Abstract
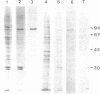
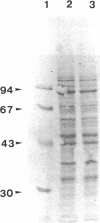
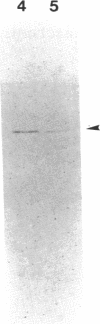
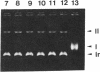
DNA topoisomerase I was purified to near homogeneity from a clonal line of human lymphoblastic leukemia cells, RPMI 8402, that is resistant to camptothecin, a cytotoxic alkaloid from Camptotheca acuminata, and compared with that of the parent wild-type cells. As assayed by relaxation of the supercoiled plasmid DNA and by formation of enzyme-linked DNA breaks, the purified enzyme from the resistant cells was shown to be greater than 125-fold as resistant to camptothecin as the wild-type enzyme, comparable to a cellular resistance index of about 300. Therefore, the cellular resistance appears to be due to the resistance of the enzyme. The amount of the immunoreactive enzyme protein in whole extract appeared to be reduced to less than half that of the wild-type enzyme. These results establish that DNA topoisomerase I is the cellular target of camptothecin and that DNA topoisomerase I is essential for the survival of mammalian cells.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abelson H. T., Penman S. Selective interruption of high molecular weight RNA synthesis in HeLa cells by camptothecin. Nat New Biol. 1972 May 31;237(74):144–146. doi: 10.1038/newbio237144a0. [DOI] [PubMed] [Google Scholar]
- Andersen A. H., Gocke E., Bonven B. J., Nielsen O. F., Westergaard O. Topoisomerase I has a strong binding preference for a conserved hexadecameric sequence in the promoter region of the rRNA gene from Tetrahymena pyriformis. Nucleic Acids Res. 1985 Mar 11;13(5):1543–1557. doi: 10.1093/nar/13.5.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonven B. J., Gocke E., Westergaard O. A high affinity topoisomerase I binding sequence is clustered at DNAase I hypersensitive sites in Tetrahymena R-chromatin. Cell. 1985 Jun;41(2):541–551. doi: 10.1016/s0092-8674(85)80027-1. [DOI] [PubMed] [Google Scholar]
- Bosmann H. B. Camptothecin inhibits macromolecular synthesis in mammalian cells but not in isolated mitochondria of E. coli. Biochem Biophys Res Commun. 1970 Dec 24;41(6):1412–1420. doi: 10.1016/0006-291x(70)90544-9. [DOI] [PubMed] [Google Scholar]
- Champoux J. J. Proteins that affect DNA conformation. Annu Rev Biochem. 1978;47:449–479. doi: 10.1146/annurev.bi.47.070178.002313. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA gyrase and the supercoiling of DNA. Science. 1980 Feb 29;207(4434):953–960. doi: 10.1126/science.6243420. [DOI] [PubMed] [Google Scholar]
- DiNardo S., Voelkel K. A., Sternglanz R., Reynolds A. E., Wright A. Escherichia coli DNA topoisomerase I mutants have compensatory mutations in DNA gyrase genes. Cell. 1982 Nov;31(1):43–51. doi: 10.1016/0092-8674(82)90403-2. [DOI] [PubMed] [Google Scholar]
- Drewinko B., Freireich E. J., Gottlieb J. A. Lethal activity of camptothecin sodium on human lymphoma cells. Cancer Res. 1974 Apr;34(4):747–750. [PubMed] [Google Scholar]
- Fleischmann G., Pflugfelder G., Steiner E. K., Javaherian K., Howard G. C., Wang J. C., Elgin S. C. Drosophila DNA topoisomerase I is associated with transcriptionally active regions of the genome. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6958–6962. doi: 10.1073/pnas.81.22.6958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gellert M., Menzel R., Mizuuchi K., O'Dea M. H., Friedman D. I. Regulation of DNA supercoiling in Escherichia coli. Cold Spring Harb Symp Quant Biol. 1983;47(Pt 2):763–767. doi: 10.1101/sqb.1983.047.01.087. [DOI] [PubMed] [Google Scholar]
