et al. (74) have reported that NO, either exogenously administered or endogenously generated from cytokine-treated cells, selectively induced HO-1 expression and CO release in VSMCs. The mechanism responsible for the induction of HO-1 expression by NO included the activation of Nrf2 that was independent of the MAPK pathways but is dependent on oxidative stress (74). Moreover, the induction of HO-1 by NO functioned in an autocrine manner to limit SMC apoptosis (74). To date, the interrelationship between HO-1 and NO seems to be dependent on type of inflammation/injury and tissues and is not completely elucidated in vascular inflammation.
HO-1 has been demonstrated to stimulate cell cycle progression and proliferation in vascular endothelium (66, 143). The mechanism by which HO-1 is able to stimulate the growth of vascular endothelium is not known; however, the ability of HO-1 to stimulate the synthesis of VEGF from vascular cells may contribute to its proliferative action (71). Additionally, HO-1 can directly affect cell viability by blocking programmed cell death. Soares et al. (236) first demonstrated that the overexpression of HO-1 prevents apoptosis of ECs. Recent in vitro data have revealed that HO-1 induction reverses IL-18-mediated EC death, reducing IL-18-dependent vascular injury and inflammation (269). Further, HO-1-derived CO may contribute to the reendothelialization of the vessel wall at sites of vascular injury by stimulating EC growth and by protecting ECs from apoptosis (269). In contrast, a pro-apoptotic effect of CO in ECs has also been reported (261). The reasons for these divergent outcomes are not known but may reflect differences in the dose and duration of CO exposure and/or the vascular source of ECs. CO abrogates apoptosis by activating a discrete signaling pathway, the p38 MAPK pathway, in vascular ECs (31). Hence, the protective effects of HO-1 on vascular inflammation, to some degree, seem to closely implicate with the EC responses through various mechanisms.
V. Control of Vascular Diseases by HO-1/CO
The HO system present in organisms from bacteria to eukaryotes is the main enzyme that can degrade heme, playing a critical role in heme and iron homeostasis. There is the possibility that heme could be degraded through HO-independent pathways. In fact, NADPH-cytochrome p450 reductase has been demonstrated to destroy heme into dipyrrolic propentdyopents and other products (224, 296). The majority of heme is present in hemoglobin, whereas other heme proteins include myoglobin, mitochondrial and microsomal cytochromes, and various catalytic enzymes such as NOSs, catalase, and respiratory burst oxidase (107, 208). These heme proteins play a critical role in many physiological processes, including oxygen transport, mitochondrial respiration, and signal transduction (208). However, free heme is cytotoxic at 1 M concentrations in the presence of ROS (18). In the presence of H2O2, the heme ring is split open releasing free iron witch amplifies ROS production via Fenton chemistry (17–19). The cell either dies or survives by induction of cytoprotective pathways such as HO-1 and ferritin.
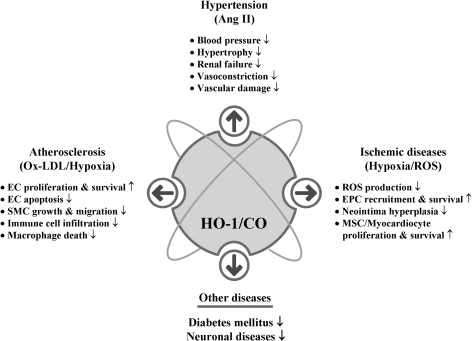
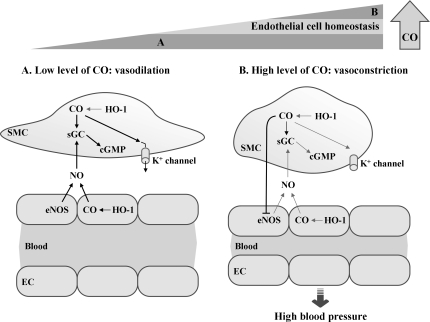
We know that CO, which is generated endogenously during cellular metabolism where heme amount is high (152), is indispensible for the homeostasis of living organisms. Still prolonged exposure to high concentrations of CO seems deleterious especially as seen in chronic disease states such as asthma and diabetes (205, 291), and it is apparent that a physiological dose of CO [reviewed in (233)], exerts beneficial roles such as vasodilatory, antiapoptotic, and antiinflammatory effects. CO now emerges as a key signaling molecule that regulates numerous vascular processes such as blood pressure (BP), vessel tone, smooth muscle proliferation, platelet aggregation, neurotransmission, and stress response, suggesting a crucial role of CO in maintaining physiological homeostasis. Restoration of physiologic CO levels exerts a beneficial effect in many disease settings, such as I/R injury, atherosclerosis, septic shock, hypertension, and metabolic syndrome (73, 170).
A. Ischemic diseases
Evidence suggests that the HO-1/CO system plays an important role against coronary artery I/R injury (Fig. 6). A study demonstrated that the lack of HO-1 makes reendothelialization at the site of injury difficult because of the reduction of EPC recruitment and differentiation compared with wild-type EPCs (67). EPC number also correlates with the extent of ischemia in stroke or myocardial infarction (68, 150). Ischemia results in an increase of growth factors such as SDF-1 and VEGF that lead to increased EPC number and formation of new blood vessels in the injured tissue (64), leading to increased neovascularization for postischemic repair. It has been shown that recruitment of EPCs from bone marrow to periphery is dependent on the establishment of the SDF-1 gradient in a model of heart (14) and hind limb ischemia (64). This gradient is caused both by increased SDF-1 in ischemic tissues (44), and the decrease in SDF-1 in the bone marrow (64, 264), pointing to the mechanisms operating between the peripheral tissues and bone marrow. Although initial human trials have shown only modest short-term benefits in the setting of coronary artery disease (CAD) (215), the therapeutic efficacy of EPC has drawn much attention to improve neovascularization and subsequent recovery of ischemic tissues.
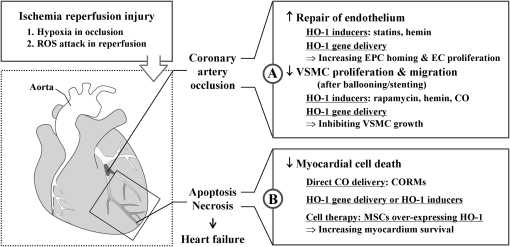
FIG. 6.
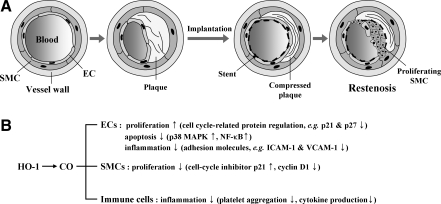
Roles of HO-1/CO in ischemia-reperfusion injury. Ischemia is caused by blockage of blood supply to tissues. Heart, brain, and kidneys are most vulnerable to hypoxia, but secondary damage by ROS after reperfusion leads to more severe damage. (A) Occlusion of coronary artery is usually caused by thrombosis from atherosclerotic plaque rupture. Ballooning and/or stenting can open the blood vessel at the occlusion site, but this treatment can also damage endothelial layer by physical stress. HO-1/CO can stimulate ECs to proliferate in the lesions and enhance EPCs recruitment at the injury site. In contrast, HO-1/CO inhibits SMC proliferation and migration, which is important for the prevention of restenosis. Recovery of damaged myocardium after ischemia/reperfusion injury can be enhanced by treatment with HO-1 inducers, vector containing HO-1 gene, and CORMs. (B) Hypoxia-induced HO-1 expression helps the tissue to resist oxidative stress. Direct delivery of CO into the heart or cardiac injection of vector containing HO-1 induces proliferation and survival of cardiomyocytes and mesenchymal stem cells. The cells overexpressing HO-1 decreases tissue necrosis and repairs injured tissues. ↑, increased; ↓, decreased; CORM, CO-releasing molecule; EPC, endothelial precursor cell; SMC, smooth muscle cell.
Importantly, regulatory mechanism of cell cycle by HO-1 appears different between ECs and SMCs. In SMCs, HO-1 expression decreased cell cycle progression, whereas in ECs, HO-1 expression increased cell cycle progression (2, 143). The mechanisms underlying the HO-1 cell-specific effect on cell cycle progression within the vascular wall are yet to be explored. Nevertheless, such a difference in regulation of cell cycle by HO-1 led to the inhibition of neointimal hyperplasia (135). Since restenosis caused by VSMC proliferation occurs within 1–3 months of stent implantation, favorable and differential effects of HO-1/CO on ECs and VSMCs make them attractive therapeutics to prevent restenosis after percutaneous coronary intervention.
Although there is no clinical evidence about a therapeutic role of CO, this gas might be therapeutically used for protecting cells from reoxygenation injury. The importance of CO in vascular biology leads many investigators to study the mechanisms of the action of CO and to find CORMs (206). CORM-3 has been shown to generate vasodilation of aorta ex vivo and reduce BP in vivo (46, 85, 173). Administration of CORM-3 reduces infarct size in vivo when given at the time of reperfusion (101). Intriguingly, a study showed that combined treatment with CO and BV increased survival up to 80% with a significant decrease of myocardial injury and improved cardiac function, whereas single treatment with either CO or BV did not alter the survival of heart grafts in a rat model (147).
BR may also display cytoprotective properties in the cardiovascular system. BR at physiological levels can provide cardioprotection by suppressing oxidation of lipid membranes and preventing EC death caused by hydrogen peroxide [reviewed in (245)]. Administration of BR or its precursor BV to rodents can suppress I/R injury (219, 256). It has been demonstrated that low serum BR levels correlate with an increased risk for CAD and oxidative metabolites of BR are detected in atherosclerotic lesions (92, 127, 255). However, there is no clinical evidence supporting the hypothesis that the protection provided by BR or BV in the ischemic myocardium could be clinically significant.
HO-1 may also have therapeutic benefits during chronic heart failure as well. Upregulation of HO-1 during heart failure mitigated pathologic left ventricular remodeling and reduced myocardial hypertrophy, oxidative stress, and inflammation (222, 223, 298). Further, HO-1 induction increases adult cardiomyocyte tolerance by reducing apoptosis to ischemia after in vivo transplantation (258), indicating the role of HO-1 in repairing infarcted myocardium. Tang et al. (258) have also shown that HO-1 transduction in bone marrow-derived mesenchymal stem cells (MSCs) improved cell survival, attenuated left ventricular remodeling, and improved functional recovery after myocardial infarction in hearts transplanted with MSCs. In this case, preconditioning with HO-1 acts to retain functional viability in vivo in adult cardiomyocyte cellular grafts after implantation. Thus, autologous atrial cardiomyocytes or MSCs could be useful cell sources and HO-1 can be used to improve the function of cardiomyocytes and MSCs to treat infarction.
Understanding the inherited factors such as candidate gene expression or finding novel genes that influence susceptibility for developing diseases may lead to find better comprehensive therapies (110). A very important case with HO-1 deficiency in human revealed that low level of BR and extremely high level of heme in serum by lack of heme catabolism (287), which caused severe malfunctions and advanced plaques development with damaged ECs, providing a direct evidence for crucial role of HO-1 in pathophysiology of cardiovascular disease. HO-1 expression in monocytes and lymphocytes from patients with CAD is significantly higher than in patients without CAD (50, 144). The HO-1 expression levels showed significant differences in order: the highest in acute myocardial infarction, followed by unstable angina pectoris, and finally by stable angina pectoris.
Nevertheless, the ultimate goal in the treatment of cardiovascular disease is the timely delivery of the best therapeutic agents to protect the heart from the deleterious effects of prolonged ischemia or the effects of repeated challenges of ischemia. Numerous studies using animal models have been developed and proved the role of HO-1/CO in I/R injury. HO-1 null mice during hypoxia show enhanced ventricular dilatation, infarction, and thrombosis (294). In addition, isolated hearts from heterozygote HO-1-knockout mice demonstrate an increased susceptibility to I/R injury compared to hearts from controls (295). A strategy for tissue protection has been developed using an adeno-associated vector system under the control of the erythropoietin hypoxia response elements for ischemia-regulated expression of the therapeutic human ho-1 gene (196). A single administration of this vector several weeks in advance of I/R injury produced a rapid and timely induction of human HO-1 during ischemia, which resulted in a dramatic reduction in tissue damage. In addition, HO-1 overexpression prevented long-term pathologic tissue remodeling and normalized tissue function (196).
HO-1 inducers and ho-1 gene delivery do not seem to induce organ-specific gene expression. As expected, biochemical inducers administered intraperitoneally or subcutaneously induced HO-1 in most organs and intracardiac injection of the ho-1 gene altered other organs, including lungs, kidneys, liver, and brain (94, 220). Thus, a key to future therapeutic application of HO-1 for humans is to properly target and regulate HO-1 expression at a specific site to allow maximal cytoprotection and minimum adverse effects at the site of the lesion.
B. Hypertension
HO and CO participate in the homeostatic control of cardiovascular functions, including the regulation of BP (185). Studies regarding HO-1/CO effects on hypertension can be approached in two ways. One is whether the increase of HO-1 is a consequence of increased BP. The other is whether the administration of exogenous CO or the induction of HO-1 by chemical inducers or gene delivery reduces hypertension.
Angiotensin II (Ang II) is a peptide that is known to increase BP when present continuously in the bloodstream. Ang II, depending on the kind of tissue, can stimulate cell proliferation, hypertrophy, apoptosis, and ROS production, and also cause oxidative DNA damage (Fig. 7). Ang II-induced hypertension is prevented by losartan or hydralazine accompanied by reduction of HO-1 expression, which is diminished by HO inhibitors (115). The interplay between Ang II and HO-1 was observed both in vitro in VSMCs (115) and ECs (2) and in vivo in vessels (115), hearts (83), and kidneys (6). Unexpectedly, weaker HO-1 induction inhibited Ang II-induced hypertrophy (108), whereas stronger HO-1 induction had no effect on hypertrophy (83).
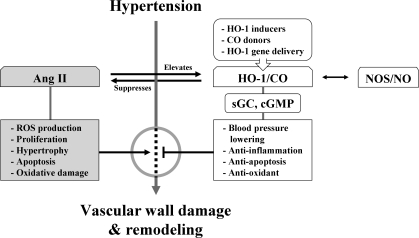
FIG. 7.
Role of HO-1/CO in hypertension. As Ang II in blood stream causes hypertension, chronic exposure of Ang II in spontaneously hypertensive rats induces hypertension. CO, like NO, activates sGC and cGMP pathway and can open potassium channels in VSMCs. This reduces vascular contractility and BP. Hypertension increases soluble VEGFs, soluble endoglins, and tumor necrosis factor-α, accompanied by low level of HO-1 activity. Upregulating HO-1 expression in myofibroblasts and infiltrated inflammatory cells reduces BP. Upregulation of aortic HO-1 protects tissue damage from high BP by reducing inflammatory response. HO-1 inducers or HO-1 gene/CO delivery may efficiently protect tissue damages by vascular inflammation and vascular remodeling. Ang II, angiotensin II; BP, blood pressure; sGC, soluble guanylyl cyclase; VEGF, vascular endothelial growth factor; VSMC, vascular SMC.
Most of our understanding of the metabolism of CO in hypertension is from studies in spontaneously hypertensive rats (SHRs), in which age is an important determinant factor for the manifestation of the hypertension (183). Using a hypertensive rat model with chronic Ang II infusion, Ishizaka et al. (115) reported that pressure overload upregulated aortic HO-1 expression and activity. Through its antioxidant and antiinflammatory properties, increased aortic HO-1 may act favorably against the tissue damage elicited by Ang II and pressure overload. Results of using another hypertensive rat model with chronic norepinephrine infusion demonstrated that HO-1 is upregulated in myofibroblasts and infiltrated inflammatory cells of the heart of Ang II-induced hypertensive rats and inhibits BP elevation (118). Upregulation of HO-1 expression by hemin or stannous chloride has been reported to be successful in decreasing BP (118). The BP-lowering effect was mediated by CO but not by other HO-1 by-products (118). BP and the level of HO-1/CO are correlated with reciprocal influence, implying that an impaired HO/CO system might constitute one of the pathogenic mechanisms of hypertension.
Administration of ZnPP, a HO inhibitor, increased total peripheral resistance and mean BP in Sprague-Dawley rats (117). Other studies reported that daily injection of ZnPP for 4 days resulted in a striking increase in BP in prehypertensive (4-week-old) and young (8-week-old) SHRs, whereas ZnPP did not affect BP in adult Wistar-Kyoto rats (20 weeks) or age-matched SHRs (184, 185). Other nonspecific effects of metalloporphyrins include the inhibition of muscle relaxation and suppression of cyclic adenosine monophosphate and cGMP. These inhibitory effects may be consequential to the interaction of metalloporphyrins with membrane receptors or their downstream signal transduction pathways.
One of the ultimate goals of the use of HO-1 inducers is to increase CO delivery to the injured tissues. When hypertension was induced by NOS inhibitor in Lewis rats, BP was significantly decreased by a CO donor (173). In addition, when adult SHRs were administrated with HO substrates, BP was decreased within an hour and sustained for 1–2 h. Since this time cannot be enough to induce HO-1, BP reduction could be attributable to CO's effect, probably by HO-2 activity.
Delivery of the ho-1 gene by retroviral vectors is capable of long-term expression but also have some limitations, such as poor efficiency in infecting nondividing cells and unknown immunological response (228). Most HO inducers (e.g., heme analogs or heme derivatives) are administered intraperitoneally, whereas the ho-1 gene is usually delivered by intracardiac injection. When the ho-1 gene delivery was accomplished with a single injection of ho-1 retroviral vectors, this single gene delivery has proven to be sufficient to upregulate expression of HO-1 and decrease BP at different ages in the following weeks and months in SHRs (220). Thus, development of proper HO-1/CO delivery strategies may help to augment the antihypertensive effect and possibly to decrease BP to normotensive values.
Hypertension is characterized by increased vascular contractility, concomitant increase in oxidative stress (286), and enhanced vascular inflammation and vascular remodeling (Fig. 7). An upregulated HO-1/CO system would not only normalize the endogenous CO concentration, but also increase the production of BV and BR, two potent antioxidants. Antiinflammatory and antioxidant protection of cardiovascular tissues by CO will also protect the primary and secondary damage inflicted on tissues by hypertension.
C. Atherosclerosis
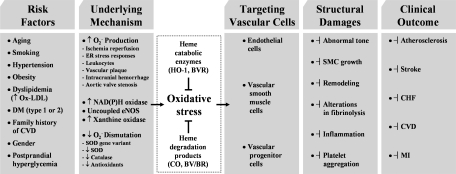
Well-known risk factors for the development of atherosclerosis are smoking, hypertension, hyperlipidemia, and diabetes. With the risk factors, vascular cells in the vessel wall can produce ROS, which can initiate endothelial dysfunction by altering the cellular redox state and subsequently induces vascular inflammation (168). Therefore, understanding the adaptive and/or protective responses of the vasculature to oxidative stress are important in preventing atherogenesis (25). During the atherosclerotic process (Fig. 8), one of the most protective proteins is the enzyme HO-1. HO-1 is upregulated at transcriptional level by Ox-LDL (112), high BP (115), and shear stress in blood vessels (51) and a multitude of systemic inflammatory processes (218). A report demonstrated that HO-1 expression and its by-product, BR, were present only in ECs from advanced atherosclerotic lesions but not in cells from early lesions or normal arteries (171). The finding that the higher level of HO-1 in ECs with a greater disease was observed might indicate that HO-1 expression could be a defense mechanism.
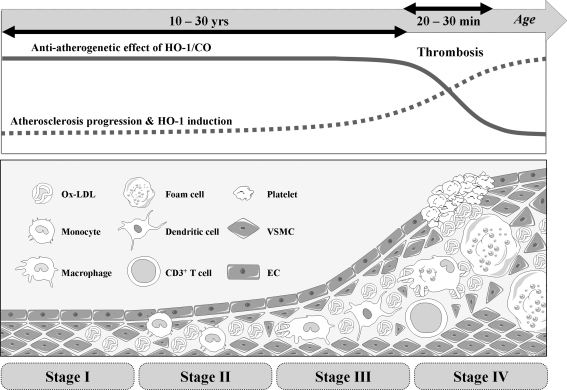
FIG. 8.
