Abstract
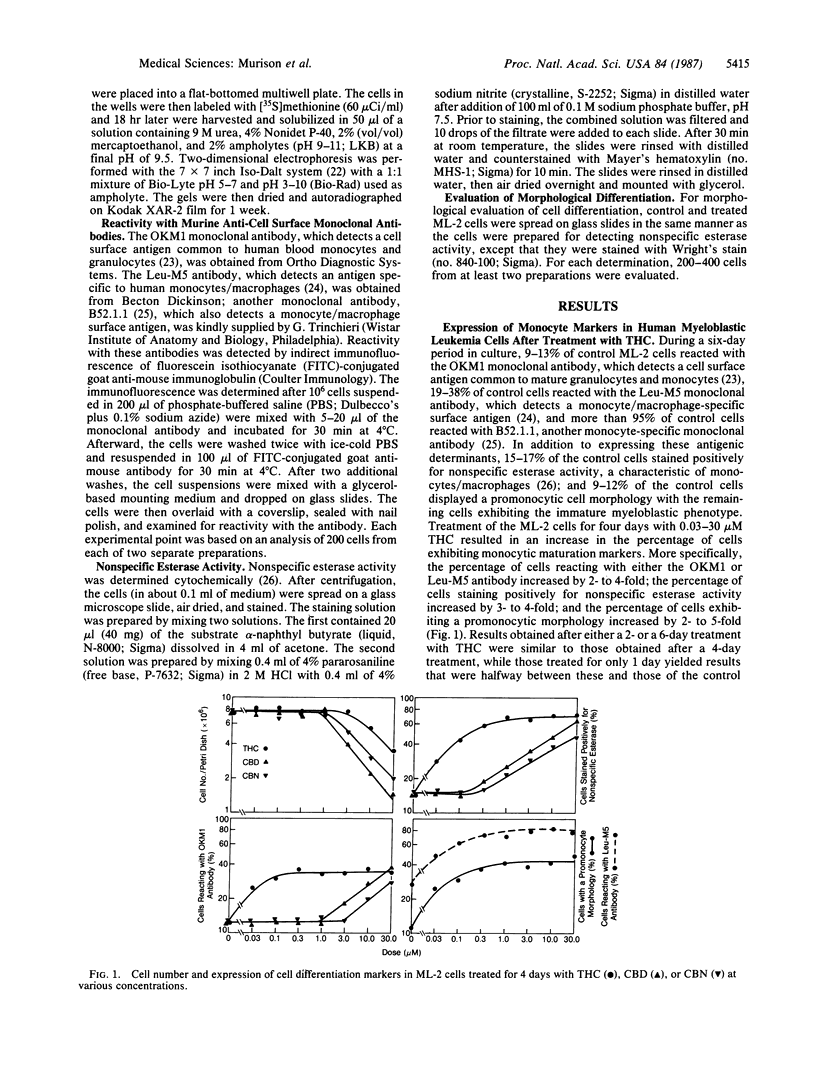
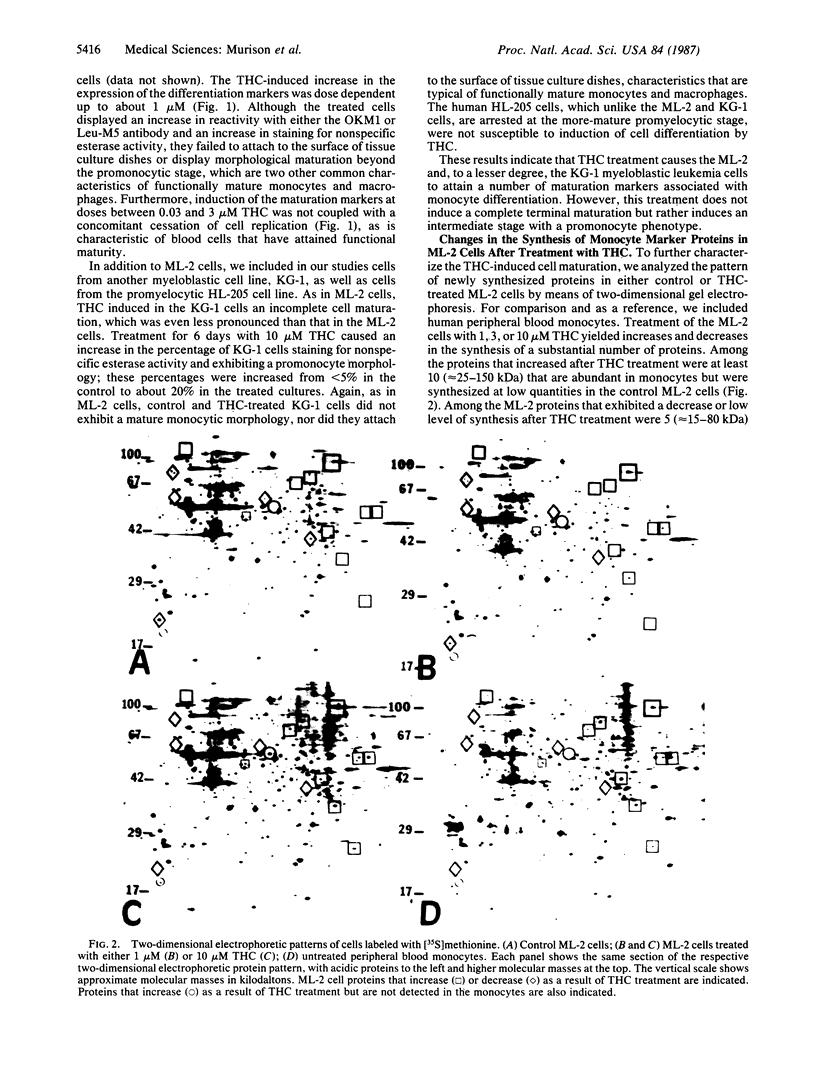
Monocyte maturation markers were induced in cultured human myeloblastic ML-2 leukemia cells after treatment for 1-6 days with 0.03-30 microM delta 9-tetrahydrocannabinol (THC), the major psychoactive component of marijuana. After a 2-day or longer treatment, 2- to 5-fold increases were found in the percentages of cells exhibiting reactivity with either the murine OKM1 monoclonal antibody or the Leu-M5 monoclonal antibody, staining positively for nonspecific esterase activity, and displaying a promonocyte morphology. The increases in these differentiation markers after treatment with 0.03-1 microM THC were dose dependent. At this dose range, THC did not cause an inhibition of cell growth. The THC-induced cell maturation was also characterized by specific changes in the patterns of newly synthesized proteins. Pronounced among these changes was an increase in the synthesis of at least 10 proteins that are found abundantly in monocytes. The THC-induced differentiation did not, however, result in cells with a highly developed mature monocyte phenotype; the THC-treated cells failed to exhibit other monocyte markers such as attachment to the surface of tissue culture dishes or morphological maturation beyond the promonocyte stage. However, treatment of these "incompletely" matured cells with either phorbol 12-myristate 13-acetate or 1 alpha,25-dihydroxycholecalciferol, which are inducers of differentiation in myeloid leukemia cells (including ML-2 cells), produced cells with a mature monocyte morphology. Two other cannabinoids, cannabidiol and cannabinol, which were more cytotoxic than THC at comparable doses, also caused an increase in the expression of maturation markers, but at doses higher than those required for THC. The ML-2 cell system described here may be a useful tool for deciphering critical biochemical events that lead to the cannabinoid-induced "incomplete" cell differentiation of ML-2 cells and other related cell types. Findings obtained from this system may have important implications for studies of cannabinoid effects on normal human bone-marrow progenitor cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agurell S., Halldin M., Lindgren J. E., Ohlsson A., Widman M., Gillespie H., Hollister L. Pharmacokinetics and metabolism of delta 1-tetrahydrocannabinol and other cannabinoids with emphasis on man. Pharmacol Rev. 1986 Mar;38(1):21–43. [PubMed] [Google Scholar]
- Anderson N. G., Anderson N. L. Analytical techniques for cell fractions. XXI. Two-dimensional analysis of serum and tissue proteins: multiple isoelectric focusing. Anal Biochem. 1978 Apr;85(2):331–340. doi: 10.1016/0003-2697(78)90229-4. [DOI] [PubMed] [Google Scholar]
