Abstract
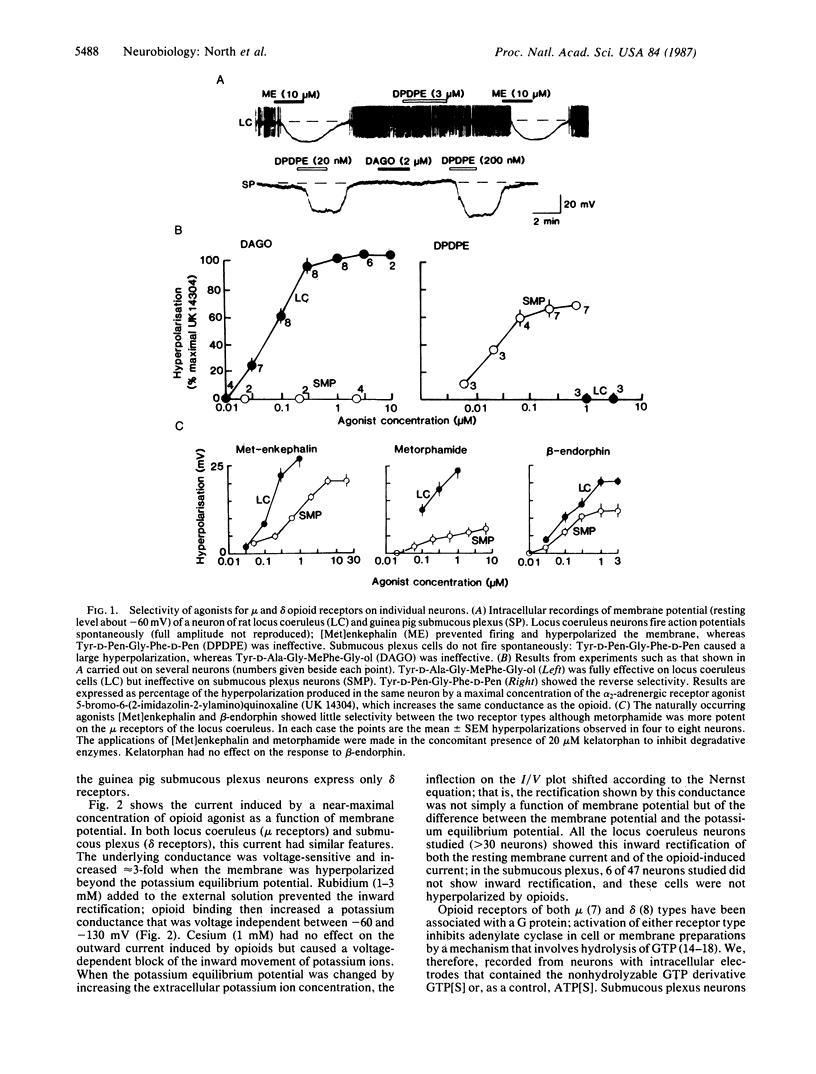
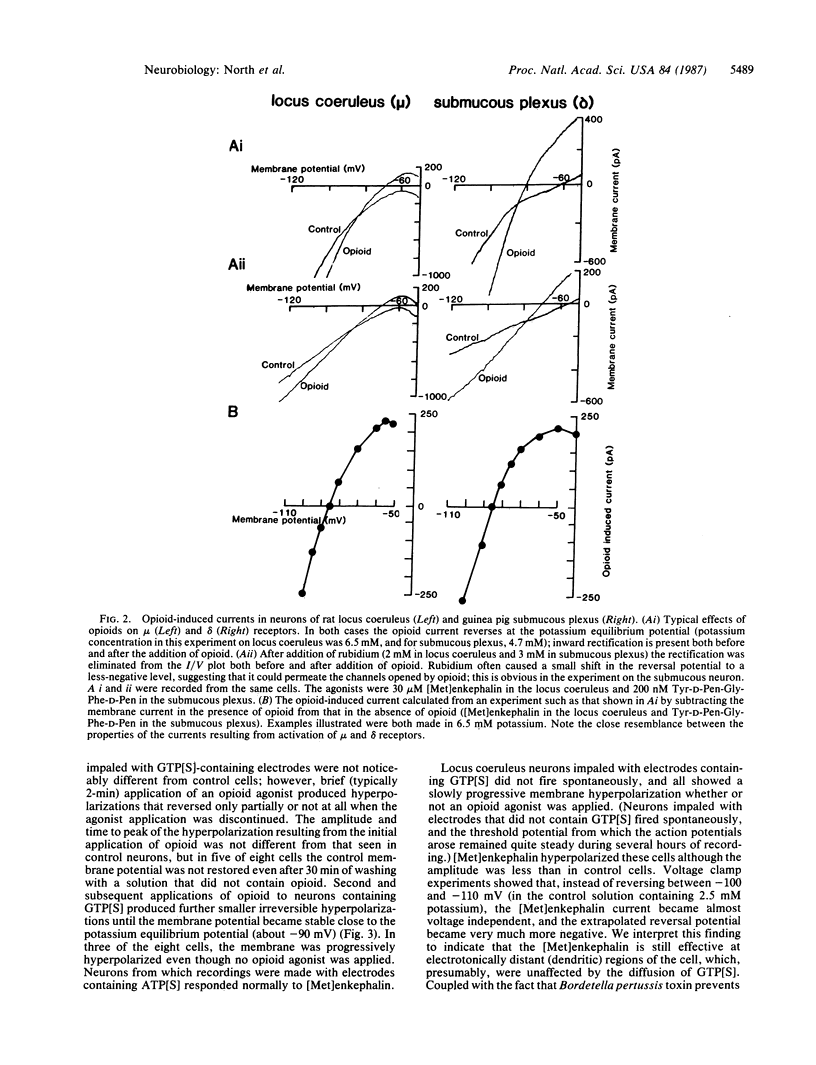
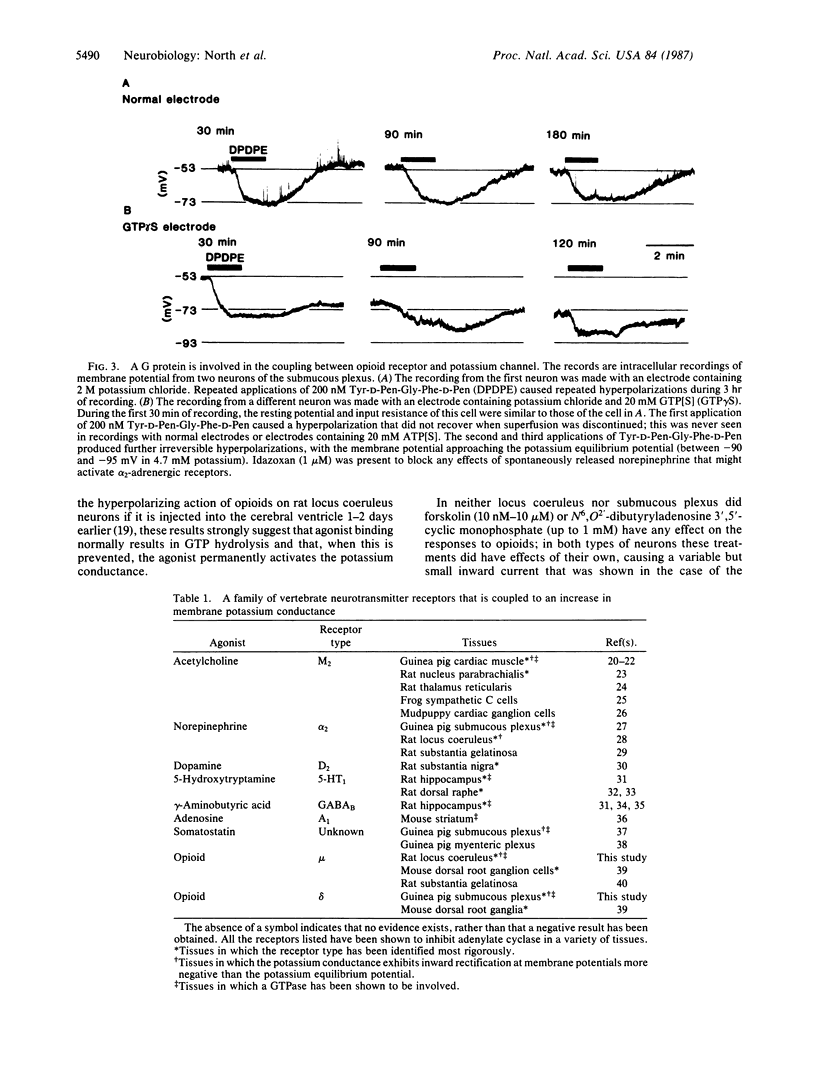
The effects of agonists at mu and delta opioid receptors were compared by measuring membrane currents under voltage clamp from neurons of the rat nucleus locus coeruleus and guinea pig submucous plexus. In each tissue, the appropriate selective agonist (Tyr-D-Ala-Gly-MePhe-Gly-ol for mu receptors in locus coeruleus or Tyr-D-Pen-Gly-Phe-D-Pen for delta receptors in submucous plexus) increased the conductance of an inwardly rectifying potassium conductance and strongly hyperpolarized the membrane. The properties of the potassium conductance affected by the two opioids could not be distinguished. Experiments with intracellular application of guanosine 5'-[gamma-thio]triphosphate indicated that a guanine nucleotide-binding regulatory protein was involved in the coupling between opioid receptor and potassium channel, but there was no evidence for activation of either cAMP-dependent protein kinase or protein kinase C. It is noted that a number of vertebrate neurotransmitter receptors are coupled to potassium channels. The potassium conductance associated with these channels has properties similar to the conductance activated by mu and delta opioids; this family includes the following receptors: acetylcholine M2, norepinephrine alpha 2, dopamine D2, 5-hydroxytryptamine 5-HT1, adenosine A1, gamma-aminobutyric acid GABAB, and somatostatin. It is suggested that this conductance is a conserved neuronal effector coupled to one of the receptor types that mediates the effects of each of several major transmitters. The mu and delta opioid receptors appear to be unusual in that both utilize this same effector mechanism.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aghajanian G. K., Lakoski J. M. Hyperpolarization of serotonergic neurons by serotonin and LSD: studies in brain slices showing increased K+ conductance. Brain Res. 1984 Jul 2;305(1):181–185. doi: 10.1016/0006-8993(84)91137-5. [DOI] [PubMed] [Google Scholar]
- Aghajanian G. K., Wang Y. Y. Pertussis toxin blocks the outward currents evoked by opiate and alpha 2-agonists in locus coeruleus neurons. Brain Res. 1986 Apr 23;371(2):390–394. doi: 10.1016/0006-8993(86)90382-3. [DOI] [PubMed] [Google Scholar]