Role of HO-1/CO in atherosclerosis. Atherosclerosis initiates from the oxidation of accumulated lipoprotein in subendothelial layer of blood vessel. Oxidized LDL induces production of inflammatory cytokines and metalloproteinases, which can breakdown extracellular matrix network. As disease progresses, inflammatory cells are infiltrated and especially monocytes are differentiated to foam cells. SMCs in media are proliferated and migrated to make intima. As the atherosclerotic lesion progress, ECs and SMCs gradually express HO-1, which is mainly induced by oxidized LDL and hypoxia, whereas cells at early stage barely express HO-1. Delivering HO-1/CO to the atherosclerotic lesion helps stabilizing the lesion by enhancing EPC recruitment, EC proliferation/survival, and inhibition of VSMC growth/migration. However, delivering HO-1/CO in advanced lesions does not show much protective effects. LDL, low-density lipoprotein.
HO-1 is expressed in vascular ECs and macrophages in the early stages of atherosclerotic lesion formation and in foam cells and VSMCs residing in the necrotic core of advanced lesions (113, 114, 274). HO-1 in vascular ECs, VSMCs, and macrophages is markedly upregulated by Ox-LDL, but not by native LDL in ECs and VSMCs (112, 274, 290). In atherosclerotic lesions, HO-1 has been found to be colocalized with oxidized phospholipids, supporting that HO-1 is induced by oxidized phospholipids in vivo. HO-1 contributes to the balance of pro-oxidant and antioxidant factors in the vascular wall through multiple mechanisms.
Most importantly, studies with a 6-year-old boy who had HO-1 deficiency revealed that intravascular hemolysis and EC injury were prominent in association with hyperlipidemia (128, 287). This might be caused by deposition of iron derived from methemoglobin in plasma. In turn, Ox-LDL with iron might increase susceptibility of ECs to oxidative insults, eventually leading to the development of fatty streaks and fibrous plaques in the aorta (128, 287). These studies strongly suggested that HO-1 plays a crucial role in protecting vessels from oxidative insults in human. Additionally, importance of HO-1 in human vascular diseases came from promoter polymorphism analysis in studying the 5′-flanking sequence of the human ho-1 gene (52, 53, 76, 78, 124). A cohort study by Exner et al. (78) revealed that patients with short (25 guanine-thymine dinucleotide [GT]) dinucleotide repeats in the ho-1 promoter region on either allele had often significantly less developed restenosis compare to patients with longer (≥25 GT) dinucleotide repeats. Another study that assessed microsatellite polymorphisms in the ho-1 gene promoter showed that patients with type 2 diabetes carrying longer (≥25 GT) repeats had higher oxidative stress and increased susceptibility to the development of atherosclerosis and CAD (53). They both found that shorter dinucleotide repeats was associated with higher transcriptional activity and thus higher expression levels of HO-1, indicating a protective role of HO-1 in vascular diseases, such as atherosclerosis. Another report, a single nucleotide polymorphism in the ho-1 promoter, suggests that the AA genotype had significantly higher basal promoter activity that was independent of the length of (GT)n repeats (191). These studies claim the potential role of ho-1 gene regulation in atherosclerotic disease processes.
Many investigators have developed the strategy of delivering the ho-1 gene with a vector and generating transgenic mice to study the long-term and specific protective effects of HO-1 against atherosclerosis and vascular injury. Studies have demonstrated that ECs that were transduced by retroviral vector containing HO-1 in rat model of diabetes resisted to ROS attack (3, 213). Delivery of the ho-1 gene in rat carotid artery after balloon injury reduced neointimal hyperplasia by inhibiting VSMC proliferation in media and neointima layer (267). Transduction of adenoviral vector containing HO-1 to pig femoral artery directly reduced vasoconstriction through sGC and cGMP and inhibited vascular cell proliferation through p21 induction (69).
Overexpression of HO-1 was able to decrease atherosclerotic lesion formation by Western diet in apolipoprotein-E (apoE)−/− mice (122). Using apoE−/−HO-1−/− mice, Yet et al. (293) demonstrated that HO-1 played a protective role in atherosclerotic lesion formation. When apoE−/− mice were fed with a Western diet for 8 weeks, atherosclerotic lesions developed and apoE−/−HO-1−/− mice had larger and more advanced lesions than apoE−/− mice despite similarly elevated total plasma cholesterol. The lesions in apoE−/−HO-1−/− mice were complicated with fibrous caps, comparable to plaques seen in apoE−/− mice on a Western diet for longer periods (12 weeks). These results provide strong evidence for a beneficial effect of HO-1 on experimental atherosclerosis. Also, vein grafts from HO-1−/− mice showed a robust neointimal hyperplasia derived from VSMCs as compared with that from wild-type mice and VSMCs from HO-1−/− mice were more susceptible to H2O2-induced cell death than those from wild-type mice (293). Nrf2−/− mice showed a decreased susceptibility to apoE-mediated atherosclerotic plaque formation (249), which is surprising in light of the crucial role of Nrf2 in HO-1 expression. Although Nrf2 stimulates antiatherosclerotic HO-1 expression, this transcription factor can also stimulate pro-atherosclerotic CD36 expression (249), which may explain why Nrf2 disruption in mice attenuates apoE-mediated atherosclerosis.
Together, the above results strongly support that HO-1 plays a protective role in experimental atherosclerotic vascular disease. This is very important because when evaluating the potential for HO-1 as a therapeutic target for atherosclerosis, we should also assess the ability of HO-1 to ameliorate vascular complications, not only in atherosclerosis but also after coronary artery bypass surgery or percutaneous transluminal angioplasty/stenting, namely, neointimal hyperplasia or restenosis (Fig. 9).
FIG. 9.
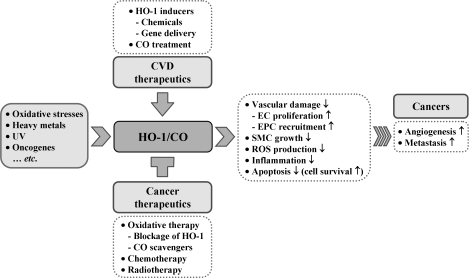
Role of HO-1 in cardiovascular disease and cancer. HO-1 is induced by many different stimuli and has protective effect on vascular cell damage. Most of tumor cells express high levels of HO-1. Increase of angiogenesis, inflammation, and decrease of apoptosis in cancer cells facilitate metastasis. High level of HO-1 expression hampers chemotherapy or radiotherapy in patients with cancer, whereas HO-1 deficient cancer cells can be killed efficiently by the same therapies. This figure summarizes different treatment effects of HO-1 between cardiovascular disease and cancer. ↑, increased; ↓, decreased.
As described, statins, inhibitors of the 3-hydroxy-3-methylglutaryl coenzyme A reductase, exert pleiotropic activities (144): one of which is HO-1 induction and subsequent reduction of ROS formation (98). Lee et al. (140) first demonstrated that simvastatin at micromolar concentration induced HO-1 expression in rat and human VSMCs as well as in VSMCs in the tunica media after intraperitoneal injection in mice, but not in ECs or macrophages (149). Ali et al. (9) have shown that atorvastatin and simvastatin at low micromolar concentration upregulated HO-1 expression in ECs with potential link between HO-1 and Kruppel-like factor 2 by laminar flow. Heeba et al. (105) have identified a novel mechanism related to induction of HO-1 by statins, which is mediated through the NO pathway and could explain some of effects of these drugs. Activation of the HO-1 pathway by statins inhibited inflammatory disorders and protected the endothelium from oxidative damage by improving eNOS functional activity and decreasing lipid peroxide formation. Habeos et al. (102) have provided evidence supporting that some of the beneficial-reported effects of statins may be attributed to the activation of Keap1/Nrf2 signaling and the concomitant increase of HO-1.
Although the effect of statins on HO-1 induction seems to be significant in vitro, the pharmacological relevance remains to be confirmed, because the physiological concentrations of statins used in clinical trials were not likely to induce HO-1. Rapamycin has been reported to block cell cycle progression in VSMCs; therefore, rapamycin- or its derivative-coated coronary stents are most popularly used to treat obstructive coronary artery lesions (166). Rapamycin has been shown to induce HO-1 expression and suppress growth factor-dependent VSMC growth (270), and a question is raised whether HO-1 expression could be involved in the reduction of restenosis rate by rapamycin.
Combination therapy of statins with antihypertensive and immunosuppressive rapamycin may also be considered to induce HO-1. Another drug, probucol, is likely to have the beneficial effect on atherosclerosis and restenosis not only by inhibition of lipid oxidation but also by induction of HO-1 (65, 284). Recent study suggests that lipid-lowering fibrates and insulin-sensitizing thiazolidinediones can be used as potential therapeutics since these drugs induce HO-1 expression via activation of peroxisome proliferator-activated receptor-α (136). Besides the aforementioned drugs, naturally occurring polyphenols are also able to induce HO-1 expression (242, 252), suggesting that a considerable part of the protective effects of fruit consumption against cardiovascular diseases could be mediated through HO-1 pathway.
Paradoxically, HO-1 inducers could be both stimulators and inhibitors of a particular disease process. For example, pro-athrogenic stimuli (e.g., TNF-α, LPS, and hypoxia) or antiatherogenic stimuli (e.g., IL-10) have been reported to induce HO-1 (123, 139, 260). One thing is clear that preemptive HO-1/CO shows protective roles in any type of vascular diseases, and therefore it can be useful as therapeutic means (15). Regardless of their capabilities, most of the HO-1 inducers, such as hemin or tin compound, appear to show cellular and tissue toxicity, such as severe nephrotoxicity. In addition to direct gene delivery, therefore, drugs that are clinically used to treat cardiovascular diseases should be carefully studied.
D. Diabetes mellitus
Diabetes mellitus (DM) is characterized by hyperglycemia, insulin resistance, and a relative impairment in insulin secretion. Insulin resistance, a key factor in the pathogenesis of DM, is frequently accompanied by hypertension, high serum LDL, low serum high-density lipoprotein, and high serum triglyceride levels, which promote the development of atherosclerotic cardiovascular disease (4). Recent studies suggest that upregulation of HO-1 system may be used for the prevention of DM. An enduring antidiabetic effect of the HO-1 inducer, hemin, on Zucker diabetic-fatty rat, a model of insulin-resistant DM, has been evaluated (181). Hemin treatment improved glucose tolerance, reduced insulin intolerance, and lowered insulin resistance. The comparative effects of the HO-1 inducer, hemin, and the HO blocker, chromium mesoporphyrin, on insulin sensitivity/glucose metabolism have been examined, and an antidiabettic effect of hemin has been further confirmed (179). Hemin therapy lowered BP and increased plasma insulin and the insulin-sensitizing protein adiponectin with slight but significant reduction of glycemia, while chromium mesoporphyrin abolished the hemin effects. Hemin also enhanced insulin sensitivity and improved glucose metabolism in insulin-resistant Goto-Kakizaki rats (180) and in adult SHRs (182), suggesting the hypothesis that upregulation of HO-1 may reverse the detrimental effects of diabetes. This hypothesis has been supported by a finding that diminished upregulation of visceral adipose HO-1 correlated with waist-to-hip ratio and insulin resistance in human obesity (232). A putative mechanism underlying the antidiabetic effects of HO-1 may include the antioxidant and antiinflammatory actions of HO-1 (4).
E. Therapeutic potential of HO-1/CO in vascular diseases
Given that cardiovascular disease risk conditions converge in the contribution to oxidative stress and oxidative stress leads to endothelial dysfunction, this background may provide the rationale for seeking pharmacological modulation of oxidative stress pathways in the treatment of cardiovascular diseases. According to Calabrese et al. (40), the ability of a cell to counteract stressful conditions requires the activation of survival pathways as well as the production of molecules endowed with antioxidant activity, which is under control of such protective gene products as HSP32, HSP70, thioredoxin, and sirtuin, all of which are encoded by so-called vitagens (36, 38). HO-1, also referred to as HSP32, is naturally induced by cellular stresses, which leads to the production of molecules with antioxidant, antiapoptotic, and immunomodulatory properties (154). Although the administration of exogenous CO alone may have potent cytoprotective effects in cell culture systems or in animal models, the coordinated response of all products may be necessary for the best outcome; because BR or BV are known to be strong antioxidants (154). Many clinically used drugs have been shown to induce HO-1 in various cell types and in animal disease models. HO-1/CO efficiently functions to recover the damaged tissues in I/R injury, hypertension, and atherosclerosis mainly by improving EPC/EC functions and inhibiting proliferation of SMCs (Fig. 10). As our understanding of the cellular and molecular events of HO-1/CO regarding cardiovascular diseases and other diseases continues to expand, we may be able to find more treatment targets for the preservation of tissues from oxidant injury and ultimately archive better outcome of patients.
FIG. 10.
HO-1/CO roles in vascular diseases. HO-1/CO has been shown to have antiinflammatory, antiapoptotic, and antioxidant effects on various cell types and its functions are further proven in a number of animal disease models. HO-1/CO is intimately involved in the vascular disease. HO-1/CO increases EPC recruitment and proliferation as well as EC proliferation and survival. HO-1/CO reduces BP by inhibiting vasoconstriction. HO-1/CO enhances proliferation and survival of myocardiocytes and mesenchymal stem cells. ↑, increased; ↓, decreased; MSC, mesenchymal stem cell.
VI. Effect of HO-1 on Angiogenesis
The development of the vascular system begins with an assembly from bone marrow-derived precursor cells that shape the first primitive plexus of vessels, a process called vasculogenesis. Subsequently, new vessels are established from preexisting vessels along with their migration and proliferation for the generation of tube-like structures, a process called angiogenesis. As the new vessels are formed, the endothelial layer endothelium forms an important interface between tissue and blood to control the transportation of nutrients and O2 from the blood to all cells. ECs adapt to oxygen tension using various O2-sensing mechanisms, such as HO-2 and eNOS (45). The O2-sensing mechanisms regulate several stages of vessel formation, ranging from EC fate decision to vasculogenesis and angiogenesis.
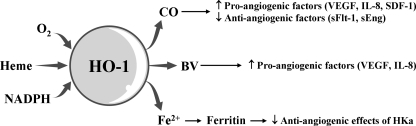
HO-1 induction could be a critical event for angiogenesis. CO synthesized by the catalytic reaction of HO-1 induces the production of angiogenic mediators, such as VEGF, IL-8, and SDF-1 and decreases the antiangiogenic mediators such as soluble VEGF receptor 1 and soluble endoglin, consequently resulting in the promotion of EC proliferation, migration, and antiapoptotic response (60, 70) (Fig. 10). BV stimulates the induction of pro-angiogenic factors, such as VEGF and IL-8 in human keratinocytes cells (148). Ferritin, Fe2+ sequestering protein, binds to HKa (cleaved high molecular weight kininogen) and antagonizes the antiangiogenic effects of HKa (58) (Fig. 11).
FIG. 11.
Potential pro-angiogenic effects of HO-1-mediated reaction products. HO-1 catalyzes the oxidative degradation of heme to CO, BV, and Fe2+. These three products possess the potential pro-angiogenic effects by inducing the pro-angiogenic mediators or by antagonizing the antiangiogenic factor. ↑, increased; ↓, decreased.
Genetic and gene transfer studies have shed light on the distinct role of HO-1 in vascular functions such as angiogenesis and endothelial dysfunction. Genetic overexpression of HO-1 enhances VEGF synthesis and augments formation of vascular capillaries, improving the blood flow in ischemic tissues, and this effect is completely abolished by treating the animals with the HO activity inhibitor, ZnPP (66, 250). Recent study demonstrated that VEGF stimulates HO-1 induction in ECs and that this induction is required for VEGF-dependent angiogenesis because the inhibition of HO-1 activity by SnPP or ZnPP abrogates the formation of blood vessels in a Matrigel assay in vivo (35). Further, Deshane et al. (67) demonstrated that aortic rings isolated from HO-1-deficient mice are unable to form capillary sprouts ex vivo in response to the chemokine SDF-1, which plays a role in the recruitment of EPCs to home to sites of injury (289) and facilitate repair and that this defect is reversed by treatment with CORM (67). This study further confirmed the functional significance of HO-1 in angiogenesis in HO-1−/− mouse models of Matrigel plug and wound healing. Deficiency of HO-1 is closely associated with impairment of neovascularization and wound healing in injured tissue. These findings demonstrate an important role for HO-1 in VEGF- and SDF-1-mediated angiogenesis for vascular remodeling and repair.
Angiogenesis can occur both prenatally as well as postnatally. Mice lacking the functional HO-1 gene demonstrate the low survival percentage (20% of expected HO-1-deficient mice) of embryos, probably by suppressing embryonic angiogenesis (209), whereas the functional loss of VEGF induces the impaired angiogenesis during embryonic development and is lethal in the mouse embryos between days 8.5 and 9.5 (81). In addition, VEGF treatment increases angiogenesis, simultaneously with HO-1 expression during chick embryo development, and VEGF-stimulated angiogenesis in chick embryos is markedly attenuated by the HO inhibitor zinc mesoporphyrin (80). These suggest that cross-talk between VEGF and HO-1 plays an important role in prenatal angiogenesis and that VEGF possesses more potential angiogenic activity than HO-1. On the other hand, inhibition of HO-1 activity by SnPP or genetic knockdown of HO-1 also leads to the suppression of neovascularization in wounded tissues and the retardation of wound closure in an adult animal model (97), indicating that HO-1 is also involved in postnatal neovascularization. HO-1-deficient human patients showed that both intravascular hemolysis and EC dysfunction are prominent, which are similar clinical symptoms to those of HO-1 knockout mice (128, 287). These findings demonstrate that the HO-1 pathway participates to the biological process of both prenatal and postnatal angiogenesis.
In addition to the adoptive function of ECs for hypoxia by inducing angiogenesis to ensure a continuous supply of O2, ECs also consume O2 to generate the signaling molecules like ROS and NO. Low levels of NO are beneficial for cardiovascular functions, such as vasodilation, inhibition of platelet aggregation, and angiogenesis (241). Like NO, HO-1-derived CO may also possess signaling properties for vascular protection, antiinflammation, and vascular remodeling, presumably by inhibiting the release of pro-inflammatory cytokines in LPS-stimulated macrophages through augmentation of the interaction between caveolin-1 and toll-like receptor-4 (278), platelet aggregation through the activation of the enzyme guanylyl cyclase and subsequent generation of cGMP (221), and cytotoxic ROS production through downregulation of NADPH oxidase activity (277). Thus, the inducible effect of HO-1/CO on vascular inflammation and function plays a quite important role in the pathophysiological condition.
The definitive link of HO-1 to pathological angiogenesis has been suggested by recent studies. HO-1/CO can be associated with several vascular disorders such as cancer and vascular restenosis (70). The persistent angiogenesis has been known to be a hallmark of pathologic status, such as inflammation and tumors. Several human tumors express high levels of HO-1, which are closely linked to tumor angiogenesis, supplying necessary nutrients and O2 for tumor growth and providing a route for tumor invasion and metastasis (279). HO-1/CO-enhanced VEGF production may lead to the formation of leaky and immature vessels in pathological tumor angiogenesis. SDF-1, which can be induced by HO-1 expression (145), also promotes tumor cell growth, migration, and invasion by inducing angiogenesis (100), and SDF-1-induced neovascularization was strongly reduced in aortic rings from HO-1−/− mice compared with those of wild-type animals (67). Therefore, HO-1 can be involved in pathological tumor angiogenesis, and regulation of HO-1 expression and activity may be potential therapeutic strategy for cancer treatment.
In a study using HO-2-null mice, Seta and colleagues have demonstrated that a constitutive isoform of HO-2 is also important in inflammation and angiogenesis (231). HO-2-deficient mice are characterized by increased production of pro-angiogenic chemokines (e.g., MIP-2 and MCP-1) and elevated angiogenesis in corneal wound region. The interesting feature of HO-2-null mice is the downregulation of HO-1 expression, which is associated with the exaggerated production of pro-angiogenic chemokines and subsequent induction of inflammatory corneal angiogenesis. Another constitutive isoform, HO-3, is a nonfunctional enzyme and remains incompletely characterized (162).
A. Cross-talk between the HO-1/CO and NOS/NO pathways
CO and NO, produced by the catalytic reactions by HO and NOS, share similar properties: (a) they are retrograde messengers which are highly diffusible; (b) they act as neurotransmitters by elevation of GMP production; (c) they affect adjacent cells without acting through a surface receptor; (d) they are extremely short-lived. Thus, CO and NO can serve as transcellular messengers. Because of the concept that the endogenously derived gas NO could exert a significant biological function in vascular physiology and pathology, CO has gained much attention as a molecule with many similar chemical and biological features.
