Abstract
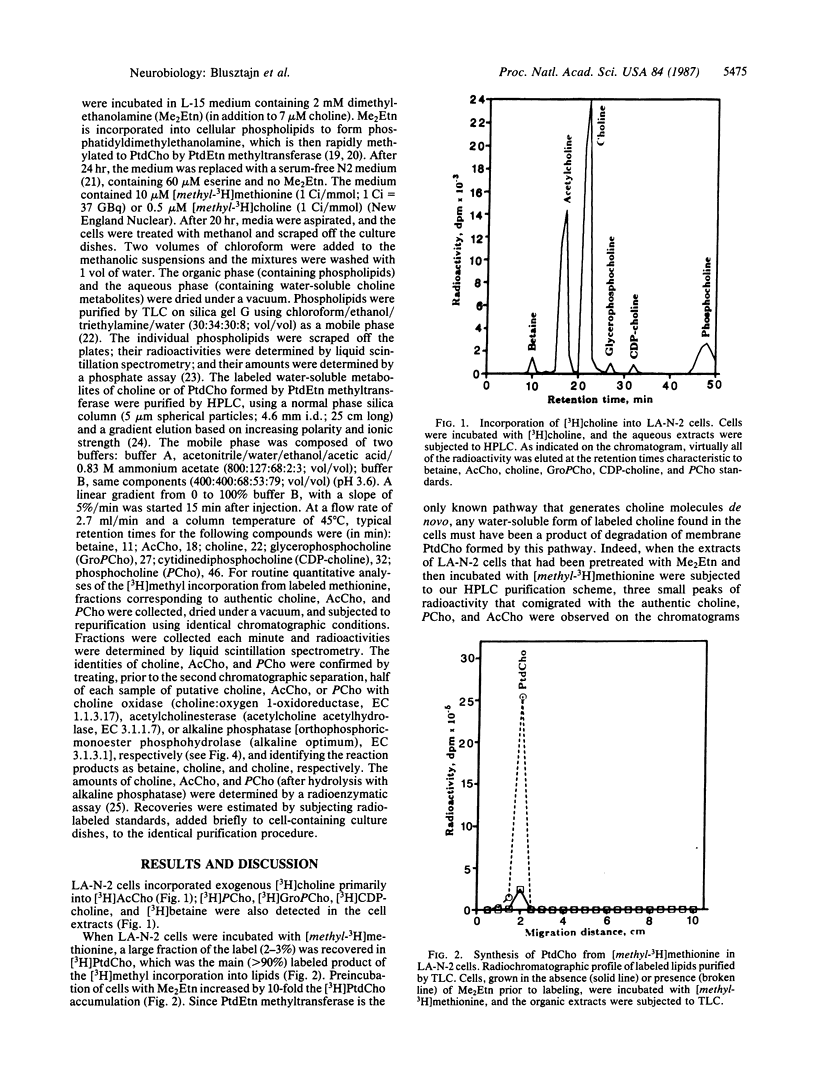
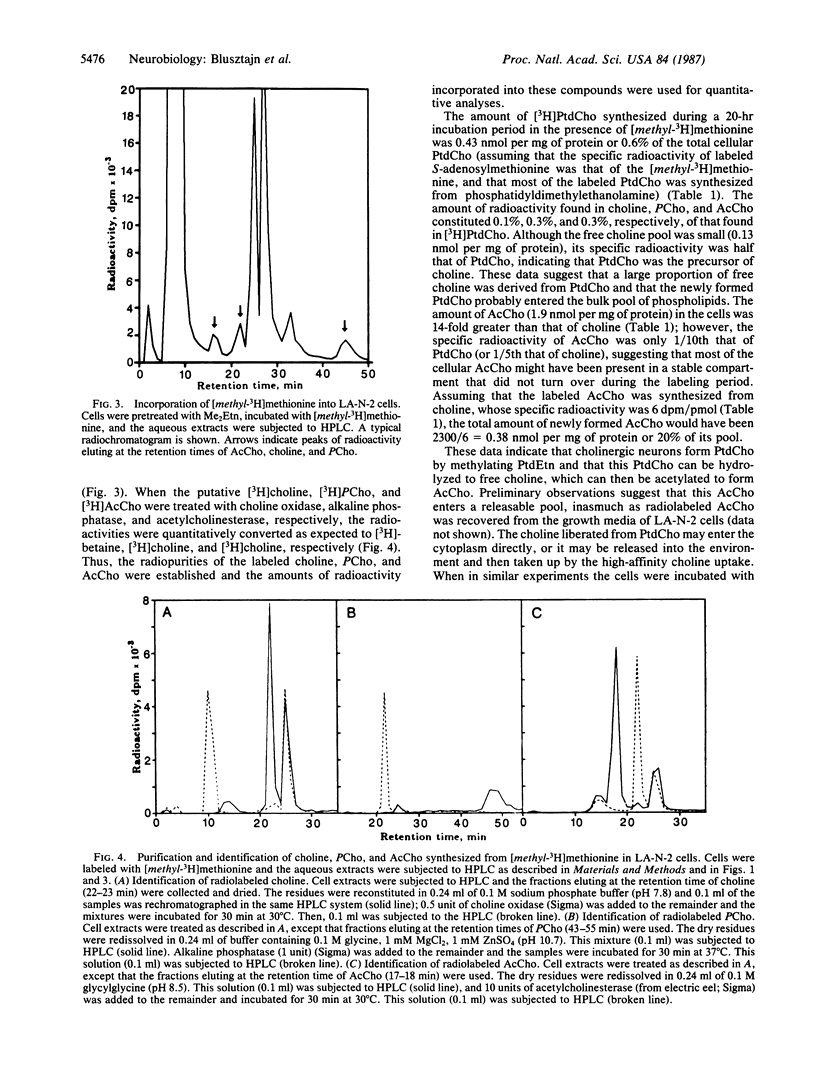
Cholinergic neurons are unique among cells since they alone utilize choline not only as a component of major membrane phospholipids, such as phosphatidylcholine (Ptd-Cho), but also as a precursor of their neurotransmitter acetylcholine (AcCho). It has been hypothesized that choline-phospholipids might serve as a storage pool of choline for AcCho synthesis. The selective vulnerability of cholinergic neurons in certain neurodegenerative diseases (e.g., Alzheimer disease, motor neuron disorders) might result from the abnormally accelerated liberation of choline (to be used as precursor of AcCho) from membrane phospholipids, resulting in altered membrane composition and function and compromised neuronal viability. However, the proposed metabolic link between membrane turnover and AcCho synthesis has been difficult to demonstrate because of the heterogeneity of the preparations used. Here we used a population of purely cholinergic cells (human neuroblastoma, LA-N-2), incubated in the presence of [methyl-3H]methionine to selectively label PtdCho synthesized by methylation of phosphatidylethanolamine, the only pathway of de novo choline synthesis. PtdCho, purified by thin-layer chromatography, contained 90% of the label incorporated into lipids, demonstrating that LA-N-2 cells contained phosphatidylethanolamine N-methyltransferase. Three peaks of radioactive material that cochromatographed with authentic Ac-Cho, choline, and phosphocholine were observed when the water-soluble metabolites of the [3H]PtdCho were purified by high-performance liquid chromatography. Their identities were ascertained by subjecting them to enzymatic modifications with acetylcholinesterase, choline oxidase, and alkaline phosphatase, respectively. The results demonstrate that AcCho can be synthesized from choline derived from the degradation of endogenous PtdCho formed de novo by methylation of phosphatidylethanolamine.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abra R. M., Quinn P. J. A novel pathway for phosphatidylcholine catabolism in rat brain homogenates. Biochim Biophys Acta. 1975 Mar 24;380(3):436–441. doi: 10.1016/0005-2760(75)90111-3. [DOI] [PubMed] [Google Scholar]
- Blusztajn J. K., Wurtman R. J. Choline biosynthesis by a preparation enriched in synaptosomes from rat brain. Nature. 1981 Apr 2;290(5805):417–418. doi: 10.1038/290417a0. [DOI] [PubMed] [Google Scholar]
- Blusztajn J. K., Zeisel S. H., Wurtman R. J. Synthesis of lecithin (phosphatidylcholine) from phosphatidylethanolamine in bovine brain. Brain Res. 1979 Dec 28;179(2):319–327. doi: 10.1016/0006-8993(79)90447-5. [DOI] [PubMed] [Google Scholar]
