The WD40-repeat protein Han11 functions as a scaffold protein to control HIPK2 and MEKK1 kinase functions
Han11 (also called WDR68/DCAF7) is described as novel scaffolding protein that regulates osmotic stress in both human cells and C.elegans by virtue of its interaction with MEKK1, Dyrk1 and HIPK2.
Keywords: Han11, HIPK2, MAP kinase, MEKK1, scaffold protein
Abstract
Protein kinases are organized in hierarchical networks that are assembled and regulated by scaffold proteins. Here, we identify the evolutionary conserved WD40-repeat protein Han11 as an interactor of the kinase homeodomain-interacting protein kinase 2 (HIPK2). In vitro experiments showed the direct binding of Han11 to HIPK2, but also to the kinases DYRK1a, DYRK1b and mitogen-activated protein kinase kinase kinase 1 (MEKK1). Han11 was required to allow coupling of MEKK1 to DYRK1 and HIPK2. Knockdown experiments in Caenorhabditis elegans showed the relevance of the Han11 orthologs Swan-1 and Swan-2 for the osmotic stress response. Downregulation of Han11 in human cells lowered the threshold and amplitude of HIPK2- and MEKK1-triggered signalling events and changed the kinetics of kinase induction. Han11 knockdown changed the amplitude and time dependence of HIPK2-driven transcription in response to DNA damage and also interfered with MEKK1-triggered gene expression and stress signalling. Impaired signal transmission also occurred upon interference with stoichiometrically assembled signalling complexes by Han11 overexpression. Collectively, these experiments identify Han11 as a novel scaffold protein regulating kinase signalling by HIPK2 and MEKK1.
Introduction
The serine/threonine kinase homeodomain-interacting protein kinase 2 (HIPK2) belongs to the HIPK family of protein kinases and serves as an important regulator of apoptosis and gene expression (D'Orazi et al, 2002; Hofmann et al, 2002; Calzado et al, 2007; Hattangadi et al, 2010). HIPK2 is composed of an N-terminal kinase domain, which is followed by an interaction domain for homeodomain transcription factors and an autoinhibitory domain. The kinase is evolutionary conserved and orthologs are even found in Caenorhabditis elegans and Drosophila melanogaster (Kim et al, 1998; Calzado et al, 2007; Rinaldo et al, 2007). Dependent on its kinase function, HIPK2 localizes mainly to subnuclear HIPK domains, but in individual cells, the kinase can also occur in cytosolic microspeckles. HIPK2 is activated in response to morphogenic signals or DNA damage and accordingly HIPK2-guided gene expression programs trigger differentiation and development or alternatively apoptosis (Aikawa et al, 2006; Rinaldo et al, 2007; Hattangadi et al, 2010). A functional role for HIPK2 in cancer is seen in a knockout model, where HIPK2−/− mice develop more skin tumours and are subjected to faster disease progression than wild-type mice after two-stage skin carcinogenesis treatment (Wei et al, 2007). Accordingly, several tumours show either missense mutations of HIPK2 or dysregulated protein levels (Li et al, 2007; Yu et al, 2009). Much has been learned about the phosphorylation substrates of HIPK2 such as p53 and STAT3 (Matsuo et al, 2001; D'Orazi et al, 2002; Hofmann et al, 2002), but its control by putative upstream kinases is not explored. The HIPK family of protein kinases belongs to the CMGC (containing CDK, MAPK, GSK and CLK families) group of protein kinases (Manning et al, 2002).
Within the CMGC group, HIPK2 is most closely related to the dual-specificity tyrosine phosphorylation-regulated (DYRK) kinases (Hofmann et al, 2000). The DYRK kinase family of serine/threonine kinases comprises various members, among which DYRK1a is the most extensively studied (Becker and Joost, 1999). The human DYRK1a gene is encoded in the Down's syndrome critical region on chromosome 21 and thus may contribute to several characteristics of this disease (Altafaj et al, 2001; Dowjat et al, 2007; Kimura et al, 2007). DYRK1a displays a broad spectrum of substrate proteins such as transcription factors, splicing factors and mediators of apoptosis. DYRK1b is expressed mostly in skeletal muscle where it is implied in skeletal muscle differentiation (Mercer et al, 2005). DYRK1a and DYRK1b bind to the evolutionary conserved Han11 protein, as revealed by coimmunoprecipitation experiments (Skurat and Dietrich, 2004).