Expression of iNOS under prooxidant conditions and the coordinate induction of HO-1 in the tissues support the concept that HO-1 could sense NO and thus be protective against ROS- and NO-mediated insults. The sequence of iNOS and HO-1 expression is supported by the following findings: (a) NO and NO-related species induce HO-1 expression in aortic vascular cells; (b) cells pretreated with NO-releasing molecules acquire increased resistance to H2O2-mediated cytotoxicity at the time when HO-1 is maximally activated; (c) in the same study, the presence of NOS inhibitors suppressed both NO production and HO-1 expression (41).
The protective functions of NO are suggested to be mediated through transcriptional activation of the Keap1/Nrf2/ARE pathway, which is responsible for the induction of >100 cytoprotective genes, including the HO-1 gene. Activation of the Keap1/Nrf2/ARE pathway leads to rapid adaptation to a variety of stress conditions and promotes cell survival (172).
Endothelial-derived CO and NO determine the supply of oxygen to tissues through angiogenesis. HO-derived CO contributes to the angiogenesis not only by increasing the synthesis of VEGF, HO-1 and SDF-1, but also by potentiating their synergistic effects on ECs (121, 146) (Fig. 12). The effect of CO on VEGF expression is shown in an animal model by Marti and Risau (161). They demonstrated that animals kept for 6 h in an atmosphere containing 0.1% CO exhibit significant induction of VEGF in various organs. In addition, genetic overexpression of HO-1 leads to the stimulation of VEGF expression in ECs (121). VEGF is also able to stimulate HO-1 expression (35) and eNOS-derived NO production (204) in ECs, consequently inducing angiogenesis (Fig. 12C). Because expression of HO-1 can protect EPCs from oxidative injury and stimulate EPC homing to injured regions for promoting angiogenesis, such a positive feedback can be of beneficial importance in EPC function. In addition, recent in vivo study shows that the cytoprotective effect of VEGF on hyperoxic lung injury is mediated by upregulation of HO-1 expression (235). In concert with CO, NO also induces angiogenesis by upregulating pro-angiogenic mediators, such as HO-1, VEGF, and IL-8 (120, 174). Treatment of ECs with an NO donor enhances the protein levels of HO-1, VEGF, and IL-8 (201), and, conversely, VEGF increases eNOS-dependent NO production in ECs (204), suggesting that NO-HO-1/CO-VEGF axis/circuit is important regulating pathway for angiogenesis.
FIG. 12.
HO-1-induced angiogenesis. (A) HO-1 is induced by NO and CO, hypoxia-induced growth factors (e.g., VEGF), and cytokines (e.g., SDF-1) in ECs. (B) Signaling pathways affected by gaseous molecules such as NO and CO. NO and CO can activate sGC, modulate p38 MAPK activities, stabilize HIF-1α protein, and increase expression of HO-1. CO is also involved in the activation of NF-κB transcription factor. (C) VEGF-induced angiogenesis is initiated through binding of VEGF to its cognate receptor VEGFR-2, which leads to the activation of eNOS and production of NO in ECs. NO upregulates HO-1 with production of CO, resulting in the increase in levels of SDF-1, VEGF, and HO-1 in ECs. SDF-1-induced angiogenesis is initiated through binding of SDF-1 to its receptor CXCR-4, which increases the HO-1 level, consequently inducing SDF-1, VEGF, and HO-1. This positive regulation of VEGF and SDF-1 promotes angiogenesis via HO-1. eNOS, endothelial NO synthase; HIF, hypoxia inducible factor; SDF-1, stromal cell–derived factor 1; VEGFR, VEGF receptor.
HO-1/CO is closely linked to the pro-angiogenic effects of SDF-1 (Fig. 12C). ho-1 gene transfer highly appears to increase neovascularization through the recruitment of circulating progenitor/stem cells and the high expression of angiogenic factors such as VEGF and SDF-1 (146). Treatment with CO (250 ppm) enhances reendothelialization after vascular injury by increasing the circulating SDF-1 in a mouse model (146). Further, SDF-1 promotes angiogenesis in EPCs and aortic ECs isolated from wild-type, but not from HO-1−/− mice, and the impaired capillary sprouts in HO-1-deficient mice are restored by exposure to CO (67), indicating that vascular remodeling by SDF-1 requires HO-1 induction and subsequent CO production. In addition, local gene transfer of SDF-1 enhances ischemia-induced vasculogenesis and angiogenesis in vivo through the elevation of VEGF expression and NO production (106), suggesting that the VEGF/eNOS pathway is critically involved in SDF-1-induced vascular remodeling. These findings indicate that a positive link among HO-1/CO, eNOS, VEGF, and SDF-1 can provide new avenues for ischemia-induced neovascularization in adults, as well as support a vital role of HO-1 and its reaction byproduct, CO, in the vascular repair through enhancing EPC mobilization.
It can be interesting to investigate whether HO-1/CO is involved in eNOS/NO-dependent angiogenic events or vice versa. NO upregulates HO-1 with production of CO (84). The high levels of CO inhibit NOS activity and NO generation, whereas the low concentration of CO induces NO release from either eNOS stimulation or a large intracellular pool of NO (254, 262) (Fig. 13). Therefore, the relationship between the HO-1/CO and eNOS/NO pathways is still complex, dynamic, and adaptable, although the effect of HO-1/CO pathway on vascular system can be cross-talk with the eNOS/NO pathway, which controls expression of angiogenic factors, including VEGF.
FIG. 13.
Crosstalk between CO and NO. Low level of CO stimulates NO release, whereas higher level of CO inhibits NO synthase. Coordinated crosstalk between CO and NO contributes to the EC homeostasis. (A) Low level of CO activates sGC to increase intracellular cGMP levels, and also activates calcium-activated K+ channels. The activation of K+ channels leads to membrane hyperpolarization, causing SMC relaxation. EC-derived NO may facilitate the vasodilatory action of CO by stimulating sGC in SMCs. (B) In cases in which CO level is significantly high in SMCs and ECs, CO can act as a negative regulator of eNOS in ECs, reducing NO production and inducing vasoconstriction.
B. Effect of HO-1/CO pathway on vascular homeostasis
CO or NO production by ECs not only enhances O2 delivery by stimulating angiogenesis but also influences O2 consumption during mitochondrial respiration by acting as a reversible inhibitor of cytochrome c oxidase in the electron transfer chain and as a guardian of cellular energy homeostasis (119). Like evidence of NO action in ECs (134, 280), the gaseous molecule CO produced from HO-1 exerts both paracrine and autocrine effects on various vascular cells, leading to the regulation of vascular tone, endothelial function, the proliferation of smooth muscle cells, and vascular inflammation (27, 206).
To maintain normal vascular system against diverse forms of stimuli, living cells should communicate with adjacent cells. Gaseous retrograde messengers, CO and NO, are highly diffusible, thereby transmitting signals from ECs to smooth muscle cells or vice versa. Both CO and NO may cause relaxation of the smooth muscle cells of vessel walls, allowing the vessels to dilate (Fig. 13). Both gaseous molecules bind to iron in the heme moiety of sGC (247, 251), a stimulator of the cGMP synthesis. Several reports show that vasodilatory properties of HO-1-derived CO are clearly linked to the production of cGMP and the activation of calcium-activated K+ channels (167, 275) (Fig. 13). The activation of K+ channels leads to membrane hyperpolarization, which in turn inhibits voltage-gated Ca2+ channels, causing SMC relaxation (276). HO-derived CO may facilitate the vasodilatory action of NO by stimulating cGMP levels in VSMCs (84, 244) (Fig. 13A). High levels of CO, however, can act as a negative regulator of eNOS activity by interacting with its catalytic heme moiety in ECs, leading to the suppression of NO production and the elevation of vasoconstriction (85, 262) (Fig. 13B). Therefore, vasodilatory property of HO-1-derived CO is not only directly dependent on cGMP production but also coordinately connected with EC-derived NO.
EC injury or apoptosis can occur, leading to the vascular dysfunction, when ECs are exposed to pro-inflammatory stimuli, nutrient deprivation, or DNA-damaging agents. Cytoprotective functions of HO-1 may be attributed to heme turnover and the concerted action of its enzymatic reaction products, such as CO, BV/BR, and iron. Each of these metabolites has their own set of physiological properties that may contribute to protection of ECs against apoptosis. Expression of HO-1 or administration of exogenous CO prevents ECs from TNF-α-initiated EC apoptosis through the activation of the p38 MAPK pathway (31). The antiapoptotic effects of CO (15 ppm) in anoxia/reoxygenation-exposed rat pulmonary artery ECs is dependent on the activation of several signaling pathways, such as phosphatidyl inositol 3-kinase and p38 MAPK pathways, and associated with decreased Fas expression and caspase-3 activity (299). HO-1/CO has been shown to block IL-18-mediated human cardiac EC death by reversing IL-18-mediated p38 MAPK and NF-κB activation (297). Although a majority of in vitro studies show the protective effects of CO on vascular endothelium, exposure of bovine pulmonary artery ECs with CO (100 ppm) induces a pro-apoptotic effect (261), implicating that the cytoprotective effects of CO can be influenced by a dose of CO and cell types. Inhibition of BR level by siRNA targeting for BVR increased the ROS production, leading to cell death in HeLa and primary neuronal cells (20). BR can protect ECs against oxidative or nitrosative stress via ROS scavenging (72). Administration of BV to various injured models, such as transplantation, I/R, and vascular balloon injury, attenuates apoptotic cell death [reviewed in (170, 176)]. Free iron produced by HO-1 induces the synthesis of ferritin (292), which also confers cytoprotective effect (18).
Further, Wang et al. showed that CO attenuates hyperoxia-induced EC apoptosis by inhibiting both extrinsic (Fas-dependent) and intrinsic (mitochondria-dependent) pathways (277). They showed that CO inhibits hyperoxia-induced extrinsic apoptosis signaling initiated by death-inducing signal complex trafficking from the Golgi apparatus to the plasma membrane and downstream activation of apical caspase-8 through the suppression of NADPH oxidase activity for ROS production (175), which is involved in intrinsic apoptosis signaling. CO also inhibits hyperoxia-induced EC death by regulating the Bcl-2-related proteins involved in inhibiting both intrinsic and extrinsic apoptotic signaling. Indeed, CO inhibits the activation of Bid and expression and mitochondrial translocation of Bax, whereas it increases Bcl-XL/Bax interaction and Bad phosphorylation, resulting in the suppression of cytochrome c release and caspase-9/-3 activation. These results indicate that HO-1/CO prevents apoptotic EC death by regulating the activation of both extrinsic and intrinsic apoptosis signal pathways.
VSMCs closely contact with vascular ECs and play a primary role in vascular tone. HO-1 expressed in the vasculature and its catalytic products CO and BV are involved in the control of SMC proliferation (207, 253). Inhibition of SMC proliferation by CO (250 ppm) is mediated by G0/G1 arrest via upregulation of G1-cyclin-dependent protein kinase inhibitor p21 and downregulation of cyclin D1 expression (240) (Fig. 13B). CO can inhibit platelet-derived growth factor–induced SMC proliferation by blocking the mitochondrial ROS-ERK-cyclin D1 pathway and the NADPH oxidase-cyclin D1 pathway (254). HO-1 activators and BR decrease platelet-derived growth factor–induced human SMC proliferation by reducing ROS production and ERK phosphorylation (253). Since exaggerated SMC proliferation is correlated with vascular inflammatory diseases, such as atherosclerosis and restenosis, expression of HO-1 and its metabolic products may play a vital role in vascular therapy.
Increases in HO-1 expression and/or HO-1 metabolic products suppress a variety of inflammatory responses in the vascular endothelium. In quiescent state, the vascular endothelium regulates vascular tone and inflammation to maintain proper vascular homeostasis (57). However, in inflammatory conditions, ECs become highly vasoconstrictive, prothrombotic, and pro-adhesive (57). Upregulated adhesion molecules in ECs, such as selectins (VCAM-1 and ICAM-1) by inflammatory stimuli induce the recruitment of inflammatory cells to the endothelium, ultimately inducing vascular dysfunction such as atherosclerosis and restenosis (28). ECs isolated from HO-1-deficient mice show higher levels of intracellular free iron, ROS and TNF-α-induced VCAM-1, ICAM-1, and E-selectin as compared with ECs isolated from wild-type mice (227). Induction of HO-1 expression inhibits hypoxia/reoxygenation-induced stasis by suppressing NF-κB-dependent expression of ICAM-1 and VCAM-1 in the skin of sickle mice (22). Similarly to the effect of HO-1 expression, exogenous CO attenuates an increase in ICAM-1 expression and polymorphonuclear leukocytes adhesion to LPS-stimulated human ECs. CO released from systemically administered chemical CO donors provides antiinflammatory effects by interfering with NF-κB activation and subsequent downregulation of pro-adhesive vascular EC phenotype in the liver of septic mice (43). This effect is involved in inhibition of the transcription factor NF-κB by blocking NF-κB RelA phosphorylation at serine 276, which is critically involved in expression of adhesion molecules such as ICAM-1 and VCAM-1, leading to the promotion of vascular inflammation and diseases (297).
C. Regulation of VEGF expression by HO-1/CO
VEGF is known as an essential mediator not only for developmental and pathological angiogenesis but also for metabolism. So far, the VEGF family is composed of at least seven members: VEGF-A, VEGF-B, VEGF-C, VEGF-D, VEGF-E, PlGF, and snake venom-derived VEGF. VEGF-A is regarded as a key regulator of angiogenesis since both VEGF-A−/− and VEGF-A+/− mice die in the embryonic stage showing defects in angiogenesis (59, 234). VEGF-A expression is mainly upregulated through the stabilization of HIF by hypoxia, cytokines, growth factors, NO, HO-1/CO, ROS, and others. HIFs are potent regulators of VEGF-A, VEGF receptor-1, and VEGF receptor-2 expression by various stimulants, including hypoxia (212, 229). Particularly, the biological gaseous molecules, CO and NO, have been shown to stabilize HIF-1α to elicit angiogenesis-associated gene expression, such as VEGF.
CO acts as an inhibitor of electron-transport complex IV (33) and results in a significant and transient burst of ROS arising from the mitochondria (55, 300), which can be involved in HIF-1 stability (45). Exposure to CO promoted the activation of HIF-1α by ROS production from mitochondria in macrophages, consequently protecting against lung I/R injury in an animal model (55). However, precise mechanisms by which CO promotes HIF-1α stability and VEGF expression in ECs remain incompletely clarified. The contribution of CO to the regulation of HIF-1α is little complicated since CO is reported to stabilize HIF-1α in normoxia whereas they promote HIF-1α degradation under hypoxia (109). These findings indicate that the effect of CO on HIF-1α activity is dependent on the oxygen availability.
A body of evidence shows that VEGF-induced angiogenesis is initiated through the induction of HO-1 [reviewed in (72)], which in turn promotes the angiogenic processes, including vascular permeability, vasodilation, EC survival, proliferation, migration, and angiogenic sprouting (47). VEGF transgenic mice induce a more than 10-fold accumulation of HO-1 mRNA in lung tissues and protect mice from lethal hypoxia (232). This protective effect is suppressed by the inhibition of HO-1 expression using siRNA and its activity using chemical inhibitors, indicating that HO-1 is an important contributor to biological functions of VEGF. Further evidence that the HO-1/CO pathway plays an important role in angiogenesis has been also confirmed by in vivo Matrigel plus assay (35). Administration with VEGF significantly increases angiogenesis in a mouse model, and this angiogenic effect was abrogated by the inhibition of HO-1 activity. The angiogenic effect of fibroblast growth factor was not affected by HO-1 inhibitors. These results suggest that VEGF and fibroblast growth factor induce angiogenesis through distinct pathways for HO-1 induction (93). The signal pathway of HO-1 induction in ECs by VEGF is likely to be associated with an increase in NO production by increasing Akt-dependent eNOS phosphorylation and association of eNOS with HSP90 (32, 91), leading to angiogenesis (Fig. 13C).
HO-2 deletion in mice affects the phenotype of ECs (23). The alteration in the VEGF pathway, including changes in expression of VEGF receptors (VEGFRs) and VEGFs, was observed in ECs from HO-2-deficient mice. Compared with wild-type cells, HO-2−/− ECs displayed an increase in the mRNA levels of pro-inflammatory VEGFR-1, whereas the mRNA levels of pathologic VEGFR-2 and −3 were markedly decreased. It was found that expression of VEGF-A, the primary ligand for VEGFR-1, was largely unchanged, whereas expression of VEGF-C and -D, ligands for VEGFR-2 and −3, was significantly reduced. This finding suggests that HO-2 activity may regulate the VEGFR-2/VEGF-B and VEGFR-3/VEGF-C pathways in ECs.
The above-menthioned observations suggest that regulation of HO-1 induction and activity by gene delivery or chemical inhibitors controls angiogenesis and may be useful for therapeutic strategies for angiogenesis-associated diseases, including I/R injury and tumor growth and metastasis.
D. Restenosis and vasculopathy
Restenosis, neointimal hyperplasia, and vasculopathy are vascular disorders after percutaneous angioplasty, coronary artery bypass grafting, lower extremity vein bypass grafting, and transplantation (225). These vascular proliferative disorders remain common problems resulting in significant long-term morbidity and mortality. Their pathogenesis is multifactorial; the most common initiating event appears to be endothelial disruption and subsequent overproliferation of the underlying smooth muscle cells (Fig. 14A).
FIG. 14.
Pathological sequences of restenosis. (A) In some cases, surgery to widen or unblock a blood vessel can cause the induction of VSMC proliferation, consequently resulting in the even more narrowed vessels than before the surgery, which is called restenosis. (B) Protective effects of HO-1 and its product CO. CO protects ECs by stimulating proliferation, inhibiting apoptosis, and reducing inflammatory responses. CO inhibits SMC proliferation, and decreases the production of inflammatory cytokines from immune cells. ↑, increased; ↓, decreased.
Many studies have demonstrated that HO-1/CO is involved in regulating the pathogenic process of vascular proliferative disorders (Fig. 14B). HO-1 gene transfer into the vessel wall or CO delivery resulted in a significant reduction in intimal hyperplasia in a rat model of allogenic aorta transplantation compared to controls by decreasing activated leukocytes, adhesion molecule expression, and VSMC accumulation in the intima (47). However, HO-1 gene transfer shows a strong inhibitory effect on leukocyte infiltration and expression of adhesion molecules and cytokines compared with CO delivery, whereas CO reveals more inhibitory effect on VSMC accumulation in the intima than HO-1 gene delivery. This study indicates that the beneficial effects of HO-1 gene expression on the reducing graft arteriosclerosis are associated with CO production, but possibly also with other end products (iron and BR), from heme degradation by HO-1. However, this distinct mechanism should be further investigated.
The differential induction of HO-1, which is certainly correlated with differential production of CO from heme by the catalytic reaction of HO-1, may account for the inhibition of VSMC proliferation, constrictive neointimal hyperplasia, and recurrent lumen narrowing. Indeed, direct exposure to CO suppresses restenosis after carotid balloon injury in rats and mice, and this protective effect is closely associated with a strong inhibition of vascular inflammation and VSMC proliferation (132, 195). This effect was elicited by the generation of cGMP, the activation of p38 MAPK, and expression of the cell cycle inhibitor p21 (195). Probucol, a drug used to prevent restenosis, reveals its beneficial effect by inhibiting the proliferation of VSMCs via induction of HO-1 (65). These findings strongly suggest that end products, mainly CO, from heme degradation by HO serve as key modulators for vascular inflammation, EC function, and VSMC proliferation, which are pathogenic factors for human vascular proliferative diseases such as restenosis, neointimal hyperplasia, and vasculopathy.