- Baczynsky W. O., Zimmerman A. M. Effects of delta 9-tetrahydrocannabinol, cannabinol and cannabidiol on the immune system in mice. II. In vitro investigation using cultured mouse splenocytes. Pharmacology. 1983;26(1):12–19. doi: 10.1159/000137764. [DOI] [PubMed] [Google Scholar]
- Breitman T. R., Selonick S. E., Collins S. J. Induction of differentiation of the human promyelocytic leukemia cell line (HL-60) by retinoic acid. Proc Natl Acad Sci U S A. 1980 May;77(5):2936–2940. doi: 10.1073/pnas.77.5.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins S. J., Ruscetti F. W., Gallagher R. E., Gallo R. C. Terminal differentiation of human promyelocytic leukemia cells induced by dimethyl sulfoxide and other polar compounds. Proc Natl Acad Sci U S A. 1978 May;75(5):2458–2462. doi: 10.1073/pnas.75.5.2458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foon K. A., Schroff R. W., Gale R. P. Surface markers on leukemia and lymphoma cells: recent advances. Blood. 1982 Jul;60(1):1–19. [PubMed] [Google Scholar]
- Gemmell M. A., Anderson N. L. Lymphocyte, monocyte, and granulocyte proteins compared by use of two-dimensional electrophoresis. Clin Chem. 1982 Apr;28(4 Pt 2):1062–1066. [PubMed] [Google Scholar]
- Gupta S., Grieco M. H., Cushman P., Jr Impairment of rosette-forming T lymphocytes in chronic marihuana smokers. N Engl J Med. 1974 Oct 24;291(17):874–877. doi: 10.1056/NEJM197410242911704. [DOI] [PubMed] [Google Scholar]
- Hemmi H., Breitman T. R. Combinations of recombinant human interferons and retinoic acid synergistically induce differentiation of the human promyelocytic leukemia cell line HL-60. Blood. 1987 Feb;69(2):501–507. [PubMed] [Google Scholar]
- Hollister L. E. Health aspects of cannabis. Pharmacol Rev. 1986 Mar;38(1):1–20. [PubMed] [Google Scholar]
- Homma Y., Henning-Chubb C. B., Huberman E. Translocation of protein kinase C in human leukemia cells susceptible or resistant to differentiation induced by phorbol 12-myristate 13-acetate. Proc Natl Acad Sci U S A. 1986 Oct;83(19):7316–7319. doi: 10.1073/pnas.83.19.7316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huberman E., Callaham M. F. Induction of terminal differentiation in human promyelocytic leukemia cells by tumor-promoting agents. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1293–1297. doi: 10.1073/pnas.76.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koeffler H. P., Bar-Eli M., Territo M. C. Phorbol ester effect on differentiation of human myeloid leukemia cell lines blocked at different stages of maturation. Cancer Res. 1981 Mar;41(3):919–926. [PubMed] [Google Scholar]
- Li C. Y., Lam K. W., Yam L. T. Esterases in human leukocytes. J Histochem Cytochem. 1973 Jan;21(1):1–12. doi: 10.1177/21.1.1. [DOI] [PubMed] [Google Scholar]