- Bhoola K. D., Pay S. Opioid inhibition of adenylate cyclase in the striatum and vas deferens of the rat. Br J Pharmacol. 1986 Sep;89(1):109–118. doi: 10.1111/j.1476-5381.1986.tb11126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgoin S., Le Bars D., Artaud F., Clot A. M., Bouboutou R., Fournie-Zaluski M. C., Roques B. P., Hamon M., Cesselin F. Effects of kelatorphan and other peptidase inhibitors on the in vitro and in vivo release of methionine-enkephalin-like material from the rat spinal cord. J Pharmacol Exp Ther. 1986 Jul;238(1):360–366. [PubMed] [Google Scholar]
- Breitwieser G. E., Szabo G. Uncoupling of cardiac muscarinic and beta-adrenergic receptors from ion channels by a guanine nucleotide analogue. Nature. 1985 Oct 10;317(6037):538–540. doi: 10.1038/317538a0. [DOI] [PubMed] [Google Scholar]
- Codina J., Yatani A., Grenet D., Brown A. M., Birnbaumer L. The alpha subunit of the GTP binding protein Gk opens atrial potassium channels. Science. 1987 Apr 24;236(4800):442–445. doi: 10.1126/science.2436299. [DOI] [PubMed] [Google Scholar]
- Collier H. O., Roy A. C. Morphine-like drugs inhibit the stimulation of E prostaglandins of cyclic AMP formation by rat brain homogenate. Nature. 1974 Mar 1;248(5443):24–27. doi: 10.1038/248024a0. [DOI] [PubMed] [Google Scholar]
- Cooper D. M., Londos C., Gill D. L., Rodbell M. Opiate receptor-mediated inhibition of adenylate cyclase in rat striatal plasma membranes. J Neurochem. 1982 Apr;38(4):1164–1167. doi: 10.1111/j.1471-4159.1982.tb05365.x. [DOI] [PubMed] [Google Scholar]
- Demoliou-Mason C. D., Barnard E. A. Distinct subtypes of the opioid receptor with allosteric interactions in brain membranes. J Neurochem. 1986 Apr;46(4):1118–1128. doi: 10.1111/j.1471-4159.1986.tb00626.x. [DOI] [PubMed] [Google Scholar]
- Dodd J., Horn J. P. Muscarinic inhibition of sympathetic C neurones in the bullfrog. J Physiol. 1983 Jan;334:271–291. doi: 10.1113/jphysiol.1983.sp014494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantozzi R., Mullikin-Kilpatrick D., Blume A. J. Irreversible inactivation of the opiate receptors in the neuroblastoma x glioma hybrid NG108-15 by chlornaltrexamine. Mol Pharmacol. 1981 Jul;20(1):8–15. [PubMed] [Google Scholar]