VII. Conclusion
Optimal vascular health depends on a delicate balance in the vascular wall of pro-oxidative and antioxidant cellular mechanisms. Presence of more than one major cardiovascular risk factor creates an environment in which the principal oxidative systems such as NADH/NADPH oxidase, xanthine oxidase, and the uncoupling of eNOS are activated in the vascular wall in addition to increased production and decreased dismutation of superoxide. As a result, functional and structural alterations develop in the vascular cells. Moreover, inflammation can potentiate the deleterious effects of oxidative stress on the vasculature. These contribute to the development of clinical sequences such as atherosclerosis, myocardial infarction, stroke, congestive heart failure, and cognitive dysfunction related to vascular damage (Fig. 15). However, the HO system could attenuate/block the progression of vascular diseases via their antioxidant, antiinflammatory, and antiproliferative effects.
FIG. 15.
Critical roles of oxidative stress and inflammation in the progression of vascular diseases, and their attenuation by the HO system. CHF, congestive heart failure; CVD, cardiovascular disease; DM, diabetes mellitus; MI, myocardial infarcton; ↑, increased; ↓, decreased; ⊣, attenuated.
Laminar shear stress (LSS) may protect vascular endothelium against atherogenesis. LSS increases NO biosynthesis, prolongs EC survival, and generates an anticoagulant, antiadhesive cell surface [reviewed in (212)]. In contrast, disturbed blood flow, with low shear reversing or oscillatory flow patterns, may promote atherogenesis; ECs exposed to disturbed blood flow exhibit reduced eNOS levels, increased apoptosis, oxidative stress, permeability to LDL, and leukocyte adhesion (203). Recently, Ali et al. (10) have presented evidence that LSS increases endothelial responsiveness to statins and that HO-1 induction represents an important component of the vasculoprotective profile of these drugs. They found that treatment of mice with atorvastatin induced HO-1 expression in the aortic endothelium and that this occurred preferentially at sites exposed to LSS. The mechanisms underlying a synergistic relationship between LSS and statins include maximal Akt phosphorylation, dependence upon eNOS, Kruppel-like factor 2, and Nrf2 activation. This study may have important implications for the efficacy of statins in patients with CAD, and also emphasize the need for novel therapies, such as those targeting HO-1/CO system, to optimize vasculoprotection.
The strategies to target HO system as described in this review may offer promising therapeutic approaches to clinicians for the effective management of a number of vascular disease states that have, in the past, proved difficult to treat. Also, this review has laid a foundation to prepare the researcher for translation of these current discoveries into new therapeutic modalities to combat vascular diseases.
Abbreviations Used
- Ang II
angiotensin II
- AP-1
activator protein-1
- apoE
apolipoprotein-E
- ARE
antioxidant response element
- Bach1
bric-à-brac, tramtrack and broad complex and cap ’n’ collar homology 1
- BP
blood pressure
- BR
bilirubin
- BV
biliverdin
- BVR
biliverdin reductase
- CAD
coronary artery disease
- cGMP
cyclic guanine monophosphate
- CO
carbon monoxide
- CORM
CO-releasing molecule
- COX
cyclooxygenase
- CREB
cyclic adenosine monophosphate-responsive element-binding protein
- DM
diabetes mellitus
- EC
endothelial cell
- eNOS
endothelial nitric oxide synthase
- EPC
endothelial precursor cell
- ER
endoplasmic reticulum
- ERK
extracellular regulated kinases
- HIF
hypoxia inducible factor
- HKa
high molecular weight kininogen
- Hmox
heme oxygenase gene
- HO
heme oxygenase
- HSP
heat shock protein
- I/R
ischemia/reperfusion
- ICAM-1
intercellular adhesion molecule 1
- IL
interleukin
- iNOS
inducible nitric oxide synthase
- JNK
c-Jun N-terminal kinases
- Keap1
Kelch-like ECH-associated protein 1
- LDL
low-density lipoprotein
- LPS
lipopolysaccharide
- LSS
laminar shear stress
- Maf
Musculo-aponeurotic fibrosarcoma
- MAPK
mitogen-activated protein kinase
- MARE
Maf recognition elements
- MCP-1
monocyte chemotatic protein 1
- MIP
macrophage inflammatory protein
- MSC
mesenchymal stem cell
- NADPH
nicotinamide adenine dinucleotide phosphate
- NF-κB
nuclear factor-κB
- NO
nitric oxide
- Nrf2
nuclear factor E2-related factor 2
- Ox-LDL
oxidized low-density lipoprotein
- PI3K
phosphatidyl inositol 3-kinase
- PKA
protein kinase A
- PKC
protein kinase C
- ROS
reactive oxygen species
- SDF-1
stromal cell–derived factor-1
- sGC
soluble guanylyl cyclase
- SHR
spontaneously hypertensive rat
- siRNA
small interfering RNA
- SnPP
tin protoporphyrin
- StRE
stress response element
- TNF
tumor necrosis factor
- VCAM-1
vascular cell adhesion molecule 1
- VEGF
vascular endothelial growth factor
- VEGFR
VEGF receptor
- VSMC
vascular smooth muscle cell
- ZnPP
zinc protoporphyrin
Footnotes
Reviewing Editors: John Belcher, Francois Boucher, Vittorio Calabrese, Damian Calay, De-Xing Hou, Giovanni Li Volti, Mahin Maines, Cesare Mancuso, Giovanni Mann, and Helena Parfenova
Acknowledgment
This work has been supported by a National Research Foundation Grant funded by the Korean Government (MOEHRD) (BRL-2010-0087350).
References
- 1.Abraham NG. Kappas A. Pharmacological and clinical aspects of heme oxygenase. Pharmacol Rev. 2008;60:79–127. doi: 10.1124/pr.107.07104. [DOI] [PubMed] [Google Scholar]
- 2.Abraham NG. Kushida T. McClung J. Weiss M. Quan S. Lafaro R. Darzynkiewicz Z. Wolin M. Heme oxygenase-1 attenuates glucose-mediated cell growth arrest and apoptosis in human microvessel endothelial cells. Circ Res. 2003;93:507–514. doi: 10.1161/01.RES.0000091828.36599.34. [DOI] [PubMed] [Google Scholar]
- 3.Abraham NG. Rezzani R. Rodella L. Kruger A. Taller D. Li Volti G. Goodman AI. Kappas A. Overexpression of human heme oxygenase-1 attenuates endothelial cell sloughing in experimental diabetes. Am J Physiol Heart Circ Physiol. 2004;287:H2468–H2477. doi: 10.1152/ajpheart.01187.2003. [DOI] [PubMed] [Google Scholar]
- 4.Abraham NG. Tsenovoy PL. McClung J. Drummond GS. Heme oxygenase: a target gene for anti-diabetic and obesity. Curr Pharm Des. 2008;14:412–421. doi: 10.2174/138161208783597371. [DOI] [PubMed] [Google Scholar]
- 5.Abuarqoub H. Foresti R. Green CJ. Motterlini R. Heme oxygenase-1 mediates the anti-inflammatory actions of 2'-hydroxychalcone in RAW 264.7 murine macrophages. Am J Physiol Cell Physiol. 2006;290:C1092–C1099. doi: 10.1152/ajpcell.00380.2005. [DOI] [PubMed] [Google Scholar]
- 6.Aizawa T. Ishizaka N. Taguchi J. Nagai R. Mori I. Tang SS. Ingelfinger JR. Ohno M. Heme oxygenase-1 is upregulated in the kidney of angiotensin II-induced hypertensive rats: possible role in renoprotection. Hypertension. 2000;35:800–806. doi: 10.1161/01.hyp.35.3.800. [DOI] [PubMed] [Google Scholar]
- 7.Alam J. Cook JL. How many transcription factors does it take to turn on the heme oxygenase-1 gene? Am J Respir Cell Mol Biol. 2007;36:166–174. doi: 10.1165/rcmb.2006-0340TR. [DOI] [PubMed] [Google Scholar]
- 8.Alam J. Den Z. Distal AP-1 binding sites mediate basal level enhancement and TPA induction of the mouse heme oxygenase-1 gene. J Biol Chem. 1992;267:21894–21900. [PubMed] [Google Scholar]
- 9.Ali F. Hamdulay SS. Kinderlerer AR. Boyle JJ. Lidington EA. Yamaguchi T. Soares MP. Haskard DO. Randi AM. Mason JC. Statin-mediated cytoprotection of human vascular endothelial cells: a role for Kruppel-like factor 2-dependent induction of heme oxygenase-1. J Thromb Haemost. 2007;5:2537–2546. doi: 10.1111/j.1538-7836.2007.02787.x. [DOI] [PubMed] [Google Scholar]
- 10.Ali F. Zakkar M. Karu K. Lidington EA. Hamdulay SS. Boyle JJ. Zloh M. Bauer A. Haskard DO. Evans PC. Mason JC. Induction of the cytoprotective enzyme heme oxygenase-1 by statins is enhanced in vascular endothelium exposed to laminar shear stress and impaired by disturbed flow. J Biol Chem. 2009;284:18882–18892. doi: 10.1074/jbc.M109.009886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Amersi F. Buelow R. Kato H. Ke B. Coito AJ. Shen XD. Zhao D. Zaky J. Melinek J. Lassman CR. Kolls JK. Alam J. Ritter T. Volk HD. Farmer DG. Ghobrial RM. Busuttil RW. Kupiec-Weglinski JW. Upregulation of heme oxygenase-1 protects genetically fat Zucker rat livers from ischemia/reperfusion injury. J Clin Investig. 1999;104:1631–1639. doi: 10.1172/JCI7903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Anwar AA. Li FY. Leake DS. Ishii T. Mann GE. Siow RC. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: role of mitogen-activated protein kinases and Nrf2. Free Radic Biol Med. 2005;39:227–236. doi: 10.1016/j.freeradbiomed.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 13.Ashino T. Yamanaka R. Yamamoto M. Shimokawa H. Sekikawa K. Iwakura Y. Shioda S. Numazawa S. Yoshida T. Negative feedback regulation of lipopolysaccharide-induced inducible nitric oxide synthase gene expression by heme oxygenase-1 induction in macrophages. Mol Immunol. 2008;45:2106–2115. doi: 10.1016/j.molimm.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Askari AT. Unzek S. Popovic ZB. Goldman CK. Forudi F. Kiedrowski M. Rovner A. Ellis SG. Thomas JD. DiCorleto PE. Topol EJ. Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet. 2003;362:697–703. doi: 10.1016/S0140-6736(03)14232-8. [DOI] [PubMed] [Google Scholar]
- 15.Bach FH. Heme oxygenase-1: a therapeutic amplification funnel. FASEB J. 2005;19:1216–1219. doi: 10.1096/fj.04-3485cmt. [DOI] [PubMed] [Google Scholar]
- 16.Balla G. Jacob HS. Balla J. Rosenberg M. Nath K. Apple F. Eaton JW. Vercellotti GM. Ferritin: a cytoprotective antioxidant strategem of endothelium. J Biol Chem. 1992;267:18148–18153. [PubMed] [Google Scholar]
- 17.Balla G. Vercellotti G. Muller-Eberhard U. Eaton JW. Jacob HS. Exposure of endothelial cells to free heme potentiates damage mediated by granulocytes and toxic oxygen species. Lab Invest. 1991;64:648–655. [PubMed] [Google Scholar]
- 18.Balla J. Jacob HS. Balla G. Nath K. Eaton JW. Vercellotti GM. Endothelial-cell heme uptake from heme proteins: induction of sensitization and desensitization to oxidant damage. Proc Natl Acad Sci USA. 1993;90:9285–9289. doi: 10.1073/pnas.90.20.9285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Balla J. Vercellotti GM. Jeney V. Yachie A. Varga Z. Jacob HS. Eaton JW. Balla G. Heme, heme oxygenase, and ferritin: how the vascular endothelium survives (and dies) in an iron-rich environment. Antioxid Redox Signal. 2007;9:1–19. doi: 10.1089/ars.2007.1787. [DOI] [PubMed] [Google Scholar]
- 20.Baranano DE. Rao M. Ferris CD. Snyder SH. Biliverdin reductase: a major physiologic cytoprotectant. Proc Natl Acad Sci USA. 2002;99:16093–16098. doi: 10.1073/pnas.252626999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Belcher JD. Beckman JD. Balla G. Balla J. Vercellotti G. Heme degradation and vascular injury. Antioxid Redox Signal. 2010;12:233–248. doi: 10.1089/ars.2009.2822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Belcher JD. Mahaseth H. Welch TE. Otterbein LE. Hebbel RP. Vercellotti GM. Heme oxygenase-1 is a modulator of inflammation and vaso-occlusion in transgenic sickle mice. J Clin Invest. 2006;116:808–816. doi: 10.1172/JCI26857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bellner L. Martinelli L. Halilovic A. Patil K. Puri N. Dunn MW. Regan RF. Schwartzman ML. Heme oxygenase-2 deletion causes endothelial cell activation marked by oxidative stress, inflammation, and angiogenesis. J Pharmacol Exp Ther. 2009;331:925–932. doi: 10.1124/jpet.109.158352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Berendji-Grun D. Kolb-Bachofen V. Kroncke KD. Nitric oxide inhibits endothelial IL-1beta-induced ICAM-1 gene expression at the transcriptional level decreasing Sp1 and AP-1 activity. Mol Med. 2001;7:748–754. [PMC free article] [PubMed] [Google Scholar]
- 25.Berliner JA. Heinecke JW. The role of oxidized lipoproteins in atherogenesis. Free Radic Biol Med. 1996;20:707–727. doi: 10.1016/0891-5849(95)02173-6. [DOI] [PubMed] [Google Scholar]
- 26.Beutler B. Greenwald D. Hulmes JD. Chang M. Pan YC. Mathison J. Ulevitch R. Cerami A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature. 1985;316:552–554. doi: 10.1038/316552a0. [DOI] [PubMed] [Google Scholar]
- 27.Bilban M. Haschemi A. Wegiel B. Chin BY. Wagner O. Otterbein LE. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med. 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- 28.Blankenberg S. Barbaux S. Tiret L. Adhesion molecules and atherosclerosis. Atherosclerosis. 2003;170:191–203. doi: 10.1016/s0021-9150(03)00097-2. [DOI] [PubMed] [Google Scholar]
- 29.Boehning D. Moon C. Sharma S. Hurt KJ. Hester LD. Ronnett GV. Shugar D. Snyder SH. Carbon monoxide neurotransmission activated by CK2 phosphorylation of heme oxygenase-2. Neuron. 2003;40:129–137. doi: 10.1016/s0896-6273(03)00596-8. [DOI] [PubMed] [Google Scholar]
- 30.Boyle EM., Jr. Kovacich JC. Hebert CA. Canty TG., Jr. Chi E. Morgan EN. Pohlman TH. Verrier ED. Inhibition of interleukin-8 blocks myocardial ischemia-reperfusion injury. J Thorac Cardiovasc Surg. 1998;116:114–121. doi: 10.1016/S0022-5223(98)70249-1. [DOI] [PubMed] [Google Scholar]
- 31.Brouard S. Otterbein LE. Anrather J. Tobiasch E. Bach FH. Choi AM. Soares MP. Carbon monoxide generated by heme oxygenase 1 suppresses endothelial cell apoptosis. J Exp Med. 2000;192:1015–1026. doi: 10.1084/jem.192.7.1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brouet A. Sonveaux P. Dessy C. Balligand JL. Feron O. Hsp90 ensures the transition from the early Ca2+-dependent to the late phosphorylation-dependent activation of the endothelial nitric-oxide synthase in vascular endothelial growth factor-exposed endothelial cells. J Biol Chem. 2001;276:32663–32669. doi: 10.1074/jbc.M101371200. [DOI] [PubMed] [Google Scholar]
- 33.Brown SD. Piantadosi CA. In vivo binding of carbon monoxide to cytochrome c oxidase in rat brain. J Appl Physiol. 1990;68:604–610. doi: 10.1152/jappl.1990.68.2.604. [DOI] [PubMed] [Google Scholar]
- 34.Bussolati B. Mason JC. Dual role of VEGF-induced heme-oxygenase-1 in angiogenesis. Antiox Redox Signal. 2006;8:1153–1163. doi: 10.1089/ars.2006.8.1153. [DOI] [PubMed] [Google Scholar]
- 35.Bussolati B. Ahmed A. Pemberton H. Landis RC. Di Carlo F. Haskard DO. Mason JC. Bifunctional role for VEGF-induced heme oxygenase-1 in vivo: induction of angiogenesis and inhibition of leukocytic infiltration. Blood. 2004;103:761–766. doi: 10.1182/blood-2003-06-1974. [DOI] [PubMed] [Google Scholar]
- 36.Calabrese V. Cornelius C. Dinkova-Kostova AT. Calabrese EJ. Vitagenes, cellular stress response, and acetylcarnitine: relevance to hormesis. Biofactors. 2009;35:146–160. doi: 10.1002/biof.22. [DOI] [PubMed] [Google Scholar]
- 37.Calabrese V. Cornelius C. Mancuso C. Pennisi G. Calafato S. Bellia F. Bates TE. Giuffrida Stella AM. Schapira T. Dinkova Kostova AT. Rizzarelli E. Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem Res. 2008;33:2444–2471. doi: 10.1007/s11064-008-9775-9. [DOI] [PubMed] [Google Scholar]
- 38.Calabrese V. Cornelius C. Rizzarelli E. Owen JB. Dinkova-Kostova AT. Butterfield DA. Nitric oxide in cell survival: a janus molecule. Antioxid Redox Signal. 2009;11:2717–2739. doi: 10.1089/ars.2009.2721. [DOI] [PubMed] [Google Scholar]
- 39.Calabrese V. Guagliano E. Sapienza M. Panebianco M. Calafato S. Puleo E. Pennisi G. Mancuso C. Butterfield DA. Stella AG. Redox regulation of cellular stress response in aging and neurodegenerative disorders: role of vitagenes. Neurochem Res. 2007;32:757–773. doi: 10.1007/s11064-006-9203-y. [DOI] [PubMed] [Google Scholar]
- 40.Calabrese V. Mancuso CM. Rizzarelli E. Butterfield DA. Stella AM. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat Rev Neurosci. 2007;8:766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 41.Calabrese V. Ravagna A. Colombrita C. Guagliano E. Scapagnini G. Calvani M. Butterfield DA. Giuffrida Stella AM. Acetylcarnitine induces heme oxigenase in rat astrocytes and protects against oxidative stress: involvement of the transcription factor Nrf2. J Neurosci Res. 2005;79:509–521. doi: 10.1002/jnr.20386. [DOI] [PubMed] [Google Scholar]
- 42.Camhi SL. Alam J. Otterbein L. Sylvester SL. Choi AM. Induction of heme oxygenase-1 gene expression by lipopolysaccharide is mediated by AP-1 activation. Am J Respir Cell Mol Biol. 1995;13:387–398. doi: 10.1165/ajrcmb.13.4.7546768. [DOI] [PubMed] [Google Scholar]
- 43.Cepinskas G. Katada K. Bihari A. Potter RF. Carbon monoxide liberated from carbon monoxide-releasing molecule CORM-2 attenuates inflammation in the liver of septic mice. Am J Physiol Gastrointest Liver Physiol. 2008;294:G184–G191. doi: 10.1152/ajpgi.00348.2007. [DOI] [PubMed] [Google Scholar]
- 44.Ceradini DJ. Kulkarni AR. Callaghan MJ. Tepper OM. Bastidas N. Kleinman ME. Capla JM. Galiano RD. Levine JP. Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med. 2004;10:858–864. doi: 10.1038/nm1075. [DOI] [PubMed] [Google Scholar]