- Gocke E., Bonven B. J., Westergaard O. A site and strand specific nuclease activity with analogies to topoisomerase I frames the rRNA gene of Tetrahymena. Nucleic Acids Res. 1983 Nov 25;11(22):7661–7678. doi: 10.1093/nar/11.22.7661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottlieb J. A., Guarino A. M., Call J. B., Oliverio V. T., Block J. B. Preliminary pharmacologic and clinical evaluation of camptothecin sodium (NSC-100880). Cancer Chemother Rep. 1970 Dec;54(6):461–470. [PubMed] [Google Scholar]
- Horwitz M. S., Horwitz S. B. Intracellular degradation of HeLa and adenovirus type 2 DNA induced by camptothecin. Biochem Biophys Res Commun. 1971 Nov 5;45(3):723–727. doi: 10.1016/0006-291x(71)90476-1. [DOI] [PubMed] [Google Scholar]
- Hsiang Y. H., Hertzberg R., Hecht S., Liu L. F. Camptothecin induces protein-linked DNA breaks via mammalian DNA topoisomerase I. J Biol Chem. 1985 Nov 25;260(27):14873–14878. [PubMed] [Google Scholar]
- Huang C. C., Han C. S., Yue X. F., Shen C. M., Wang S. W., Wu F. G., Xu B. Cytotoxicity and sister chromatid exchanges induced in vitro by six anticancer drugs developed in the People's Republic of China. J Natl Cancer Inst. 1983 Oct;71(4):841–847. [PubMed] [Google Scholar]
- Ishii K., Futaki S., Uchiyama H., Nagasawa K., Andoh T. Mechanism of inhibition of mammalian DNA topoisomerase I by heparin. Biochem J. 1987 Jan 1;241(1):111–119. doi: 10.1042/bj2410111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K., Hasegawa T., Fujisawa K., Andoh T. Rapid purification and characterization of DNA topoisomerase I from cultured mouse mammary carcinoma FM3A cells. J Biol Chem. 1983 Oct 25;258(20):12728–12732. [PubMed] [Google Scholar]
- Ishii K., Katase A., Andoh T., Seno N. Inhibition of topoisomerase I by heparin. Biochem Biophys Res Commun. 1982 Jan 29;104(2):541–547. doi: 10.1016/0006-291x(82)90671-4. [DOI] [PubMed] [Google Scholar]
- Javaherian K., Liu L. F. Association of eukaryotic DNA topoisomerase I with nucleosomes and chromosomal proteins. Nucleic Acids Res. 1983 Jan 25;11(2):461–472. doi: 10.1093/nar/11.2.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessel D. Effects of camptothecin on RNA synthesis in leukemia L1210 cells. Biochim Biophys Acta. 1971 Aug 26;246(2):225–232. doi: 10.1016/0005-2787(71)90131-6. [DOI] [PubMed] [Google Scholar]
- Li L. H., Fraser T. J., Olin E. J., Bhuyan B. K. Action of camptothecin on mammalian cells in culture. Cancer Res. 1972 Dec;32(12):2643–2650. [PubMed] [Google Scholar]
- Liu L. F. DNA topoisomerases--enzymes that catalyse the breaking and rejoining of DNA. CRC Crit Rev Biochem. 1983;15(1):1–24. doi: 10.3109/10409238309102799. [DOI] [PubMed] [Google Scholar]
- Liu L. F., Davis J. L., Calendar R. Novel topologically knotted DNA from bacteriophage P4 capsids: studies with DNA topoisomerases. Nucleic Acids Res. 1981 Aug 25;9(16):3979–3989. doi: 10.1093/nar/9.16.3979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel R., Gellert M. Regulation of the genes for E. coli DNA gyrase: homeostatic control of DNA supercoiling. Cell. 1983 Aug;34(1):105–113. doi: 10.1016/0092-8674(83)90140-x. [DOI] [PubMed] [Google Scholar]