- Bottenstein J. E., Sato G. H. Growth of a rat neuroblastoma cell line in serum-free supplemented medium. Proc Natl Acad Sci U S A. 1979 Jan;76(1):514–517. doi: 10.1073/pnas.76.1.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen D. M., Benton J. S., Spillane J. A., Smith C. C., Allen S. J. Choline acetyltransferase activity and histopathology of frontal neocortex from biopsies of demented patients. J Neurol Sci. 1982 Dec;57(2-3):191–202. doi: 10.1016/0022-510x(82)90026-0. [DOI] [PubMed] [Google Scholar]
- Chalifour R., Kanfer J. N. Fatty acid activation and temperature perturbation of rat brain microsomal phospholipase D. J Neurochem. 1982 Aug;39(2):299–305. doi: 10.1111/j.1471-4159.1982.tb03946.x. [DOI] [PubMed] [Google Scholar]
- Collier B., Poon P., Salehmoghaddam S. The formation of choline and of acetylcholine by brain in vitro. J Neurochem. 1972 Jan;19(1):51–60. doi: 10.1111/j.1471-4159.1972.tb01252.x. [DOI] [PubMed] [Google Scholar]
- Crews F. T., Hirata F., Axelrod J. Identification and properties of methyltransferases that synthesize phosphatidylcholine in rat brain synaptosomes. J Neurochem. 1980 Jun;34(6):1491–1498. doi: 10.1111/j.1471-4159.1980.tb11229.x. [DOI] [PubMed] [Google Scholar]
- Dainous F., Kanfer J. N. Effect of modification of membrane phospholipid composition on phospholipid methylation in aggregating cell culture. J Neurochem. 1986 Jun;46(6):1859–1864. doi: 10.1111/j.1471-4159.1986.tb08505.x. [DOI] [PubMed] [Google Scholar]
- Goldberg A. M., McCaman R. E. The determination of picomole amounts of acetylcholine in mammalian brain. J Neurochem. 1973 Jan;20(1):1–8. doi: 10.1111/j.1471-4159.1973.tb12097.x. [DOI] [PubMed] [Google Scholar]
- Lefresne P., Guyenet P., Glowinski J. Acetylcholine synthesis from (2- 14 C)pyruvate in rat striatal slices. J Neurochem. 1973 Apr;20(4):1083–1097. doi: 10.1111/j.1471-4159.1973.tb00079.x. [DOI] [PubMed] [Google Scholar]
- Liscovitch M., Freese A., Blusztajn J. K., Wurtman R. J. High-performance liquid chromatography of water-soluble choline metabolites. Anal Biochem. 1985 Nov 15;151(1):182–187. doi: 10.1016/0003-2697(85)90069-7. [DOI] [PubMed] [Google Scholar]
- Maire J. C., Wurtman R. J. Effects of electrical stimulation and choline availability on the release and contents of acetylcholine and choline in superfused slices from rat striatum. J Physiol (Paris) 1985;80(3):189–195. [PubMed] [Google Scholar]
- Mozzi R., Porcellati G. Conversion of phosphatidylethanolamine to phosphatidylcholine in rat brain by the methylation pathway. FEBS Lett. 1979 Apr 15;100(2):363–366. doi: 10.1016/0014-5793(79)80370-1. [DOI] [PubMed] [Google Scholar]