- 45.Chandel NS. Maltepe E. Goldwasser E. Mathieu CE. Simon MC. Schumacker PT. Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA. 1998;95:11715–11720. doi: 10.1073/pnas.95.20.11715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chatterjee PK. Water-soluble carbon monoxide-releasing molecules: helping to elucidate the vascular activity of the “silent killer.”. Br J Pharmacol. 2004;142:391–393. doi: 10.1038/sj.bjp.0705826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chauveau C. Bouchet D. Roussel JC. Mathieu P. Braudeau C. Renaudin K. Tesson L. Soulillou JP. Iyer S. Buelow R. Anegon I. Gene transfer of heme oxygenase-1 and carbon monoxide delivery inhibit chronic rejection. Am J Transplant. 2002;2:581–592. doi: 10.1034/j.1600-6143.2002.20702.x. [DOI] [PubMed] [Google Scholar]
- 48.Chen JC. Huang KC. Lin WW. HMG-CoA reductase inhibitors upregulate heme oxygenase-1 expression in murine RAW264.7 macrophages via ERK, p38 MAPK and protein kinase G pathways. Cell Signal. 2006;18:32–39. doi: 10.1016/j.cellsig.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 49.Chen S. Kapturczak MH. Wasserfall C. Glushakova OY. Campbell-Thompson M. Deshane JS. Joseph R. Cruz PE. Hauswirth WW. Madsen KM. Croker BP. Berns KI. Atkinson MA. Flotte TR. Tisher CC. Agarwal A. Interleukin 10 attenuates neointimal proliferation and inflammation in aortic allografts by a heme oxygenase-dependent pathway. Proc Natl Acad Sci USA. 2005;102:7251–7256. doi: 10.1073/pnas.0502407102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen SM. Li YG. Wang DM. Study on changes of heme oxygenase-1 expression in patients with coronary heart disease. Clin Cardiol. 2005;28:197–201. doi: 10.1002/clc.4960280410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen XL. Varner SE. Rao AS. Grey JY. Thomas S. Cook CK. Wasserman MA. Medford RM. Jaiswal AK. Kunsch C. Laminar flow induction of antioxidant response element-mediated genes in endothelial cells. A novel anti-inflammatory mechanism. J Biol Chem. 2003;278:703–711. doi: 10.1074/jbc.M203161200. [DOI] [PubMed] [Google Scholar]
- 52.Chen YH. Chau LY. Lin MW. Chen LC. Yo MH. Chen JW. Lin SJ. Heme oxygenase-1 gene promotor microsatellite polymorphism is associated with angiographic restenosis after coronary stenting. Eur Heart J. 2004;25:39–47. doi: 10.1016/j.ehj.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 53.Chen YH. Lin SJ. Lin MW. Tsai HL. Kuo SS. Chen JW. Charng MJ. Wu TC. Chen LC. Ding YA. Pan WH. Jou YS. Chau LY. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Hum Genet. 2002;111:1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- 54.Chen ZH. Saito Y. Yoshida Y. Sekine A. Noguchi N. Niki E. 4-Hydroxynonenal induces adaptive response and enhances PC12 cell tolerance primarily through induction of thioredoxin reductase 1 via activation of Nrf2. J Biol Chem. 2005;280:41921–41927. doi: 10.1074/jbc.M508556200. [DOI] [PubMed] [Google Scholar]
- 55.Chin BY. Jiang G. Wegiel B. Wang HJ. Macdonald T. Zhang XC. Gallo D. Cszimadia E. Bach FH. Lee PJ. Otterbein LE. Hypoxia-inducible factor 1α stabilization by carbon monoxide results in cytoprotective preconditioning. Proc Natl Acad Sci USA. 2007;104:5109–5114. doi: 10.1073/pnas.0609611104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Choi BM. Pae HO. Kim YM. Chung HT. Nitric oxide-mediated cytoprotection of hepatocytes from glucose deprivation-induced cytotoxicity: involvement of heme oxygenase-1. Hepatology. 2003;37:810–823. doi: 10.1053/jhep.2003.50114. [DOI] [PubMed] [Google Scholar]
- 57.Cines DB. Pollak ES. Buck CA. Loscalzo J. Zimmerman GA. McEver RP. Pober JS. Wick TM. Konkle BA. Schwartz BS. Barnathan ES. McCrae KR. Hug BA. Schmidt AM. Stern DM. Endothelial cells in physiology and in the pathophysiology of vascular disorders. Blood. 1998;91:3527–3561. [PubMed] [Google Scholar]
- 58.Coffman LG. Parsonage D. D'Agostino R., Jr. Torti FM. Torti SV. Regulatory effects of ferritin on angiogenesis. Proc Natl Acad Sci USA. 2009;106:570–575. doi: 10.1073/pnas.0812010106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cross MJ. Claesson-Welsh L. FGF and VEGF function in angiogenesis: signalling pathways, biological responses and therapeutic inhibition. Trends Pharmacol Sci. 2001;22:201–207. doi: 10.1016/s0165-6147(00)01676-x. [DOI] [PubMed] [Google Scholar]
- 60.Cudmore M. Ahmad S. Al-Ani B. Fujisawa T. Coxall H. Chudasama K. Devey LR. Wigmore SJ. Abbas A. Hewett PW. Ahmed A. Negative regulation of soluble Flt-1 and soluble endoglin release by heme oxygenase-1. Circulation. 2007;115:1789–1797. doi: 10.1161/CIRCULATIONAHA.106.660134. [DOI] [PubMed] [Google Scholar]
- 61.Cvetanovic M. Ucker DS. Innate immune discrimination of apoptotic cells: repression of proinflammatory macrophage transcription is coupled directly to specific recognition. J Immunol. 2004;172:880–889. doi: 10.4049/jimmunol.172.2.880. [DOI] [PubMed] [Google Scholar]
- 62.Datla SR. Dusting GJ. Mori TA. Taylor CJ. Croft KD. Jiang F. Induction of heme oxygenase-1 in vivo suppresses NADPH oxidase derived oxidative stress. Hypertension. 2007;50:636–642. doi: 10.1161/HYPERTENSIONAHA.107.092296. [DOI] [PubMed] [Google Scholar]
- 63.De Caterina R. Libby P. Peng HB. Thannickal VJ. Rajavashisth TB. Gimbrone MA., Jr. Shin WS. Liao JK. Nitric oxide decreases cytokine-induced endothelial activation. Nitric oxide selectively reduces endothelial expression of adhesion molecules and proinflammatory cytokines. J Clin Investig. 1995;96:60–68. doi: 10.1172/JCI118074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Falco E. Porcelli D. Torella AR. Straino S. Iachininoto MG. Orlandi A. Truffa S. Biglioli P. Napolitano M. Capogrossi MC. Pesce M. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood. 2004;104:3472–3482. doi: 10.1182/blood-2003-12-4423. [DOI] [PubMed] [Google Scholar]
- 65.Deng YM. Wu BJ. Witting PK. Stocker R. Probucol protects against smooth muscle cell proliferation by upregulating heme oxygenase-1. Circulation. 2004;110:1855–1860. doi: 10.1161/01.CIR.0000142610.10530.25. [DOI] [PubMed] [Google Scholar]
- 66.Deramaudt BM. Braunstein S. Remy P. Abraham NG. Gene transfer of human heme oxygenase-1 into coronary endothelial cells potentially promotes angiogenesis. J Cell Biochem. 1998;68:121–127. doi: 10.1002/(sici)1097-4644(19980101)68:1<121::aid-jcb12>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 67.Deshane J. Chen S. Caballero S. Grochot-Przeczek A. Was H. Li Calzi S. Lach R. Hock TD. Chen B. Hill-Kapturczak N. Siegal GP. Dulak J. Jozkowicz A. Grant MB. Agarwal A. Stromal cell-derived factor 1 promotes angiogenesis via a heme oxygenase 1-dependent mechanism. J Exp Med. 2007;204:605–618. doi: 10.1084/jem.20061609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dimmeler S. Zeiher AM. Vascular repair by circulating endothelial progenitor cells: the missing link in atherosclerosis? J Mol Med. 2004;82:671–677. doi: 10.1007/s00109-004-0580-x. [DOI] [PubMed] [Google Scholar]
- 69.Duckers HJ. Boehm M. True AL. Yet SF. San H. Park JL. Clinton Webb R. Lee ME. Nabel GJ. Nabel EG. Heme oxygenase-1 protects against vascular constriction and proliferation. Nat Med. 2001;7:693–698. doi: 10.1038/89068. [DOI] [PubMed] [Google Scholar]
- 70.Dulak J. Deshane J. Jozkowicz A. Agarwal A. Heme oxygenase-1 and carbon monoxide in vascular pathobiology: focus on angiogenesis. Circulation. 2008;117:231–241. doi: 10.1161/CIRCULATIONAHA.107.698316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dulak J. Jozkowicz A. Foresti R. Kasza A. Frick M. Huk I. Green CJ. Pachinger O. Weidinger F. Motterlini R. Heme oxygenase activity modulates vascular endothelial growth factor synthesis in vascular smooth muscle cells. Antioxid Redox Signal. 2002;4:229–240. doi: 10.1089/152308602753666280. [DOI] [PubMed] [Google Scholar]
- 72.Dulak J. Loboda A. Jozkowicz A. Effect of heme oxygenase-1 on vascular function and disease. Curr Opin Lipidol. 2008;19:505–512. doi: 10.1097/MOL.0b013e32830d81e9. [DOI] [PubMed] [Google Scholar]
- 73.Durante W. Johnson FK. Johnson RA. Role of carbon monoxide in cardiovascular function. J Cell Mol Med. 2006;10:672–686. doi: 10.1111/j.1582-4934.2006.tb00427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Durante W. Kroll MH. Christodoulides N. Peyton KJ. Schafer AI. Nitric oxide induces heme oxygenase-1 gene expression and carbon monoxide production in vascular smooth muscle cells. Circ Res. 1997;80:557–564. doi: 10.1161/01.res.80.4.557. [DOI] [PubMed] [Google Scholar]
- 75.Elbirt KK. Whitmarsh AJ. Davis RJ. Bonkovsky HL. Mechanism of sodium arsenite-mediated induction of heme oxygenase-1 in hepatoma cells. Role of mitogen-activated protein kinases. J Biol Chem. 1998;273:8922–8931. doi: 10.1074/jbc.273.15.8922. [DOI] [PubMed] [Google Scholar]
- 76.Endler G. Exner M. Schillinger M. Marculescu R. Sunder-Plassmann R. Raith M. Jordanova N. Wojta J. Mannhalter C. Wagner OF. Huber K. A microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with increased bilirubin and HDL levels but not with coronary artery disease. Thromb Haemost. 2004;91:155–161. doi: 10.1160/TH03-05-0291. [DOI] [PubMed] [Google Scholar]
- 77.Erwig LP. Kluth DC. Rees AJ. Macrophages in renal inflammation. Curr Opin Nephrol Hypertens. 2001;10:341–347. doi: 10.1097/00041552-200105000-00007. [DOI] [PubMed] [Google Scholar]
- 78.Exner M. Schillinger M. Minar E. Mlekusch W. Schlerka G. Haumer M. Mannhalter C. Wagner O. Heme oxygenase-1 gene promoter microsatellite polymorphism is associated with restenosis after percutaneous transluminal angioplasty. J Endovasc Ther. 2001;8:433–440. doi: 10.1177/152660280100800501. [DOI] [PubMed] [Google Scholar]
- 79.Fadok VA. Bratton DL. Konowal A. Freed PW. Westcott JY. Henson PM. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J Clin Invest. 1998;101:890–898. doi: 10.1172/JCI1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fernandez M. Bonkovsky HL. Vascular endothelial growth factor increases heme oxygenase-1 protein expression in the chick embryo chorioallantoic membrane. Br J Pharmacol. 2003;139:634–640. doi: 10.1038/sj.bjp.0705272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ferrara N. Carver-Moore K. Chen H. Dowd M. Lu L. O'Shea KS. Powell-Braxton L. Hillan KJ. Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- 82.Florczyk UM. Jozkowicz A. Dulak J. Biliverdin reductase: new features of an old enzyme and its potential therapeutic significance. Pharmacol Rep. 2008;60:38–48. [PMC free article] [PubMed] [Google Scholar]
- 83.Foo RS. Siow RC. Brown MJ. Bennett MR. Heme oxygenase-1 gene transfer inhibits angiotensin II-mediated rat cardiac myocyte apoptosis but not hypertrophy. J Cell Physiol. 2006;209:1–7. doi: 10.1002/jcp.20723. [DOI] [PubMed] [Google Scholar]
- 84.Foresti R. Motterlini R. The heme oxygenase pathway and its interaction with nitric oxide in the control of cellular homeostasis. Free Radic Res. 1999;31:459–475. doi: 10.1080/10715769900301031. [DOI] [PubMed] [Google Scholar]
- 85.Foresti R. Hammad J. Clark JE. Johnson TR. Mann BE. Friebe A. Green CJ. Motterlini R. Vasoactive properties of CORM-3, a novel water-soluble carbon monoxide-releasing molecule. Br J Pharmacol. 2004;142:453–460. doi: 10.1038/sj.bjp.0705825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Förstermann U. Oxidative stress in vascular disease: causes, defense mechanisms and potential therapies. Nat Clin Pract Cardiovasc Med. 2008;5:338–349. doi: 10.1038/ncpcardio1211. [DOI] [PubMed] [Google Scholar]
- 87.Freire-de-Lima CG. Nascimento DO. Soares MB. Bozza PT. Castro-Faria-Neto HC. de Mello FG. DosReis GA. Lopes MF. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature. 2000;403:199–203. doi: 10.1038/35003208. [DOI] [PubMed] [Google Scholar]
- 88.Fürst R. Blumenthal SB. Kiemer AK. Zahler S. Vollmar AM. Nuclear factor-kappaB-independent anti-inflammatory action of salicylate in human endothelial cells: induction of heme oxygenase-1 by the c-Jun N-terminal kinase/activator protein-1 pathway. J Pharmacol Exp Ther. 2006;318:389–394. doi: 10.1124/jpet.106.102251. [DOI] [PubMed] [Google Scholar]
- 89.Fürst R. Brueckl C. Kuebler WM. Zahler S. Krötz F. Görlach A. Vollmar AM. Kiemer AK. Atrial natriuretic peptide induces mitogen-activated protein kinase phosphatase-1 in human endothelial cells via Rac1 and NAD(P)H oxidase/Nox2-activation. Circ Res. 2005;96:43–53. doi: 10.1161/01.RES.0000151983.01148.06. [DOI] [PubMed] [Google Scholar]
- 90.Galkina E. Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Garcia-Cardena G. Fan R. Shah V. Sorrentino R. Cirino G. Papapetropoulos A. Sessa WC. Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature. 1998;392:821–824. doi: 10.1038/33934. [DOI] [PubMed] [Google Scholar]
- 92.Ghem C. Sarmento-Leite RE. de Quadros AS. Rossetto S. Gottschall CA. Serum bilirubin concentration in patients with an established coronary artery disease. Int Heart J. 2010;51:86–91. doi: 10.1536/ihj.51.86. [DOI] [PubMed] [Google Scholar]
- 93.Giavazzi R. Sennino B. Coltrini D. Garofalo A. Dossi R. Ronca R. Tosatti MP. Presta M. Distinct role of fibroblast growth factor-2 and vascular endothelial growth factor on tumor growth and angiogenesis. Am J Pathol. 2003;162:1913–1926. doi: 10.1016/S0002-9440(10)64325-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Goodman AI. Quan S. Yang L. Synghal A. Abraham NG. Functional expression of human heme oxygenase-1 gene in renal structure of spontaneously hypertensive rats. Exp Biol Med (Maywood) 2003;228:454–458. doi: 10.1177/15353702-0322805-04. [DOI] [PubMed] [Google Scholar]
- 95.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- 96.Gozzelino R. Jeney V. Soares MP. Mechanisms of cell protection by heme oxygenase-1. Annu Rev Pharmacol Toxicol. 2010;50:323–354. doi: 10.1146/annurev.pharmtox.010909.105600. [DOI] [PubMed] [Google Scholar]
- 97.Grochot-Przeczek A. Lach R. Mis J. Skrzypek K. Gozdecka M. Sroczynska P. Dubiel M. Rutkowski A. Kozakowska M. Zagorska A. Walczynski J. Was H. Kotlinowski J. Drukala J. Kurowski K. Kieda C. Herault Y. Dulak J. Jozkowicz A. Heme oxygenase-1 accelerates cutaneous wound healing in mice. PLoS One. 2009;4:e5803. doi: 10.1371/journal.pone.0005803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grosser N. Erdmann K. Hemmerle A. Berndt G. Hinkelmann U. Smith G. Schroder H. Rosuvastatin upregulates the antioxidant defense protein heme oxygenase-1. Biochem Biophys Res Commun. 2004;325:871–876. doi: 10.1016/j.bbrc.2004.10.123. [DOI] [PubMed] [Google Scholar]
- 99.Gueler F. Park JK. Rong S. Kirsch T. Lindschau C. Zheng W. Elger M. Fiebeler A. Fliser D. Luft FC. Haller H. Statins attenuate ischemia-reperfusion injury by inducing heme oxygenase-1 in infiltrating macrophages. Am J Pathol. 2007;170:1192–1199. doi: 10.2353/ajpath.2007.060782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Guleng B. Tateishi K. Ohta M. Kanai F. Jazag A. Ijichi H. Tanaka Y. Washida M. Morikane K. Fukushima Y. Yamori T. Tsuruo T. Kawabe T. Miyagishi M. Taira K. Sata M. Omata M. Blockade of the stromal cell-derived factor-1/CXCR4 axis attenuates in vivo tumor growth by inhibiting angiogenesis in a vascular endothelial growth factor-independent manner. Cancer Res. 2005;65:5864–5871. doi: 10.1158/0008-5472.CAN-04-3833. [DOI] [PubMed] [Google Scholar]
- 101.Guo Y. Stein AB. Wu WJ. Tan W. Zhu X. Li QH. Dawn B. Motterlini R. Bolli R. Administration of a CO-releasing molecule at the time of reperfusion reduces infarct size in vivo. Am J Physiol Heart Circ Physiol. 2004;286:H1649–H1653. doi: 10.1152/ajpheart.00971.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Habeos IG. Ziros PG. Chartoumpekis D. Psyrogiannis A. Kyriazopoulou V. Papavassiliou AG. Simvastatin activates Keap1/Nrf2 signaling in rat liver. J Mol Med. 2008;86:1279–1285. doi: 10.1007/s00109-008-0393-4. [DOI] [PubMed] [Google Scholar]
- 103.Halliwell B. Gutteridge JM. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hayashi S. Takamiya R. Yamaguchi T. Matsumoto K. Tojo SJ. Tamatani T. Kitajima M. Makino N. Ishimura Y. Suematsu M. Induction of heme oxygenase-1 suppresses venular leukocyte adhesion elicited by oxidative stress: role of bilirubin generated by the enzyme. Circ Res. 1999;85:663–671. doi: 10.1161/01.res.85.8.663. [DOI] [PubMed] [Google Scholar]
- 105.Heeba G. Moselhy ME. Hassan M. Khalifa M. Gryglewski R. Malinski T. Anti-atherogenic effect of statins: role of nitric oxide, peroxynitrite and haem oxygenase-1. Br J Pharmacol. 2009;156:1256–1266. doi: 10.1111/j.1476-5381.2009.00125.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hiasa K. Ishibashi M. Ohtani K. Inoue S. Zhao Q. Kitamoto S. Sata M. Ichiki T. Takeshita A. Egashira K. Gene transfer of stromal cell-derived factor-1 enhances ischemic vasculogenesis and angiogenesis via vascular endothelial growth factor/endothelial nitric oxide synthase-related pathway: next-generation chemokine therapy for therapeutic neovascularization. Circulation. 2004;109:2454–2461. doi: 10.1161/01.CIR.0000128213.96779.61. [DOI] [PubMed] [Google Scholar]
- 107.Hill-Kapturczak N. Chang SH. Agarwal A. Heme oxygenase and the kidney. DNA Cell Biol. 2002;21:307–321. doi: 10.1089/104454902753759726. [DOI] [PubMed] [Google Scholar]