- Fischmeister R., Hartzell H. C. Mechanism of action of acetylcholine on calcium current in single cells from frog ventricle. J Physiol. 1986 Jul;376:183–202. doi: 10.1113/jphysiol.1986.sp016148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman A. G. G proteins and dual control of adenylate cyclase. Cell. 1984 Mar;36(3):577–579. doi: 10.1016/0092-8674(84)90336-2. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Snyder S. H., Kuhar M. J., Young W. S., 3rd Differentiation of delta and mu opiate receptor localizations by light microscopic autoradiography. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6239–6243. doi: 10.1073/pnas.77.10.6239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gähwiler B. H., Brown D. A. GABAB-receptor-activated K+ current in voltage-clamped CA3 pyramidal cells in hippocampal cultures. Proc Natl Acad Sci U S A. 1985 Mar;82(5):1558–1562. doi: 10.1073/pnas.82.5.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartzell H. C., Kuffler S. W., Stickgold R., Yoshikami D. Synaptic excitation and inhibition resulting from direct action of acetylcholine on two types of chemoreceptors on individual amphibian parasympathetic neurones. J Physiol. 1977 Oct;271(3):817–846. doi: 10.1113/jphysiol.1977.sp012027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katayama Y., North R. A. The action of somatostatin on neurones of the myenteric plexus of the guinea-pig ileum. J Physiol. 1980 Jun;303:315–323. doi: 10.1113/jphysiol.1980.sp013287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lands A. M., Arnold A., McAuliff J. P., Luduena F. P., Brown T. G., Jr Differentiation of receptor systems activated by sympathomimetic amines. Nature. 1967 May 6;214(5088):597–598. doi: 10.1038/214597a0. [DOI] [PubMed] [Google Scholar]
- Lord J. A., Waterfield A. A., Hughes J., Kosterlitz H. W. Endogenous opioid peptides: multiple agonists and receptors. Nature. 1977 Jun 9;267(5611):495–499. doi: 10.1038/267495a0. [DOI] [PubMed] [Google Scholar]
- McCormick D. A., Prince D. A. Acetylcholine induces burst firing in thalamic reticular neurones by activating a potassium conductance. 1986 Jan 30-Feb 5Nature. 319(6052):402–405. doi: 10.1038/319402a0. [DOI] [PubMed] [Google Scholar]
- McLean S., Rothman R. B., Herkenham M. Autoradiographic localization of mu- and delta-opiate receptors in the forebrain of the rat. Brain Res. 1986 Jul 16;378(1):49–60. doi: 10.1016/0006-8993(86)90285-4. [DOI] [PubMed] [Google Scholar]