- MECHOULAM R., GAONI Y. A TOTAL SYNTHESIS OF DL-DELTA-1-TETRAHYDROCANNABINOL, THE ACTIVE CONSTITUENT OF HASHISH. J Am Chem Soc. 1965 Jul 20;87:3273–3275. doi: 10.1021/ja01092a065. [DOI] [PubMed] [Google Scholar]
- Murao S. I., Fukumoto Y., Katayama M., Maeda H., Sugiyama T., Huberman E. Recombinant gamma-interferon and lipopolysaccharide enhance 1,25-dihydroxyvitamin D3-induced cell differentiation in human promyelocytic leukemia (HL-60) cells. Jpn J Cancer Res. 1985 Jul;76(7):596–602. [PubMed] [Google Scholar]
- Murao S., Epstein A. L., Clevenger C. V., Huberman E. Expression of maturation-specific nuclear antigens in differentiating human myeloid leukemia cells. Cancer Res. 1985 Feb;45(2):791–795. [PubMed] [Google Scholar]
- Nye J. S., Seltzman H. H., Pitt C. G., Snyder S. H. High-affinity cannabinoid binding sites in brain membranes labeled with [3H]-5'-trimethylammonium delta 8-tetrahydrocannabinol. J Pharmacol Exp Ther. 1985 Sep;234(3):784–791. [PubMed] [Google Scholar]
- Rovera G., Santoli D., Damsky C. Human promyelocytic leukemia cells in culture differentiate into macrophage-like cells when treated with a phorbol diester. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2779–2783. doi: 10.1073/pnas.76.6.2779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs L. Control of normal cell differentiation and the phenotypic reversion of malignancy in myeloid leukaemia. Nature. 1978 Aug 10;274(5671):535–539. doi: 10.1038/274535a0. [DOI] [PubMed] [Google Scholar]
- Schwarting R., Stein H., Wang C. Y. The monoclonal antibodies alpha S-HCL 1 (alpha Leu-14) and alpha S-HCL 3 (alpha Leu-M5) allow the diagnosis of hairy cell leukemia. Blood. 1985 Apr;65(4):974–983. [PubMed] [Google Scholar]
- Silverman A. Y., Darnell B. J., Montiel M. M., Smith C. G., Asch R. H. Response of rhesus monkey lymphocytes to short-term administration of THC. Life Sci. 1982 Jan 4;30(1):107–115. doi: 10.1016/0024-3205(82)90642-7. [DOI] [PubMed] [Google Scholar]
- Stöckbauer P., Gahmberg C. G., Andersson L. C. Changes in cell surface glycoproteins and antigens during differentiation of the human myeloid leukemia cell lines ML-1, ML-2, and HL-60. Cancer Res. 1985 Jun;45(6):2821–2826. [PubMed] [Google Scholar]
- Trinchieri G., Kobayashi M., Rosen M., Loudon R., Murphy M., Perussia B. Tumor necrosis factor and lymphotoxin induce differentiation of human myeloid cell lines in synergy with immune interferon. J Exp Med. 1986 Oct 1;164(4):1206–1225. doi: 10.1084/jem.164.4.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson E. S., Murphy J. C., Turner C. E. Allergenic properties of naturally occurring cannabinoids. J Pharm Sci. 1983 Aug;72(8):954–955. doi: 10.1002/jps.2600720831. [DOI] [PubMed] [Google Scholar]