- 108.Hu CM. Chen YH. Chiang MT. Chau LY. Heme oxygenase-1 inhibits angiotensin II-induced cardiac hypertrophy in vitro and in vivo. Circulation. 2004;110:309–316. doi: 10.1161/01.CIR.0000135475.35758.23. [DOI] [PubMed] [Google Scholar]
- 109.Huang LE. Willmore WG. Gu J. Goldberg MA. Bunn HF. Inhibition of hypoxia-inducible factor 1 activation by carbon monoxide and nitric oxide. Implications for oxygen sensing and signaling. J Biol Chem. 1999;274:9038–9044. doi: 10.1074/jbc.274.13.9038. [DOI] [PubMed] [Google Scholar]
- 110.Idriss NK. Blann AD. Lip GY. Hemoxygenase-1 in cardiovascular disease. J Am Coll Cardiol. 2008;52:971–978. doi: 10.1016/j.jacc.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 111.Igarashi K. Sun J. The heme-Bach1 pathway in the regulation of oxidative stress response and erythroid differentiation. Antioxid Redox Signal. 2006;8:107–118. doi: 10.1089/ars.2006.8.107. [DOI] [PubMed] [Google Scholar]
- 112.Ishikawa K. Navab M. Leitinger N. Fogelman AM. Lusis AJ. Induction of heme oxygenase-1 inhibits the monocyte transmigration induced by mildly oxidized LDL. J Clin Invest. 1997;100:1209–1216. doi: 10.1172/JCI119634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ishikawa K. Sugawara D. Goto J. Watanabe Y. Kawamura K. Shiomi M. Itabe H. Maruyama Y. Heme oxygenase-1 inhibits atherogenesis in Watanabe heritable hyperlipidemic rabbits. Circulation. 2001;104:1831–1836. doi: 10.1161/hc3901.095897. [DOI] [PubMed] [Google Scholar]
- 114.Ishikawa K. Sugawara D. Wang X. Suzuki K. Itabe H. Maruyama Y. Lusis AJ. Heme oxygenase-1 inhibits atherosclerotic lesion formation in ldl-receptor knockout mice. Circ Res. 2001;88:506–512. doi: 10.1161/01.res.88.5.506. [DOI] [PubMed] [Google Scholar]
- 115.Ishizaka N. de Leon H. Laursen JB. Fukui T. Wilcox JN. De Keulenaer G. Griendling KK. Alexander RW. Angiotensin II-induced hypertension increases heme oxygenase-1 expression in rat aorta. Circulation. 1997;96:1923–1929. doi: 10.1161/01.cir.96.6.1923. [DOI] [PubMed] [Google Scholar]
- 116.Jison ML. Munson PJ. Barb JJ. Suffredini AF. Talwar S. Logun C. Raghavachari N. Beigel JH. Shelhamer JH. Danner RL. Gladwin MT. Blood mononuclear cell gene expression profiles characterize the oxidant, hemolytic, and inflammatory stress of sickle cell disease. Blood. 2004;104:270–280. doi: 10.1182/blood-2003-08-2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Johnson RA. Lavesa M. Askari B. Abraham NG. Nasjletti A. A heme oxygenase product, presumably carbon monoxide, mediates a vasodepressor function in rats. Hypertension. 1995;25:166–169. doi: 10.1161/01.hyp.25.2.166. [DOI] [PubMed] [Google Scholar]
- 118.Johnson RA. Lavesa M. DeSeyn K. Scholer MJ. Nasjletti A. Heme oxygenase substrates acutely lower blood pressure in hypertensive rats. Am J Physiol. 1996;271:H1132–H1138. doi: 10.1152/ajpheart.1996.271.3.H1132. [DOI] [PubMed] [Google Scholar]
- 119.Jones CI., 3rd Han Z. Presley T. Varadharaj S. Zweier JL. Ilangovan G. Alevriadou BR. Endothelial cell respiration is affected by the oxygen tension during shear exposure: role of mitochondrial peroxynitrite. Am J Physiol Cell Physiol. 2008;295:C180–C191. doi: 10.1152/ajpcell.00549.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jozkowicz A. Cooke JP. Guevara I. Huk I. Funovics P. Pachinger O. Weidinger F. Dulak J. Genetic augmentation of nitric oxide synthase increases the vascular generation of VEGF. Cardiovasc Res. 2001;51:773–783. doi: 10.1016/s0008-6363(01)00344-3. [DOI] [PubMed] [Google Scholar]
- 121.Jozkowicz A. Huk I. Nigisch A. Weigel G. Dietrich W. Motterlini R. Dulak J. Heme oxygenase and angiogenic activity of endothelial cells: stimulation by carbon monoxide and inhibition by tin protoporphyrin-IX. Antioxid Redox Signal. 2003;5:155–162. doi: 10.1089/152308603764816514. [DOI] [PubMed] [Google Scholar]
- 122.Juan SH. Lee TS. Tseng KW. Liou JY. Shyue SK. Wu KK. Chau LY. Adenovirus-mediated heme oxygenase-1 gene transfer inhibits the development of atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2001;104:1519–1525. doi: 10.1161/hc3801.095663. [DOI] [PubMed] [Google Scholar]
- 123.Kacimi R. Chentoufi J. Honbo N. Long CS. Karliner JS. Hypoxia differentially regulates stress proteins in cultured cardiomyocytes: role of the p38 stress-activated kinase signaling cascade, and relation to cytoprotection. Cardiovasc Res. 2000;46:139–150. doi: 10.1016/s0008-6363(00)00007-9. [DOI] [PubMed] [Google Scholar]
- 124.Kaneda H. Ohno M. Taguchi J. Togo M. Hashimoto H. Ogasawara K. Aizawa T. Ishizaka N. Nagai R. Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler Thromb Vasc Biol. 2002;22:1680–1685. doi: 10.1161/01.atv.0000033515.96747.6f. [DOI] [PubMed] [Google Scholar]
- 125.Kapitulnik J. Maines MD. Pleiotropic functions of biliverdin reductase: cellular signaling and generation of cytoprotective and cytotoxic bilirubin. Trends Pharmacol Sci. 2009;30:129–137. doi: 10.1016/j.tips.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 126.Kaspar JW. Niture SK. Jaiswal AK. Nrf2:INrf2 (Keap1) signaling in oxidative stress. Free Radic Biol Med. 2009;47:1304–1309. doi: 10.1016/j.freeradbiomed.2009.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kawamura K. Ishikawa K. Wada Y. Kimura S. Matsumoto H. Kohro T. Itabe H. Kodama T. Maruyama Y. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:155–160. doi: 10.1161/01.ATV.0000148405.18071.6a. [DOI] [PubMed] [Google Scholar]
- 128.Kawashima A. Oda Y. Yachie A. Koizumi S. Nakanishi I. Heme oxygenase-1 deficiency: the first autopsy case. Hum Pathol. 2002;33:125–130. doi: 10.1053/hupa.2002.30217. [DOI] [PubMed] [Google Scholar]
- 129.Kemp PJ. Hemeoxygenase-2 as an O2 sensor in K+ channel-dependent chemotransduction. Biochem Biophys Res Commun. 2005;338:648–652. doi: 10.1016/j.bbrc.2005.08.110. [DOI] [PubMed] [Google Scholar]
- 130.Kim HP. Ryter SW. Choi AM. CO as a cellular signaling molecule. Annu Rev Pharmacol Toxicol. 2006;46:411–449. doi: 10.1146/annurev.pharmtox.46.120604.141053. [DOI] [PubMed] [Google Scholar]
- 131.Kim HP. Wang X. Zhang J. Suh GY. Benjamin IJ. Ryter SW. Choi AM. Heat shock protein-70 mediates the cytoprotective effect of carbon monoxide: involvement of p38 beta MAPK and heat shock factor-1. J Immunol. 2005;175:2622–2629. doi: 10.4049/jimmunol.175.4.2622. [DOI] [PubMed] [Google Scholar]
- 132.Kim JE. Kang YJ. Lee KY. Choi HC. Isoproterenol inhibits angiotensin II-stimulated proliferation and reactive oxygen species production in vascular smooth muscle cells through heme oxygenase-1. Biol Pharm Bull. 2009;32:1047–1052. doi: 10.1248/bpb.32.1047. [DOI] [PubMed] [Google Scholar]
- 133.Kim KM. Pae HO. Zheng M. Park R. Kim YM. Chung HT. Carbon monoxide induces heme oxygenase-1 via activation of protein kinase R-like endoplasmic reticulum kinase and inhibits endothelial cell apoptosis triggered by endoplasmic reticulum stress. Circ Res. 2007;101:919–927. doi: 10.1161/CIRCRESAHA.107.154781. [DOI] [PubMed] [Google Scholar]
- 134.Kim YM. Bombeck CA. Billiar TR. Nitric oxide as a bifunctional regulator of apoptosis. Circ Res. 1999;84:253–256. doi: 10.1161/01.res.84.3.253. [DOI] [PubMed] [Google Scholar]
- 135.Kong D. Melo LG. Mangi AA. Zhang L. Lopez-Ilasaca M. Perrella MA. Liew CC. Pratt RE. Dzau VJ. Enhanced inhibition of neointimal hyperplasia by genetically engineered endothelial progenitor cells. Circulation. 2004;109:1769–1775. doi: 10.1161/01.CIR.0000121732.85572.6F. [DOI] [PubMed] [Google Scholar]
- 136.Kronke G. Kadl A. Ikonomu E. Bluml S. Furnkranz A. Sarembock IJ. Bochkov VN. Exner M. Binder BR. Leitinger N. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2007;27:1276–1282. doi: 10.1161/ATVBAHA.107.142638. [DOI] [PubMed] [Google Scholar]
- 137.Kruger AL. Peterson SJ. Schwartzman ML. Fusco H. McClung JA. Weiss M. Shenouda S. Goodman AI. Goligorsky MS. Kappas A. Abraham NG. Up-regulation of heme oxygenase provides vascular protection in an animal model of diabetes through its antioxidant and antiapoptotic effects. J Pharmacol Exp Ther. 2006;319:1144–1152. doi: 10.1124/jpet.106.107482. [DOI] [PubMed] [Google Scholar]
- 138.Lee PJ. Alam J. Wiegand GW. Choi AM. Overexpression of heme oxygenase-1 in human pulmonary epithelial cells results in cell growth arrest and increased resistance to hyperoxia. Proc Natl Acad Sci USA. 1996;93:10393–10398. doi: 10.1073/pnas.93.19.10393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Lee TS. Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat Med. 2002;8:240–246. doi: 10.1038/nm0302-240. [DOI] [PubMed] [Google Scholar]
- 140.Lee TS. Chang CC. Zhu Y. Shyy JY. Simvastatin induces heme oxygenase-1: a novel mechanism of vessel protection. Circulation. 2004;110:1296–1302. doi: 10.1161/01.CIR.0000140694.67251.9C. [DOI] [PubMed] [Google Scholar]
- 141.Lerner-Marmarosh N. Miralem T. Gibbs PE. Maines MD. Human biliverdin reductase is an ERK activator; hBVR is an ERK nuclear transporter and is required for MAPK signaling. Proc Natl Acad Sci USA. 2008;105:6870–6875. doi: 10.1073/pnas.0800750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Li C. Hossieny P. Wu BJ. Qawasmeh A. Beck K. Stocker R. Pharmacologic induction of heme oxygenase-1. Antioxid Redox Signal. 2007;9:2227–2239. doi: 10.1089/ars.2007.1783. [DOI] [PubMed] [Google Scholar]
- 143.Li Volti G. Wang J. Traganos F. Kappas A. Abraham N. Differential effect of heme oxygenase-1 in endothelial and smooth muscle cell cycle progression. Biochem Biophys Res Commun. 2002;296:1077–1082. doi: 10.1016/s0006-291x(02)02054-5. [DOI] [PubMed] [Google Scholar]
- 144.Liao JK. Laufs U. Pleiotropic effects of statins. Annu Rev Pharmacol Toxicol. 2005;45:89–118. doi: 10.1146/annurev.pharmtox.45.120403.095748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Lin HH. Chen YH. Chang PF. Lee YT. Yet SF. Chau LY. Heme oxygenase-1 promotes neovascularization in ischemic heart by coinduction of VEGF and SDF-1. J Mol Cell Cardiol. 2008;45:44–55. doi: 10.1016/j.yjmcc.2008.04.011. [DOI] [PubMed] [Google Scholar]
- 146.Lin HH. Chen YH. Yet SF. Chau LY. Heme oxygenase-1/carbon monoxide enhances reendothelialization after vascular injury via promoting mobilization of circulating endothelial progenitor cells. J Thromb Haemost. 2009;7:1401–1408. doi: 10.1111/j.1538-7836.2009.03478.x. [DOI] [PubMed] [Google Scholar]
- 147.Llesuy SF. Tomaro ML. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta. 1994;1223:9–14. doi: 10.1016/0167-4889(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 148.Loboda A. Jazwa A. Grochot-Przeczek A. Rutkowski AJ. Cisowski J. Agarwal A. Jozkowicz A. Dulak J. Heme oxygenase-1 and the vascular bed: from molecular mechanisms to therapeutic opportunities. Antioxid Redox Signal. 2008;10:1767–1812. doi: 10.1089/ars.2008.2043. [DOI] [PubMed] [Google Scholar]
- 149.Loboda A. Jazwa A. Jozkowicz A. Dorosz J. Balla J. Molema G. Dulak J. Atorvastatin prevents hypoxia-induced inhibition of endothelial nitric oxide synthase expression but does not affect heme oxygenase-1 in human microvascular endothelial cells. Atherosclerosis. 2006;187:26–30. doi: 10.1016/j.atherosclerosis.2006.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Losordo DW. Dimmeler S. Therapeutic angiogenesis and vasculogenesis for ischemic disease. Part I: angiogenic cytokines. Circulation. 2004;109:2487–2491. doi: 10.1161/01.CIR.0000128595.79378.FA. [DOI] [PubMed] [Google Scholar]
- 151.Maines MD. Miralem T. Lerner-Marmarosh N. Shen J. Gibbs PE. Human biliverdin reductase, a previously unknown activator of protein kinase C betaII. J Biol Chem. 2007;282:8110–8122. doi: 10.1074/jbc.M513427200. [DOI] [PubMed] [Google Scholar]
- 152.Maines MD. The heme oxygenase system: a regulator of second messenger gases. Annu Rev Pharmacol Toxicol. 1997;37:517–554. doi: 10.1146/annurev.pharmtox.37.1.517. [DOI] [PubMed] [Google Scholar]
- 153.Mancuso C. Heme oxygenase and its products in the nervous system. Antioxid Redox Signal. 2004;6:878–887. doi: 10.1089/ars.2004.6.878. [DOI] [PubMed] [Google Scholar]
- 154.Mancuso C. Barone E. The heme oxygenase/biliverdin reductase pathway in drug research and development. Curr Drug Metab. 2009;10:579–594. doi: 10.2174/138920009789375405. [DOI] [PubMed] [Google Scholar]
- 155.Mancuso C. Bonsignore A. Capone C. Di Stasio E. Pani G. Albumin-bound bilirubin interacts with nitric oxide by a redox mechanism. Antioxid Redox Signal. 2006;8:487–494. doi: 10.1089/ars.2006.8.487. [DOI] [PubMed] [Google Scholar]
- 156.Mancuso C. Kostoglou-Athanassiou I. Forsling ML. Grossman AB. Preziosi P. Navarra P. Minotti G. Activation of heme oxygenase and consequent carbon monoxide formation inhibits the release of arginine vasopressin from rat hypothalamic explants. Molecular linkage between heme catabolism and neuroendocrine function. Brain Res Mol Brain Res. 1997;50:267–276. doi: 10.1016/s0169-328x(97)00197-6. [DOI] [PubMed] [Google Scholar]
- 157.Mancuso C. Pani G. Calabrese V. Bilirubin: an endogenous scavenger of nitric oxide and reactive nitrogen species. Redox Rep. 2006;11:207–213. doi: 10.1179/135100006X154978. [DOI] [PubMed] [Google Scholar]
- 158.Mancuso C. Perluigi M. Cini C. De Marco C. Giuffrida Stella AM. Calabrese V. Heme oxygenase and cyclooxygenase in the central nervous system: a functional interplay. J Neurosci Res. 2006;84:1385–1391. doi: 10.1002/jnr.21049. [DOI] [PubMed] [Google Scholar]
- 159.Mancuso C. Pistritto G. Tringali G. Grossman AB. Preziosi P. Navarra P. Evidence that carbon monoxide stimulates prostaglandin endoperoxide synthase activity in rat hypothalamic explants and in primary cultures of rat hypothalamic astrocytes. Brain Res Mol Brain Res. 1997;45:294–300. doi: 10.1016/s0169-328x(96)00258-6. [DOI] [PubMed] [Google Scholar]
- 160.Mantovani A. Sozzani S. Locati M. Allavena P. Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 161.Marti HH. Risau W. Systemic hypoxia changes the organ-specific distribution of vascular endothelial growth factor and its receptors. Proc Natl Acad Sci USA. 1998;95:15809–15814. doi: 10.1073/pnas.95.26.15809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.McCoubrey WK., Jr. Huang TJ. Maines MD. Isolation and characterization of a cDNA from the rat brain that encodes hemoprotein heme oxygenase-3. Eur J Biochem. 1997;247:725–732. doi: 10.1111/j.1432-1033.1997.00725.x. [DOI] [PubMed] [Google Scholar]
- 163.Minamino T. Christou H. Hsieh CM. Liu Y. Dhawan V. Abraham NG. Perrella MA. Mitsialis SA. Kourembanas S. Targeted expression of heme oxygenase-1 prevents the pulmonary inflammatory and vascular responses to hypoxia. Proc Natl Acad Sci USA. 2001;98:8798–8803. doi: 10.1073/pnas.161272598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Miralem T. Hu Z. Torno MD. Lelli KM. Maines MD. Small interference RNA-mediated gene silencing of human biliverdin reductase, but not that of heme oxygenase-1, attenuates arsenite-mediated induction of the oxygenase and increases apoptosis in 293A kidney cells. J Biol Chem. 280:17084–17092. doi: 10.1074/jbc.M413121200. [DOI] [PubMed] [Google Scholar]
- 165.Mizuguchi S. Stephen J. Bihari R. Markovic N. Suehiro S. Capretta A. Potter RF. Cepinskas G. CORM-3-derived CO modulates polymorphonuclear leukocyte migration across the vascular endothelium by reducing levels of cell surface-bound elastase. Am J Physiol Heart Circ Physiol. 2009;297:H920–H929. doi: 10.1152/ajpheart.00305.2009. [DOI] [PubMed] [Google Scholar]
- 166.Morice MC. Serruys PW. Sousa JE. Fajadet J. Ban Hayashi E. Perin M. Colombo A. Schuler G. Barragan P. Guagliumi G. Molnar F. Falotico R. A randomized comparison of a sirolimus-eluting stent with a standard stent for coronary revascularization. N Engl J Med. 2002;346:1773–1780. doi: 10.1056/NEJMoa012843. [DOI] [PubMed] [Google Scholar]
- 167.Morita T. Perrella MA. Lee ME. Kourembanas S. Smooth muscle cell-derived carbon monoxide is a regulator of vascular cGMP. Proc Natl Acad Sci USA. 1995;92:1475–1479. doi: 10.1073/pnas.92.5.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Morita T. Heme oxygenase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2005;25:1786–1795. doi: 10.1161/01.ATV.0000178169.95781.49. [DOI] [PubMed] [Google Scholar]
- 169.Morse D. Sethi J. Carbon monoxide and human disease. Antioxid Redox Signal. 2002;4:331–338. doi: 10.1089/152308602753666389. [DOI] [PubMed] [Google Scholar]
- 170.Morse D. Lin L. Choi AM. Ryter SW. Heme oxygenase-1, a critical arbitrator of cell death pathways in lung injury and disease. Free Radic Biol Med. 2009;47:1–12. doi: 10.1016/j.freeradbiomed.2009.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Morsi WG. Shaker OG. Ismail EF. Ahmed HH. El-Serafi TI. Maklady FA. Abdel-Aziz MT. El-Asmar MF. Atta HM. HO-1 and VGEF gene expression in human arteries with advanced atherosclerosis. Clin Biochem. 2006;39:1057–1062. doi: 10.1016/j.clinbiochem.2006.08.003. [DOI] [PubMed] [Google Scholar]
- 172.Motohashi H. Yamamoto M. Nrf2-Keap1 defines a physiologically important stress response mechanism. Trend Mol Med. 2004;10:549–557. doi: 10.1016/j.molmed.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 173.Motterlini R. Clark JE. Foresti R. Sarathchandra P. Mann BE. Green CJ. Carbon monoxide-releasing molecules: characterization of biochemical and vascular activities. Circ Res. 2002;90:E17–E24. doi: 10.1161/hh0202.104530. [DOI] [PubMed] [Google Scholar]