- Pearson R. C., Sofroniew M. V., Cuello A. C., Powell T. P., Eckenstein F., Esiri M. M., Wilcock G. K. Persistence of cholinergic neurons in the basal nucleus in a brain with senile dementia of the Alzheimer's type demonstrated by immunohistochemical staining for choline acetyltransferase. Brain Res. 1983 Dec 19;289(1-2):375–379. doi: 10.1016/0006-8993(83)90046-x. [DOI] [PubMed] [Google Scholar]
- Perry E. K., Tomlinson B. E., Blessed G., Bergmann K., Gibson P. H., Perry R. H. Correlation of cholinergic abnormalities with senile plaques and mental test scores in senile dementia. Br Med J. 1978 Nov 25;2(6150):1457–1459. doi: 10.1136/bmj.2.6150.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry R. H., Candy J. M., Perry E. K., Irving D., Blessed G., Fairbairn A. F., Tomlinson B. E. Extensive loss of choline acetyltransferase activity is not reflected by neuronal loss in the nucleus of Meynert in Alzheimer's disease. Neurosci Lett. 1982 Dec 13;33(3):311–315. doi: 10.1016/0304-3940(82)90391-3. [DOI] [PubMed] [Google Scholar]
- Párducz A., Kiss Z., Joó F. Changes of the phosphatidylcholine content and the number of synaptic vesicles in relation to the neurohumoral transmission in sympathetic ganglia. Experientia. 1976 Dec 15;32(12):1520–1521. doi: 10.1007/BF01924428. [DOI] [PubMed] [Google Scholar]
- Quastel J. H., Tennenbaum M., Wheatley A. H. Choline ester formation in, and choline esterase activities of, tissues in vitro. Biochem J. 1936 Sep;30(9):1668–1681. doi: 10.1042/bj0301668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEBSTER G. R., MARPLES E. A., THOMPSON R. H. Glycerylphosphorylcholine diesterase activity of nervous tissue. Biochem J. 1957 Feb;65(2):374–377. doi: 10.1042/bj0650374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West G. J., Uki J., Herschman H. R., Seeger R. C. Adrenergic, cholinergic, and inactive human neuroblastoma cell lines with the action-potential Na+ ionophore. Cancer Res. 1977 May;37(5):1372–1376. [PubMed] [Google Scholar]
- Wilcock G. K., Esiri M. M., Bowen D. M., Smith C. C. Alzheimer's disease. Correlation of cortical choline acetyltransferase activity with the severity of dementia and histological abnormalities. J Neurol Sci. 1982 Dec;57(2-3):407–417. doi: 10.1016/0022-510x(82)90045-4. [DOI] [PubMed] [Google Scholar]
- Yavin E. Polar head group decarboxylation and methylation of phospholipids: an alternate route for phosphatidylcholine formation in cultured neuronal cells. J Neurochem. 1985 May;44(5):1451–1458. doi: 10.1111/j.1471-4159.1985.tb08782.x. [DOI] [PubMed] [Google Scholar]