- Mihara S., North R. A. Opioids increase potassium conductance in submucous neurones of guinea-pig caecum by activating delta-receptors. Br J Pharmacol. 1986 Jun;88(2):315–322. doi: 10.1111/j.1476-5381.1986.tb10207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milligan G., Simonds W. F., Streaty R. A., Tocque B., Klee W. A. Functional control of the delta-opiate receptor by the inhibitory guanine nucleotide-binding protein. Biochem Soc Trans. 1985 Dec;13(6):1110–1113. doi: 10.1042/bst0131110. [DOI] [PubMed] [Google Scholar]
- Moxham C. P., George S. T., Graziano M. P., Brandwein H. J., Malbon C. C. Mammalian beta 1- and beta 2-adrenergic receptors. Immunological and structural comparisons. J Biol Chem. 1986 Nov 5;261(31):14562–14570. [PubMed] [Google Scholar]
- Newberry N. R., Nicoll R. A. Comparison of the action of baclofen with gamma-aminobutyric acid on rat hippocampal pyramidal cells in vitro. J Physiol. 1985 Mar;360:161–185. doi: 10.1113/jphysiol.1985.sp015610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Surprenant A. Inhibitory synaptic potentials resulting from alpha 2-adrenoceptor activation in guinea-pig submucous plexus neurones. J Physiol. 1985 Jan;358:17–33. doi: 10.1113/jphysiol.1985.sp015537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Williams J. T. On the potassium conductance increased by opioids in rat locus coeruleus neurones. J Physiol. 1985 Jul;364:265–280. doi: 10.1113/jphysiol.1985.sp015743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- North R. A., Yoshimura M. The actions of noradrenaline on neurones of the rat substantia gelatinosa in vitro. J Physiol. 1984 Apr;349:43–55. doi: 10.1113/jphysiol.1984.sp015141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaffinger P. J., Martin J. M., Hunter D. D., Nathanson N. M., Hille B. GTP-binding proteins couple cardiac muscarinic receptors to a K channel. Nature. 1985 Oct 10;317(6037):536–538. doi: 10.1038/317536a0. [DOI] [PubMed] [Google Scholar]
- Sharma S. K., Nirenberg M., Klee W. A. Morphine receptors as regulators of adenylate cyclase activity. Proc Natl Acad Sci U S A. 1975 Feb;72(2):590–594. doi: 10.1073/pnas.72.2.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima M., Noma A. Mode of regulation of the ACh-sensitive K-channel by the muscarinic receptor in rabbit atrial cells. Pflugers Arch. 1984 Apr;400(4):424–431. doi: 10.1007/BF00587544. [DOI] [PubMed] [Google Scholar]