- 174.Motterlini R. Foresti R. Intaglietta M. Winslow RM. NO-mediated activation of heme oxygenase: endogenous cytoprotection against oxidative stress to endothelium. Am J Physiol. 1996;270:H107–H114. doi: 10.1152/ajpheart.1996.270.1.H107. [DOI] [PubMed] [Google Scholar]
- 175.Nakahira K. Kim HP. Geng XH. Nakao A. Wang X. Murase N. Drain PF. Sasidhar M. Nabel EG. Takahashi T. Lukacs NW. Ryter SW. Morita K. Choi AM. Carbon monoxide differentially inhibits TLR signaling pathways by regulating ROS-induced trafficking of TLRs to lipid rafts. J Exp Med. 2006;203:2377–2389. doi: 10.1084/jem.20060845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Nakao A. Kaczorowski DJ. Sugimoto R. Billiar TR. McCurry KR. Application of heme oxygenase-1, carbon monoxide and biliverdin for the prevention of intestinal ischemia/reperfusion injury. J Clin Biochem Nutr. 2008;42:78–88. doi: 10.3164/jcbn.2008013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Nakao A. Neto JS. Kanno S. Stolz DB. Kimizuka K. Liu F. Bach FH. Billiar TR. Choi AM. Otterbein LE. Murase N. Protection against ischemia/reperfusion injury in cardiac and renal transplantation with carbon monoxide, biliverdin and both. Am J Transplant. 2005;5:282–291. doi: 10.1111/j.1600-6143.2004.00695.x. [DOI] [PubMed] [Google Scholar]
- 178.Nath KA. Grande JP. Haggard JJ. Croatt AJ. Katusic ZS. Solovey A. Hebbel RP. Oxidative stress and induction of heme oxygenase-1 in the kidney in sickle cell disease. Am J Pathol. 2001;158:893–903. doi: 10.1016/S0002-9440(10)64037-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ndisang JF. Jadhav A. The heme oxygenase system attenuates pancreatic lesions and improves insulin sensitivity and glucose metabolism in deoxycorticosterone acetate hypertension. Am J Physiol Regul Integr Comp Physiol. 2010;298:R211–R223. doi: 10.1152/ajpregu.91000.2008. [DOI] [PubMed] [Google Scholar]
- 180.Ndisang JF. Jadhav A. Up-regulating the heme oxygenase system enhances insulin sensitivity and improves glucose metabolism in insulin-resistant diabetes in Goto-Kakizaki rats. Endocrinology. 2009;150:2627–2636. doi: 10.1210/en.2008-1370. [DOI] [PubMed] [Google Scholar]
- 181.Ndisang JF. Lane N. Jadhav A. The heme oxygenase system abates hyperglycemia in Zucker diabetic fatty rats by potentiating insulin-sensitizing pathways. Endocrinology. 2009;150:2098–2108. doi: 10.1210/en.2008-0239. [DOI] [PubMed] [Google Scholar]
- 182.Ndisang JF. Lane N. Syed N. Jadhav A. Up-regulating the heme oxygenase system with hemin improves insulin sensitivity and glucose metabolism in adult spontaneously hypertensive rats. Endocrinology. 2010;151:549–560. doi: 10.1210/en.2009-0471. [DOI] [PubMed] [Google Scholar]
- 183.Ndisang JF. Tabien HE. Wang R. Carbon monoxide and hypertension. J Hypertens. 2004;22:1057–1074. doi: 10.1097/00004872-200406000-00002. [DOI] [PubMed] [Google Scholar]
- 184.Ndisang JF. Wu L. Zhao W. Wang R. Induction of heme oxygenase-1 and stimulation of cGMP production by hemin in aortic tissues from hypertensive rats. Blood. 2003;101:3893–3900. doi: 10.1182/blood-2002-08-2608. [DOI] [PubMed] [Google Scholar]
- 185.Ndisang JF. Zhao W. Wang R. Selective regulation of blood pressure by heme oxygenase-1 in hypertension. Hypertension. 2002;40:315–321. doi: 10.1161/01.hyp.0000028488.71068.16. [DOI] [PubMed] [Google Scholar]
- 186.Nguyen T. Nioi P. Pickett CB. The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem. 2009;284:13291–13295. doi: 10.1074/jbc.R900010200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Nizamutdinova IT. Kim YM. Kim HJ. Seo HG. Lee JH. Chang KC. Carbon monoxide (from CORM-2) inhibits high glucose-induced ICAM-1 expression via AMP-activated protein kinase and PPAR-gamma activations in endothelial cells. Atherosclerosis. 2009;207:405–411. doi: 10.1016/j.atherosclerosis.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 188.Ockaili Ramzi. Natarajan Ramesh. Salloum Fadi. Fisher Bernard J. Jones Drew. Fowler Alpha A., III Kukreja Rakesh C. HIF-1 activation attenuates postischemic myocardial injury: role for heme oxygenase-1 in modulating microvascular chemokine generation. Am J Physiol Heart Circ Physiol. 2005;289:H542–H548. doi: 10.1152/ajpheart.00089.2005. [DOI] [PubMed] [Google Scholar]
- 189.Oh GS. Pae HO. Lee BS. Kim BN. Kim JM. Kim HR. Jeon SB. Jeon WK. Chae HJ. Chung HT. Hydrogen sulfide inhibits nitric oxide production and nuclear factor-κB via heme oxygenase-1 expression in RAW264.7 macrophages stimulated with lipopolysaccharide. Free Radic Biol Med. 2006;41:106–119. doi: 10.1016/j.freeradbiomed.2006.03.021. [DOI] [PubMed] [Google Scholar]
- 190.Omura S. Suzuki H. Toyofuku M. Ozono R. Kohno N. Igarashi K. Effects of genetic ablation of bach1 upon smooth muscle cell proliferation and atherosclerosis after cuff injury. Genes Cells. 2005;10:277–285. doi: 10.1111/j.1365-2443.2005.00832.x. [DOI] [PubMed] [Google Scholar]
- 191.Ono K. Goto Y. Takagi S. Baba S. Tago N. Nonogi H. Iwai N. A promoter variant of the heme oxygenase-1 gene may reduce the incidence of ischemic heart disease in Japanese. Atherosclerosis. 2004;173:315–319. doi: 10.1016/j.atherosclerosis.2003.11.021. [DOI] [PubMed] [Google Scholar]
- 192.Orozco LD. Kapturczak MH. Barajas B. Wang X. Weinstein MM. Wong J. Deshane J. Bolisetty S. Shaposhnik Z. Shih DM. Agarwal A. Lusis AJ. Araujo JA. Heme oxygenase-1 expression in macrophages plays a beneficial role in atherosclerosis. Circ Res. 2007;100:1703–1711. doi: 10.1161/CIRCRESAHA.107.151720. [DOI] [PubMed] [Google Scholar]
- 193.Otterbein LE. Bach FH. Alam J. Soares M. Tao Lu H. Wysk M. Davis RJ. Flavell RA. Choi AM. Carbon monoxide has anti-inflammatory effects involving the mitogen-activated protein kinase pathway. Nat Med. 2000;6:422–428. doi: 10.1038/74680. [DOI] [PubMed] [Google Scholar]
- 194.Otterbein LE. Soares MP. Yamashita K. Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- 195.Otterbein LE. Zuckerbraun BS. Haga M. Liu F. Song R. Usheva A. Stachulak C. Bodyak N. Smith RN. Csizmadia E. Tyagi S. Akamatsu Y. Flavell RJ. Billiar TR. Tzeng E. Bach FH. Choi AM. Soares MP. Carbon monoxide suppresses arteriosclerotic lesions associated with chronic graft rejection and with balloon injury. Nat Med. 2003;9:183–190. doi: 10.1038/nm817. [DOI] [PubMed] [Google Scholar]
- 196.Pachori AS. Melo LG. Hart ML. Noiseux N. Zhang L. Morello F. Solomon SD. Stahl GL. Pratt RE. Dzau VJ. Hypoxia-regulated therapeutic gene as a preemptive treatment strategy against ischemia/reperfusion tissue injury. Proc Natl Acad Sci USA. 2004;101:12282–12287. doi: 10.1073/pnas.0404616101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Pae HO. Jeong GS. Kim HS. Woo WH. Rhew HY. Kim HS. Sohn DH. Kim YC. Chung HT. Costunolide inhibits production of tumor necrosis factor-alpha and interleukin-6 by inducing heme oxygenase-1 in RAW264.7 macrophages. Inflamm Res. 2007;56:520–526. doi: 10.1007/s00011-007-7015-4. [DOI] [PubMed] [Google Scholar]
- 198.Pae HO. Kim EC. Chung HT. Integrative survival response evoked by heme oxygenase-1 and heme metabolites. J Clin Biochem Nutr. 2008;42:197–203. doi: 10.3164/jcbn.2008029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Pae HO. Lee YC. Chung HT. Heme oxygenase-1 and carbon monoxide: emerging therapeutic targets in inflammation and allergy. Recent Pat Inflamm Allergy Drug Discov. 2008;2:159–165. doi: 10.2174/187221308786241929. [DOI] [PubMed] [Google Scholar]
- 200.Pae HO. Lee YC. Jo EK. Chung HT. Subtle interplay of endogenous bioactive gases (NO, CO and H2S) in inflammation. Arch Pharm Res. 2009;32:1155–1162. doi: 10.1007/s12272-009-1806-9. [DOI] [PubMed] [Google Scholar]
- 201.Pae HO. Oh GS. Choi BM. Kim YM. Chung HT. A molecular cascade showing nitric oxide-heme oxygenase-1-vascular endothelial growth factor-interleukin-8 sequence in human endothelial cells. Endocrinology. 2005;146:2229–2238. doi: 10.1210/en.2004-1431. [DOI] [PubMed] [Google Scholar]
- 202.Pae HO. Oh GS. Lee BS. Rim JS. Kim YM. Chung HT. 3-Hydroxyanthranilic acid, one of L-tryptophan metabolites, inhibits monocyte chemoattractant protein-1 secretion and vascular cell adhesion molecule-1expression via heme oxygenase-1 induction in human umbilical vein endothelial cells. Atherosclerosis. 2006;187:274–284. doi: 10.1016/j.atherosclerosis.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 203.Pan S. Molecular mechanisms responsible for the atheroprotective effects of laminar shear stress. Antioxid Redox Signal. 2009;11:1669–1682. doi: 10.1089/ars.2009.2487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Papapetropoulos A. Garcia-Cardena G. Madri JA. Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Paredi P. Biernacki W. Invernizzi G. Kharitonov SA. Barnes PJ. Exhaled carbon monoxide levels elevated in diabetes and correlated with glucose concentration in blood: a new test for monitoring the disease? Chest. 1999;116:1007–1011. doi: 10.1378/chest.116.4.1007. [DOI] [PubMed] [Google Scholar]
- 206.Peterson SJ. Frishman WH. Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol Rev. 2009;17:99–111. doi: 10.1097/CRD.0b013e31819d813a. [DOI] [PubMed] [Google Scholar]
- 207.Peyton KJ. Reyna SV. Chapman GB. Ensenat D. Liu XM. Wang H. Schafer AI. Durante W. Heme oxygenase-1-derived carbon monoxide is an autocrine inhibitor of vascular smooth muscle cell growth. Blood. 2002;99:4443–4448. doi: 10.1182/blood.v99.12.4443. [DOI] [PubMed] [Google Scholar]
- 208.Ponka P. Cell biology of heme. Am J Med Sci. 1999;318:241–256. doi: 10.1097/00000441-199910000-00004. [DOI] [PubMed] [Google Scholar]
- 209.Poss KD. Tonegawa S. Heme oxygenase 1 is required for mammalian iron reutilization. Proc Natl Acad Sci USA. 1997;94:10919–10924. doi: 10.1073/pnas.94.20.10919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Poss KD. Tonegawa S. Reduced stress defense in heme oxygenase 1-deficient cells. Proc Natl Acad Sci USA. 1997;94:10925–10930. doi: 10.1073/pnas.94.20.10925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Qi X. Li J. Gu J. Li S. Dang Y. Wang T. Plasma levels of IL-8 predict early complications in patients with coronary heart disease after percutaneous coronary intervention. Jpn Heart J. 2003;44:451–461. doi: 10.1536/jhj.44.451. [DOI] [PubMed] [Google Scholar]
- 212.Qing G. Simon MC. Hypoxia inducible factor-2: a critical mediator of aggressive tumor phenotypes. Curr Opin Genet Dev. 2009;19:60–66. doi: 10.1016/j.gde.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Quan S. Yang L. Abraham NG. Kappas A. Regulation of human heme oxygenase in endothelial cells by using sense and antisense retroviral constructs. Proc Natl Acad Sci USA. 2001;98:12203–12208. doi: 10.1073/pnas.211399398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Rathmell JC. Thompson CB. The central effectors of cell death in the immune system. Annu Rev Immunol. 1999;17:781–828. doi: 10.1146/annurev.immunol.17.1.781. [DOI] [PubMed] [Google Scholar]
- 215.Rosenzweig A. Cardiac cell therapy—mixed results from mixed cells. N Engl J Med. 2006;355:1274–1277. doi: 10.1056/NEJMe068172. [DOI] [PubMed] [Google Scholar]
- 216.Rouse BT. Regulatory T cells in health and disease. J Intern Med. 2007;262:78–95. doi: 10.1111/j.1365-2796.2007.01836.x. [DOI] [PubMed] [Google Scholar]
- 217.Rucker M. Schafer T. Roesken F. Spitzer WJ. Bauer M. Menger MD. Reduction of inflammatory response in composite flap transfer by local stress conditioning-induced heat-shock protein 32. Surgery. 2001;129:292–301. doi: 10.1067/msy.2001.111079. [DOI] [PubMed] [Google Scholar]
- 218.Ryter SW. Alam J. Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- 219.Ryter SW. Morse D. Choi AM. Carbon monoxide and bilirubin: potential therapies for pulmonary/vascular injury and disease. Am J Respir Cell Mol Biol. 2007;36:175–182. doi: 10.1165/rcmb.2006-0333TR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Sabaawy HE. Zhang F. Nguyen X. ElHosseiny A. Nasjletti A. Schwartzman M. Dennery P. Kappas A. Abraham NG. Human heme oxygenase-1 gene transfer lowers blood pressure and promotes growth in spontaneously hypertensive rats. Hypertension. 2001;38:210–215. doi: 10.1161/01.hyp.38.2.210. [DOI] [PubMed] [Google Scholar]
- 221.Sato K. Balla J. Otterbein L. Smith RN. Brouard S. Lin Y. Csizmadia E. Sevigny J. Robson SC. Vercellotti G. Choi AM. Bach FH. Soares MP. Carbon monoxide generated by heme oxygenase-1 suppresses the rejection of mouse-to-rat cardiac transplants. J Immunol. 2001;166:4185–4194. doi: 10.4049/jimmunol.166.6.4185. [DOI] [PubMed] [Google Scholar]
- 222.Schachinger V. Erbs S. Elsasser A. Haberbosch W. Hambrecht R. Holschermann H. Yu J. Corti R. Mathey DG. Hamm CW. Suselbeck T. Assmus B. Tonn T. Dimmeler S. Zeiher AM. Intracoronary bone marrow-derived progenitor cells in acute myocardial infarction. N Engl J Med. 2006;355:1210–1221. doi: 10.1056/NEJMoa060186. [DOI] [PubMed] [Google Scholar]
- 223.Schachinger V. Erbs S. Elsasser A. Haberbosch W. Hambrecht R. Holschermann H. Yu J. Corti R. Mathey DG. Hamm CW. Suselbeck T. Werner N. Haase J. Neuzner J. Germing A. Mark B. Assmus B. Tonn T. Dimmeler S. Zeiher AM. Improved clinical outcome after intracoronary administration of bone-marrow-derived progenitor cells in acute myocardial infarction: final 1-year results of the REPAIR-AMI trial. Eur Heart J. 2006;27:2775–2783. doi: 10.1093/eurheartj/ehl388. [DOI] [PubMed] [Google Scholar]
- 224.Schaefer WH. Harris TM. Guengerich FP. Characterization of the enzymatic and nonenzymatic peroxidative degradation of iron porphyrins and cytochrome P-450 heme. Biochemistry. 1985;24:3254–3263. doi: 10.1021/bi00334a027. [DOI] [PubMed] [Google Scholar]
- 225.Schwentker A. Billiar TR. Inducible nitric oxide synthase: from cloning to therapeutic applications. World J Surg. 2002;26:772–778. doi: 10.1007/s00268-002-4051-7. [DOI] [PubMed] [Google Scholar]
- 226.Schwer CI. Mutschler M. Stoll P. Goebel U. Humar M. Hoetzel A. Schmidt R. Carbon monoxide releasing molecule-2 inhibits pancreatic stellate cell proliferation by activating p38 mitogen-activated protein kinase/heme oxygenase-1 signaling. Mol Pharmacol. 2010;77:660–669. doi: 10.1124/mol.109.059519. [DOI] [PubMed] [Google Scholar]
- 227.Seldon MP. Silva G. Pejanovic N. Larsen R. Gregoire IP. Filipe J. Anrather J. Soares MP. Heme oxygenase-1 inhibits the expression of adhesion molecules associated with endothelial cell activation via inhibition of NF-κB RelA phosphorylation at serine 276. J Immunol. 2007;179:7840–7851. doi: 10.4049/jimmunol.179.11.7840. [DOI] [PubMed] [Google Scholar]
- 228.Sellers KW. Katovich MJ. Gelband CH. Raizada MK. Gene therapy to control hypertension: current studies and future perspectives. Am J Med Sci. 2001;322:1–6. doi: 10.1097/00000441-200107000-00001. [DOI] [PubMed] [Google Scholar]
- 229.Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- 230.Serbina NV. Pamer EG. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]
- 231.Seta F. Bellner L. Rezzani R. Regan RF. Dunn MW. Abraham NG. Gronert K. Laniado-Schwartzman M. Heme oxygenase-2 is a critical determinant for execution of an acute inflammatory and reparative response. Am J Pathol. 2006;169:1612–1623. doi: 10.2353/ajpath.2006.060555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Shakeri-Manesch S. Zeyda M. Huber J. Ludvik B. Prager G. Stulnig TM. Diminished upregulation of visceral adipose heme oxygenase-1 correlates with waist-to-hip ratio and insulin resistance. Int J Obes (Lond) 2009;33:1257–1264. doi: 10.1038/ijo.2009.160. [DOI] [PubMed] [Google Scholar]
- 233.Shibahara S. The heme oxygenase dilemma in cellular homeostasis: new insights for the feedback regulation of heme catabolism. Tohoku J Exp Med. 2003;200:167–186. doi: 10.1620/tjem.200.167. [DOI] [PubMed] [Google Scholar]
- 234.Shibuya M. Vascular endothelial growth factor-dependent and -independent regulation of angiogenesis. BMB Rep. 2008;41:278–286. doi: 10.5483/bmbrep.2008.41.4.278. [DOI] [PubMed] [Google Scholar]
- 235.Siner JM. Jiang G. Cohen ZI. Shan P. Zhang X. Lee CG. Elias JA. Lee PJ. VEGF-induced heme oxygenase-1 confers cytoprotection from lethal hyperoxia in vivo. FASEB J. 2007;21:1422–1432. doi: 10.1096/fj.06-6661com. [DOI] [PubMed] [Google Scholar]
- 236.Soares MP. Lin Y. Anrather J. Csizmadia E. Takigami K. Sato K. Grey ST. Colvin RB. Choi AM. Poss KD. Bach FH. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat Med. 1998;4:1073–1077. doi: 10.1038/2063. [DOI] [PubMed] [Google Scholar]
- 237.Soares MP. Seldon MP. Gregoire IP. Vassilevskaia T. Berberat PO. Yu J. Tsui TY. Bach FH. Heme oxygenase-1 modulates the expression of adhesion molecules associated with endothelial cell activation. J Immunol. 2004;172:3553–3563. doi: 10.4049/jimmunol.172.6.3553. [DOI] [PubMed] [Google Scholar]