- Muller M. T., Pfund W. P., Mehta V. B., Trask D. K. Eukaryotic type I topoisomerase is enriched in the nucleolus and catalytically active on ribosomal DNA. EMBO J. 1985 May;4(5):1237–1243. doi: 10.1002/j.1460-2075.1985.tb03766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagata K., Guggenheimer R. A., Hurwitz J. Adenovirus DNA replication in vitro: synthesis of full-length DNA with purified proteins. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4266–4270. doi: 10.1073/pnas.80.14.4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruss G. J., Manes S. H., Drlica K. Escherichia coli DNA topoisomerase I mutants: increased supercoiling is corrected by mutations near gyrase genes. Cell. 1982 Nov;31(1):35–42. doi: 10.1016/0092-8674(82)90402-0. [DOI] [PubMed] [Google Scholar]
- Sahai B. M., Kaplan J. G. A quantitative decatenation assay for type II topoisomerases. Anal Biochem. 1986 Aug 1;156(2):364–379. doi: 10.1016/0003-2697(86)90267-8. [DOI] [PubMed] [Google Scholar]
- Spataro A., Kessel D. Studies on camptothecin-induced degradation and apparent reaggregation of DNA from L1210 cells. Biochem Biophys Res Commun. 1972 Aug 7;48(3):643–648. doi: 10.1016/0006-291x(72)90396-8. [DOI] [PubMed] [Google Scholar]
- Sternglanz R., DiNardo S., Voelkel K. A., Nishimura Y., Hirota Y., Becherer K., Zumstein L., Wang J. C. Mutations in the gene coding for Escherichia coli DNA topoisomerase I affect transcription and transposition. Proc Natl Acad Sci U S A. 1981 May;78(5):2747–2751. doi: 10.1073/pnas.78.5.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thrash C., Voelkel K., DiNardo S., Sternglanz R. Identification of Saccharomyces cerevisiae mutants deficient in DNA topoisomerase I activity. J Biol Chem. 1984 Feb 10;259(3):1375–1377. [PubMed] [Google Scholar]
- Towbin H., Gordon J. Immunoblotting and dot immunobinding--current status and outlook. J Immunol Methods. 1984 Sep 4;72(2):313–340. doi: 10.1016/0022-1759(84)90001-2. [DOI] [PubMed] [Google Scholar]
- Tricoli J. V., Sahai B. M., McCormick P. J., Jarlinski S. J., Bertram J. S., Kowalski D. DNA topoisomerase I and II activities during cell proliferation and the cell cycle in cultured mouse embryo fibroblast (C3H 10T1/2) cells. Exp Cell Res. 1985 May;158(1):1–14. doi: 10.1016/0014-4827(85)90426-4. [DOI] [PubMed] [Google Scholar]
- Trucksis M., Golub E. I., Zabel D. J., Depew R. E. Escherichia coli and Salmonella typhimurium supX genes specify deoxyribonucleic acid topoisomerase I. J Bacteriol. 1981 Aug;147(2):679–681. doi: 10.1128/jb.147.2.679-681.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uemura T., Yanagida M. Isolation of type I and II DNA topoisomerase mutants from fission yeast: single and double mutants show different phenotypes in cell growth and chromatin organization. EMBO J. 1984 Aug;3(8):1737–1744. doi: 10.1002/j.1460-2075.1984.tb02040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. C. DNA topoisomerases. Annu Rev Biochem. 1985;54:665–697. doi: 10.1146/annurev.bi.54.070185.003313. [DOI] [PubMed] [Google Scholar]
- Weisbrod S. T. Properties of active nucleosomes as revealed by HMG 14 and 17 chromatography. Nucleic Acids Res. 1982 Mar 25;10(6):2017–2042. doi: 10.1093/nar/10.6.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R. S., Kumar A., Warner J. R. Ribosome formation is blocked by camptothecin, a reversible inhibitor of RNA synthesis. Proc Natl Acad Sci U S A. 1971 Dec;68(12):3009–3014. doi: 10.1073/pnas.68.12.3009. [DOI] [PMC free article] [PubMed] [Google Scholar]