- Stiles G. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Mammalian beta-adrenergic receptors. Structural differences in beta 1 and beta 2 subtypes revealed by peptide maps. J Biol Chem. 1983 Sep 10;258(17):10689–10694. [PubMed] [Google Scholar]
- Trussell L. O., Jackson M. B. Adenosine-activated potassium conductance in cultured striatal neurons. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4857–4861. doi: 10.1073/pnas.82.14.4857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wamsley J. K. Opioid receptors: autoradiography. Pharmacol Rev. 1983 Mar;35(1):69–83. [PubMed] [Google Scholar]
- Werz M. A., Macdonald R. L. Opioid peptides with differential affinity for mu and delta receptors decrease sensory neuron calcium-dependent action potentials. J Pharmacol Exp Ther. 1983 Nov;227(2):394–402. [PubMed] [Google Scholar]
- Williams J. T., Henderson G., North R. A. Characterization of alpha 2-adrenoceptors which increase potassium conductance in rat locus coeruleus neurones. Neuroscience. 1985 Jan;14(1):95–101. doi: 10.1016/0306-4522(85)90166-6. [DOI] [PubMed] [Google Scholar]
- Williams J. T., North R. A. Opiate-receptor interactions on single locus coeruleus neurones. Mol Pharmacol. 1984 Nov;26(3):489–497. [PubMed] [Google Scholar]
- Williams J. T., North R. A., Shefner S. A., Nishi S., Egan T. M. Membrane properties of rat locus coeruleus neurones. Neuroscience. 1984 Sep;13(1):137–156. doi: 10.1016/0306-4522(84)90265-3. [DOI] [PubMed] [Google Scholar]
- Wise R. A., Jenck F., Gratton A., Quirion R. Opiate receptor subtypes associated with the brain mechanisms of feeding and reward. NIDA Res Monogr. 1986;71:165–172. [PubMed] [Google Scholar]
- Yoshimura M., Higashi H. 5-Hydroxytryptamine mediates inhibitory postsynaptic potentials in rat dorsal raphe neurons. Neurosci Lett. 1985 Jan 7;53(1):69–74. doi: 10.1016/0304-3940(85)90099-0. [DOI] [PubMed] [Google Scholar]
- Yoshimura M., North R. A. Substantia gelatinosa neurones hyperpolarized in vitro by enkephalin. Nature. 1983 Oct 6;305(5934):529–530. doi: 10.1038/305529a0. [DOI] [PubMed] [Google Scholar]