- 238.Sodhi K. Inoue K. Gotlinger KH. Canestraro M. Vanella L. Kim DH. Manthati VL. Koduru SR. Falck JR. Schwartzman ML. Abraham NG. Epoxyeicosatrienoic acid agonist rescues the metabolic syndrome phenotype of HO-2-null mice. J Pharmacol Exp Ther. 2009;331:906–916. doi: 10.1124/jpet.109.157545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Song H. Bergstrasser C. Rafat N. Höger S. Schmidt M. Endres N. Goebeler M. Hillebrands JL. Brigelius-Flohé R. Banning A. Beck G. Loesel R. Yard BA. The carbon monoxide releasing molecule (CORM-3) inhibits expression of vascular cell adhesion molecule-1 and E-selectin independently of haem oxygenase-1 expression. Br J Pharmacol. 2009;157:769–780. doi: 10.1111/j.1476-5381.2009.00215.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Song R. Mahidhara RS. Liu F. Ning W. Otterbein LE. Choi AM. Carbon monoxide inhibits human airway smooth muscle cell proliferation via mitogen-activated protein kinase pathway. Am J Respir Cell Mol Biol. 2002;27:603–610. doi: 10.1165/rcmb.4851. [DOI] [PubMed] [Google Scholar]
- 241.Sonveaux P. Jordan BF. Gallez B. Feron O. Nitric oxide delivery to cancer: why and how? Eur J Cancer. 2009;45:1352–1369. doi: 10.1016/j.ejca.2008.12.018. [DOI] [PubMed] [Google Scholar]
- 242.Sorrenti V. Mazza F. Campisi A. Di Giacomo C. Acquaviva R. Vanella L. Galvano F. Heme oxygenase induction by cyanidin-3-O-beta-glucoside in cultured human endothelial cells. Mol Nutr Food Res. 2007;51:580–586. doi: 10.1002/mnfr.200600204. [DOI] [PubMed] [Google Scholar]
- 243.Srisook K. Han SS. Choi HS. Li MH. Ueda H. Kim C. Cha YN. CO from enhanced HO activity or from CORM-2 inhibits both O2- and NO production and downregulates HO-1 expression in LPS-stimulated macrophages. Biochem Pharmacol. 2006;71:307–318. doi: 10.1016/j.bcp.2005.10.042. [DOI] [PubMed] [Google Scholar]
- 244.Stec DE. Drummond HA. Vera T. Role of carbon monoxide in blood pressure regulation. Hypertension. 2008;51:597–604. doi: 10.1161/HYPERTENSIONAHA.107.097154. [DOI] [PubMed] [Google Scholar]
- 245.Stocker R. Antioxidant activities of bile pigments. Antioxid Redox Signal. 6:841–849. doi: 10.1089/ars.2004.6.841. [DOI] [PubMed] [Google Scholar]
- 246.Stocker R. Perrella MA. Heme oxygenase-1: a novel drug target for atherosclerotic diseases? Circulation. 2006;114:2178–2189. doi: 10.1161/CIRCULATIONAHA.105.598698. [DOI] [PubMed] [Google Scholar]
- 247.Stone JR. Marletta MA. Soluble guanylate cyclase from bovine lung: activation with nitric oxide and carbon monoxide and spectral characterization of the ferrous and ferric states. Biochemistry. 1994;33:5636–5640. doi: 10.1021/bi00184a036. [DOI] [PubMed] [Google Scholar]
- 248.Sun X. Pi J. Liu W. Hudson LG. Liu KJ. Feng C. Induction of heme oxygenase 1 by arsenite inhibits cytokine-induced monocyte adhesion to human endothelial cells. Toxicol Appl Pharmacol. 2009;236:202–209. doi: 10.1016/j.taap.2009.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Sussan TE. Jun J. Thimmulappa R. Bedja D. Antero M. Gabrielson KL. Polotsky VY. Biswal S. Disruption of Nrf2, a key inducer of antioxidant defenses, attenuates ApoE-mediated atherosclerosis in mice. PLoS One. 2008;3:e3791. doi: 10.1371/journal.pone.0003791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Suzuki M. Iso-o N. Takeshita S. Tsukamoto K. Mori I. Sato T. Ohno M. Nagai R. Ishizaka N. Facilitated angiogenesis induced by heme oxygenase-1 gene transfer in a rat model of hindlimb ischemia. Biochem Biophys Res Commun. 2003;302:138–143. doi: 10.1016/s0006-291x(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 251.Sylvester JT. McGowan C. The effects of agents that bind to cytochrome P-450 on hypoxic pulmonary vasoconstriction. Circ Res. 1978;43:429–437. doi: 10.1161/01.res.43.3.429. [DOI] [PubMed] [Google Scholar]
- 252.Szabo ME. Gallyas E. Bak I. Rakotovao A. Boucher F. de Leiris J. Nagy N. Varga E. Tosaki A. Heme oxygenase-1-related carbon monoxide and flavonoids in ischemic/reperfused rat retina. Invest Ophthalmol Vis Sci. 2004;45:3727–3732. doi: 10.1167/iovs.03-1324. [DOI] [PubMed] [Google Scholar]
- 253.Taille C. Almolki A. Benhamed M. Zedda C. Megret J. Berger P. Leseche G. Fadel E. Yamaguchi T. Marthan R. Aubier M. Boczkowski J. Heme oxygenase inhibits human airway smooth muscle proliferation via a bilirubin-dependent modulation of ERK1/2 phosphorylation. J Biol Chem. 2003;278:27160–27168. doi: 10.1074/jbc.M300364200. [DOI] [PubMed] [Google Scholar]
- 254.Taille C. El-Benna J. Lanone S. Boczkowski J. Motterlini R. Mitochondrial respiratory chain and NAD(P)H oxidase are targets for the antiproliferative effect of carbon monoxide in human airway smooth muscle. J Biol Chem. 2005;280:25350–25360. doi: 10.1074/jbc.M503512200. [DOI] [PubMed] [Google Scholar]
- 255.Tanaka M. Fukui M. Tomiyasu K. Akabame S. Nakano K. Hasegawa G. Oda Y. Nakamura N. Low serum bilirubin concentration is associated with coronary artery calcification (CAC) Atherosclerosis. 2009;206:287–291. doi: 10.1016/j.atherosclerosis.2009.02.010. [DOI] [PubMed] [Google Scholar]
- 256.Tang LM. Wang YP. Wang K. Pu LY. Zhang F. Li XC. Kong LB. Sun BC. Li GQ. Wang XH. Exogenous biliverdin ameliorates ischemia-reperfusion injury in small-for-size rat liver grafts. Transplant Proc. 2007;39:1338–1344. doi: 10.1016/j.transproceed.2006.11.032. [DOI] [PubMed] [Google Scholar]
- 257.Tang YL. Qian K. Zhang YC. Shen L. Phillips MI. A vigilant, hypoxia-regulated heme oxygenase-1 gene vector in the heart limits cardiac injury after ischemia-reperfusion in vivo. J Cardiovasc Pharmacol Ther. 2005;10:251–263. doi: 10.1177/107424840501000405. [DOI] [PubMed] [Google Scholar]
- 258.Tang YL. Tang Y. Zhang YC. Qian K. Shen L. Phillips MI. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 259.Tedgui A. Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol Rev. 2006;86:515–581. doi: 10.1152/physrev.00024.2005. [DOI] [PubMed] [Google Scholar]
- 260.Terry CM. Clikeman JA. Hoidal JR. Callahan KS. Effect of tumor necrosis factor-alpha and interleukin-1 alpha on heme oxygenase-1 expression in human endothelial cells. Am J Physiol. 1998;274:H883–H891. doi: 10.1152/ajpheart.1998.274.3.H883. [DOI] [PubMed] [Google Scholar]
- 261.Thom SR. Fisher D. Xu YA. Notarfrancesco K. Ishiropoulos H. Adaptive responses and apoptosis in cells exposed to carbon monoxide. Proc Natl Acad Sci USA. 2000;97:1305–1310. doi: 10.1073/pnas.97.3.1305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Thorup C. Jones CL. Gross SS. Moore LC. Goligorsky MS. Carbon monoxide induces vasodilation and nitric oxide release but suppresses endothelial NOS. Am J Physiol. 1999;277:F882–F889. doi: 10.1152/ajprenal.1999.277.6.F882. [DOI] [PubMed] [Google Scholar]
- 263.Tobiasch E. Günther L. Bach FH. Heme oxygenase-1 protects pancreatic beta cells from apoptosis caused by various stimuli. J Invest Med. 2001;49:566–571. doi: 10.2310/6650.2001.33721. [DOI] [PubMed] [Google Scholar]
- 264.Tongers J. Knapp JM. Korf M. Kempf T. Limbourg A. Limbourg FP. Li Z. Fraccarollo D. Bauersachs J. Han X. Drexler H. Fiedler B. Wollert KC. Haeme oxygenase promotes progenitor cell mobilization, neovascularization, and functional recovery after critical hindlimb ischaemia in mice. Cardiovasc Res. 2008;78:294–300. doi: 10.1093/cvr/cvm107. [DOI] [PubMed] [Google Scholar]
- 265.Tranter M. Jones WK. Anti-inflammatory effects of HO-1 activity in vascular endothelial cells, commentary on “Carbon monoxide donors or heme oxygenase (HO-1) overexpression blocks interleukin-18-mediated NF-kappaB-PTEN-dependent human cardiac endothelial cell death.”. Free Radic Biol Med. 2008;44:261–263. doi: 10.1016/j.freeradbiomed.2007.10.051. [DOI] [PubMed] [Google Scholar]
- 266.Tsou CL. Peters W. Si Y. Slaymaker S. Aslanian AM. Weisberg SP. Mack M. Charo IF. Critical roles for CCR2 and MCP-3 in monocyte mobilization from bone marrow and recruitment to inflammatory sites. J Clin Investig. 2007;117:902–909. doi: 10.1172/JCI29919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Tulis DA. Durante W. Liu X. Evans AJ. Peyton KJ. Schafer AI. Adenovirus-mediated heme oxygenase-1 gene delivery inhibits injury-induced vascular neointima formation. Circulation. 2001;104:2710–2715. doi: 10.1161/hc4701.099585. [DOI] [PubMed] [Google Scholar]
- 268.Vachharajani TJ. Work J. Issekutz AC. Granger DN. Heme oxygenase modulates selectin expression in different regional vascular beds. Am J Physiol. 2000;278:H1613–H1617. doi: 10.1152/ajpheart.2000.278.5.H1613. [DOI] [PubMed] [Google Scholar]
- 269.Van Belle E. Bauters C. Asahara T. Isner JM. Endothelial regrowth after arterial injury: From vascular repair to therapeutics. Cardiovasc Res. 1998;38:54–68. doi: 10.1016/s0008-6363(97)00326-x. [DOI] [PubMed] [Google Scholar]
- 270.Visner GA. Lu F. Zhou H. Liu J. Kazemfar K. Agarwal A. Rapamycin induces heme oxygenase-1 in human pulmonary vascular cells: implications in the antiproliferative response to rapamycin. Circulation. 2003;107:911–916. doi: 10.1161/01.cir.0000048191.75585.60. [DOI] [PubMed] [Google Scholar]
- 271.Voll RE. Herrmann M. Roth EA. Stach C. Kalden JR. Girkontaite I. Immunosuppressive effects of apoptotic cells. Nature. 1997;390:350–351. doi: 10.1038/37022. [DOI] [PubMed] [Google Scholar]
- 272.Wagener FADTG. Da Silva J-L. Farley T. de Witte T. Kappas A. Abraham NG. Differential effects of heme oxygenase isoforms on heme-mediated endothelial ICAM-1 expression. J Pharmacol Exp Ther. 1999;291:416–423. [PubMed] [Google Scholar]
- 273.Wagener FADTG. Feldman E. Witte de T. Abraham NG. Heme induces the expression of adhesion molecules ICAM-1, VCAM-1 and E selectin in vascular endothelial cells. Proc Soc Exp Biol Med. 1997;216:456–463. doi: 10.3181/00379727-216-44197. [DOI] [PubMed] [Google Scholar]
- 274.Wang LJ. Lee TS. Lee FY. Pai RC. Chau LY. Expression of heme oxygenase-1 in atherosclerotic lesions. Am J Pathol. 1998;152:711–720. [PMC free article] [PubMed] [Google Scholar]
- 275.Wang R. Wu L. The chemical modification of KCa channels by carbon monoxide in vascular smooth muscle cells. J Biol Chem. 1997;272:8222–8226. doi: 10.1074/jbc.272.13.8222. [DOI] [PubMed] [Google Scholar]
- 276.Wang R. Wu L. Wang Z. The direct effect of carbon monoxide on KCa channels in vascular smooth muscle cells. Pflugers Arch. 1997;434:285–291. doi: 10.1007/s004240050398. [DOI] [PubMed] [Google Scholar]
- 277.Wang X. Wang Y. Kim HP. Nakahira K. Ryter SW. Choi AM. Carbon monoxide protects against hyperoxia-induced endothelial cell apoptosis by inhibiting reactive oxygen species formation. J Biol Chem. 2007;282:1718–1726. doi: 10.1074/jbc.M607610200. [DOI] [PubMed] [Google Scholar]
- 278.Wang XM. Kim HP. Nakahira K. Ryter SW. Choi AM. The heme oxygenase-1/carbon monoxide pathway suppresses TLR4 signaling by regulating the interaction of TLR4 with caveolin-1. J Immunol. 2009;182:3809–3818. doi: 10.4049/jimmunol.0712437. [DOI] [PubMed] [Google Scholar]
- 279.Was H. Cichon T. Smolarczyk R. Rudnicka D. Stopa M. Chevalier C. Leger JJ. Lackowska B. Grochot A. Bojkowska K. Ratajska A. Kieda C. Szala S. Dulak J. Jozkowicz A. Overexpression of heme oxygenase-1 in murine melanoma: increased proliferation and viability of tumor cells, decreased survival of mice. Am J Pathol. 2006;169:2181–2198. doi: 10.2353/ajpath.2006.051365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Watson T. Goon PK. Lip GY. Endothelial progenitor cells, endothelial dysfunction, inflammation, and oxidative stress in hypertension. Antioxid Redox Signal. 2008;10:1079–1088. doi: 10.1089/ars.2007.1998. [DOI] [PubMed] [Google Scholar]
- 281.Weis N. Weigert A. von Knethen A. Brone B. Heme oxygenase-1 contributes to an alternative macrophage activation profile induced by apoptotic cell supernatants. Mol Biol Cell. 2009;20:1280–1288. doi: 10.1091/mbc.E08-10-1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Whiteman M. Li L. Kostetski I. Chu SH. Siau JL. Bhatia M. Moore PK. Evidence for the formation of a novel nitrosothiol from the gaseous mediators nitric oxide and hydrogen sulphide. Biochem Biophys Res Commun. 2006;343:303–310. doi: 10.1016/j.bbrc.2006.02.154. [DOI] [PubMed] [Google Scholar]
- 283.Wilson HM. Walbaum D. Rees AJ. Macrophages and the kidney. Curr Opin Nephrol Hypertens. 2004;13:285–290. doi: 10.1097/00041552-200405000-00004. [DOI] [PubMed] [Google Scholar]
- 284.Wu BJ. Kathir K. Witting PK. Beck K. Choy K. Li C. Croft KD. Mori TA. Tanous D. Adams MR. Lau AK. Stocker R. Antioxidants protect from atherosclerosis by a heme oxygenase-1 pathway that is independent of free radical scavenging. J Exp Med. 2006;203:1117–1127. doi: 10.1084/jem.20052321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wu BJ. Midwinter RG. Cassano C. Beck K. Wang Y. Changsiri D. Gamble JR. Stocker R. Heme oxygenase-1 increases endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2009;29:1537–1542. doi: 10.1161/ATVBAHA.109.184713. [DOI] [PubMed] [Google Scholar]
- 286.Wu L. Juurlink BH. Increased methylglyoxal and oxidative stress in hypertensive rat vascular smooth muscle cells. Hypertension. 2002;39:809–814. doi: 10.1161/hy0302.105207. [DOI] [PubMed] [Google Scholar]
- 287.Yachie A. Niida Y. Wada T. Igarashi N. Kaneda H. Toma T. Ohta K. Kasahara Y. Koizumi S. Oxidative stress causes enhanced endothelial cell injury in human heme oxygenase-1 deficiency. J Clin Invest. 1999;103:129–135. doi: 10.1172/JCI4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Yachie A. Toma T. Mizuno K. Okamoto H. Shimura S. Ohta K. Kasahara Y. Koizumi S. Heme oxygenase-1 production by peripheral blood monocytes during acute inflammatory illnesses of children. Exp Biol Med. 2003;228:550–556. doi: 10.1177/15353702-0322805-26. [DOI] [PubMed] [Google Scholar]
- 289.Yamaguchi J. Kusano KF. Masuo O. Kawamoto A. Silver M. Murasawa S. Bosch-Marce M. Masuda H. Losordo DW. Isner JM. Asahara T. Stromal cell-derived factor-1 effects on ex vivo expanded endothelial progenitor cell recruitment for ischemic neovascularization. Circulation. 2003;107:1322–1328. doi: 10.1161/01.cir.0000055313.77510.22. [DOI] [PubMed] [Google Scholar]
- 290.Yamaguchi M. Sato H. Bannai S. Induction of stress proteins in mouse peritoneal macrophages by oxidized low-density lipoprotein. Biochem Biophys Res Commun. 1993;193:1198–1201. doi: 10.1006/bbrc.1993.1752. [DOI] [PubMed] [Google Scholar]
- 291.Yamaya M. Sekizawa K. Ishizuka S. Monma M. Sasaki H. Exhaled carbon monoxide levels during treatment of acute asthma. Eur Respir J. 1999;13:757–760. doi: 10.1034/j.1399-3003.1999.13d10.x. [DOI] [PubMed] [Google Scholar]
- 292.Yee EL. Pitt BR. Billiar TR. Kim YM. Effect of nitric oxide on heme metabolism in pulmonary artery endothelial cells. Am J Physiol. 1996;271:L512–L518. doi: 10.1152/ajplung.1996.271.4.L512. [DOI] [PubMed] [Google Scholar]
- 293.Yet SF. Layne MD. Liu X. Chen YH. Ith B. Sibinga NE. Perrella MA. Absence of heme oxygenase-1 exacerbates atherosclerotic lesion formation and vascular remodeling. FASEB J. 2003;17:1759–1761. doi: 10.1096/fj.03-0187fje. [DOI] [PubMed] [Google Scholar]
- 294.Yet SF. Perrella MA. Layne MD. Hsieh CM. Maemura K. Kobzik L. Wiesel P. Christou H. Kourembanas S. Lee ME. Hypoxia induces severe right ventricular dilatation and infarction in heme oxygenase-1 null mice. J Clin Invest. 1999;103:R23–R29. doi: 10.1172/JCI6163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Yoshida T. Maulik N. Ho YS. Alam J. Das DK. H(mox-1) constitutes an adaptive response to effect antioxidant cardioprotection: A study with transgenic mice heterozygous for targeted disruption of the Heme oxygenase-1 gene. Circulation. 2001;103:1695–1701. doi: 10.1161/01.cir.103.12.1695. [DOI] [PubMed] [Google Scholar]
- 296.Yoshinaga T. Sassa S. Kappas A. A comparative study of heme degradation by NADPH-cytochrome c reductase alone and by the complete heme oxygenase system. Distinctive aspects of heme degradation by NADPH-cytochrome c reductase. J Biol Chem. 1982;257:7794–7802. [PubMed] [Google Scholar]
- 297.Zabalgoitia M. Colston JT. Reddy SV. Holt JW. Regan RF. Stec DE. Rimoldi JM. Valente AJ. Chandrasekar B. Carbon monoxide donors or heme oxygenase (HO-1) overexpression blocks interleukin-18-mediated NF-κB-PTEN-dependent human cardiac endothelial cell death. Free Radic Biol Med. 2008;44:284–298. doi: 10.1016/j.freeradbiomed.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Zhang S. Lu S. Ge J. Guo J. Chen P. Li T. Zhang P. Jia Z. Ma K. Liu Y. Zhou C. Li L. Increased heme oxygenase-1 expression in infarcted rat hearts following human bone marrow mesenchymal cell transplantation. Microvasc Res. 2005;69:64–70. doi: 10.1016/j.mvr.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 299.Zhang X. Shan P. Alam J. Fu XY. Lee PJ. Carbon monoxide differentially modulates STAT1 and STAT3 and inhibits apoptosis via a phosphatidylinositol 3-kinase/Akt and p38 kinase-dependent STAT3 pathway during anoxia-reoxygenation injury. J Biol Chem. 2005;280:8714–9721. doi: 10.1074/jbc.M408092200. [DOI] [PubMed] [Google Scholar]
- 300.Zuckerbraun BS. Chin BY. Bilban M. d'Avila JC. Rao J. Billiar TR. Otterbein LE. Carbon monoxide signals via inhibition of cytochrome c oxidase and generation of mitochondrial reactive oxygen species. FASEB J. 2007;21:1099–1106. doi: 10.1096/fj.06-6644com. [DOI] [PubMed] [Google Scholar]