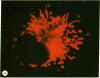
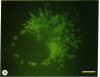
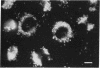
Abstract
Positively charged lipophilic compounds, such as rhodamine 123, localize in mitochondria and are selectively accumulated and retained by carcinoma cells. It has been suggested that this phenotype may be exploited for selective killing of carcinoma cells by lipophilic cations. Here we report that doubly positively charged dequalinium, which has been used for 30 years as an antimicrobial agent in over-the-counter mouthwashes, lozenges, ointments, and paints, exhibits significant anticarcinoma activity. Dequalinium is more effective than seven of eight established anticancer drugs in prolonging the survival of mice with intraperitoneally implanted mouse bladder carcinoma MB49. Dequalinium also inhibits the growth of subcutaneously implanted human colon carcinoma CX-1 in nude mice and recurrent rat colon carcinoma W163 in rats. Lipophilic cationic compounds, such as dequalinium, could comprise a unique class of anticarcinoma agents.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attwood D., Natarajan R. Micellar properties and surface activity of some bolaform drugs in aqueous solution. J Pharm Pharmacol. 1980 Jul;32(7):460–462. doi: 10.1111/j.2042-7158.1980.tb12969.x. [DOI] [PubMed] [Google Scholar]
- BABBS M., COLLIER H. O., AUSTIN W. C., POTTER M. D., TAYLOR E. P. Salts of decamethylene-bis-4-aminoquinaldinium (dequadin); a new antimicrobial agent. J Pharm Pharmacol. 1956 Feb;8(2):110–119. doi: 10.1111/j.2042-7158.1956.tb12138.x. [DOI] [PubMed] [Google Scholar]
- Bernal S. D., Lampidis T. J., McIsaac R. M., Chen L. B. Anticarcinoma activity in vivo of rhodamine 123, a mitochondrial-specific dye. Science. 1983 Oct 14;222(4620):169–172. doi: 10.1126/science.6623064. [DOI] [PubMed] [Google Scholar]
- Bernal S. D., Lampidis T. J., Summerhayes I. C., Chen L. B. Rhodamine-123 selectively reduces clonal growth of carcinoma cells in vitro. Science. 1982 Dec 10;218(4577):1117–1119. doi: 10.1126/science.7146897. [DOI] [PubMed] [Google Scholar]
- Bodden W. L., Palayoor S. T., Hait W. N. Selective antimitochondrial agents inhibit calmodulin. Biochem Biophys Res Commun. 1986 Mar 13;135(2):574–582. doi: 10.1016/0006-291x(86)90032-x. [DOI] [PubMed] [Google Scholar]
- Cain B. F., Atwell G. J., Seelye R. N. Potential antitumour agents. X. Bisquaternary salts. J Med Chem. 1969 Mar;12(2):199–206. doi: 10.1021/jm00302a001. [DOI] [PubMed] [Google Scholar]
- Davis S., Weiss M. J., Wong J. R., Lampidis T. J., Chen L. B. Mitochondrial and plasma membrane potentials cause unusual accumulation and retention of rhodamine 123 by human breast adenocarcinoma-derived MCF-7 cells. J Biol Chem. 1985 Nov 5;260(25):13844–13850. [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Bockus B. J., Chen L. B. Monitoring of relative mitochondrial membrane potential in living cells by fluorescence microscopy. J Cell Biol. 1981 Mar;88(3):526–535. doi: 10.1083/jcb.88.3.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson L. V., Walsh M. L., Chen L. B. Localization of mitochondria in living cells with rhodamine 123. Proc Natl Acad Sci U S A. 1980 Feb;77(2):990–994. doi: 10.1073/pnas.77.2.990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampidis T. J., Bernal S. D., Summerhayes I. C., Chen L. B. Selective toxicity of rhodamine 123 in carcinoma cells in vitro. Cancer Res. 1983 Feb;43(2):716–720. [PubMed] [Google Scholar]
- Mikles-Robertson F., Feuerstein B., Dave C., Porter C. W. The generality of methylglyoxal bis(guanylhydrazone)-induced mitochondrial damage and the dependence of this effect on cell proliferation. Cancer Res. 1979 Jun;39(6 Pt 1):1919–1926. [PubMed] [Google Scholar]
- Modica-Napolitano J. S., Weiss M. J., Chen L. B., Aprille J. R. Rhodamine 123 inhibits bioenergetic function in isolated rat liver mitochondria. Biochem Biophys Res Commun. 1984 Feb 14;118(3):717–723. doi: 10.1016/0006-291x(84)91453-0. [DOI] [PubMed] [Google Scholar]
- Nadakavukaren K. K., Nadakavukaren J. J., Chen L. B. Increased rhodamine 123 uptake by carcinoma cells. Cancer Res. 1985 Dec;45(12 Pt 1):6093–6099. [PubMed] [Google Scholar]
- Pedersen P. L. Tumor mitochondria and the bioenergetics of cancer cells. Prog Exp Tumor Res. 1978;22:190–274. doi: 10.1159/000401202. [DOI] [PubMed] [Google Scholar]
- Steele G., Jr, Harte P. J., Rayner A. A., Corson J. M., Madara J., Munroe A. E., King V. P., Wilson R. E. The effect of adjuvant immunotherapy on tumor recurrence after segmental resection of carcinogen-induced Wistar/Furth primary bowel adenocarcinomas. J Immunol. 1982 Jan;128(1):7–10. [PubMed] [Google Scholar]
- Summerhayes I. C., Lampidis T. J., Bernal S. D., Nadakavukaren J. J., Nadakavukaren K. K., Shepherd E. L., Chen L. B. Unusual retention of rhodamine 123 by mitochondria in muscle and carcinoma cells. Proc Natl Acad Sci U S A. 1982 Sep;79(17):5292–5296. doi: 10.1073/pnas.79.17.5292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M., Song J., Wong J. R., Weiss M. J., Chen L. B. Localization of endoplasmic reticulum in living and glutaraldehyde-fixed cells with fluorescent dyes. Cell. 1984 Aug;38(1):101–108. doi: 10.1016/0092-8674(84)90530-0. [DOI] [PubMed] [Google Scholar]
- Wright R. G., Wakelin L. P., Fieldes A., Acheson R. M., Waring M. J. Effects of ring substituents and linker chains on the bifunctional intercalation of diacridines into deoxyribonucleic acid. Biochemistry. 1980 Dec 9;19(25):5825–5836. doi: 10.1021/bi00566a026. [DOI] [PubMed] [Google Scholar]