The human anthocyanin (Han11) gene encodes a protein with four WD repeats, which consist of highly conserved repeating units of Trp-Asp (WD). WD40 repeats do not possess catalytic activity, but facilitate protein–protein interactions and thereby enable the assembly of multiprotein complexes (Hudson and Cooley, 2008). The WD40-repeat proteins include the anthocyanin11 (An11) family, which is conserved between plants and animals. In plants, the An11 protein is known to be involved in the regulation of anthocyan biosynthesis (de Vetten et al, 1997), but the function of An11 in animals is not known. A recurrent finding is the interaction of An11 with protein kinases such as DYRK1a/b (Skurat and Dietrich, 2004) but also mitogen-activated protein kinase kinase kinase 1 (MEKK1) (Bouwmeester et al, 2004).
MEKK1 is one of 21 characterized mitogen-activated protein kinase kinase kinases (MAP3Ks), which phosphorylate and activate a MAP2K, which in turn phosphorylates a MAPK such as extracellular-regulated kinase (ERK), c-Jun N-terminal kinase (JNK) and p38, thus forming a three-tiered MAPK signalling module (Uhlik et al, 2004; Cuevas et al, 2007; Pimienta and Pascual, 2007). MAP3Ks can potentially activate more than one pathway, as the specificity of the kinases increases towards downstream targets. MEKK1 is a 196-kDa protein, which can activate the JNK, ERK and p38 pathways in response to various activation signals ranging from the CD40 receptor activation to osmotic stress (Yujiri et al, 1998; Uhlik et al, 2004; Gallagher et al, 2007). MEKK1−/− embryonic stem cells show defects in JNK and ERK activation under hyperosmolar conditions (Yujiri et al, 1998). MEKK1 does not only phosphorylate and thus activate downstream kinases such as MKK4 and MKK7, but also displays ubiquitin E3-ligase activity towards ERK1/2 (Lu et al, 2002). MEKK1 also functions as a scaffold protein, which directly binds to components of the three-tier ERK module, namely Raf-1, MEK1 and ERK2 as well as to the upstream activator Ras (Russell et al, 1995; Karandikar et al, 2000). MEKK1 also uses additional scaffold proteins such as JNK/stress-activated protein kinase-associated protein 1 (JSAP1) in order to regulate the kinetics and thresholds of signalling pathways (Ito et al, 1999). Scaffolding proteins are characterized by their ability to bind to different (at least two) other signalling proteins, thus acting as platforms that allow the coordinated and ordered assembly of signalling proteins (Smith et al, 2006; Dhanasekaran et al, 2007; Shaw and Filbert, 2009). In such a way, scaffold proteins function to increase kinase specificity and restrict the ability of a kinase to phosphorylate to many downstream targets. They also function to define the quality of the signal output and to determine for example whether a given signal results in a switch-like or graded response (Bhattacharyya et al, 2006; Shaw and Filbert, 2009). Scaffold proteins help to organize signal transduction pathways as communication networks, as the classical view of MAPK signalling as a linear pipeline conveying signals from the cell surface to a distinct downstream target is replaced by the view of highly dynamic and interactive multiprotein signalling complexes (Kolch, 2005; Albert and Oltvai, 2007).
Here, we identify a role for Han11 and show that it functions as a scaffold protein with the ability to directly bind HIPK2, MEKK1 and DYRK1a/b. Knockdown of Han11 interferes with qualitative and quantitative parameters of HIPK2- and MEKK1-dependent signalling and gene expression. The general importance of Han11 for stress signalling was revealed in C. elegans where knockdown of the Han11 orthologs Swan-1 and Swan-2 disabled the osmotic stress response.
Results
Han11 is a direct interactor of HIPK2, MEKK1 and DYRK1
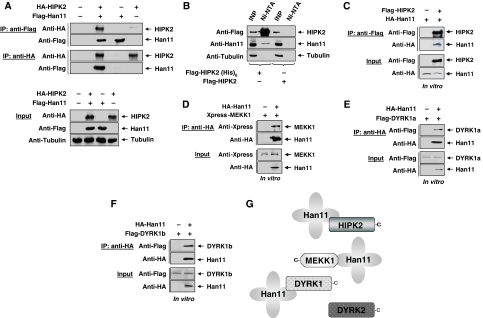
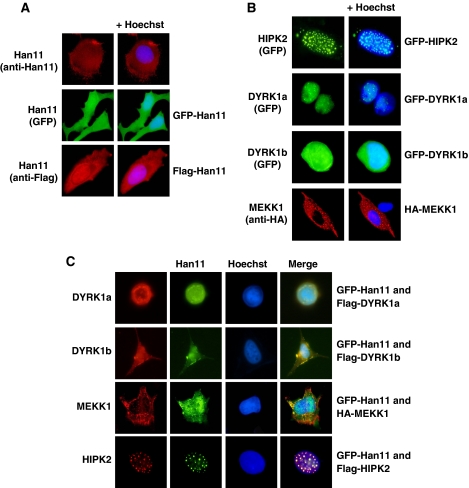
Using a kinase inactive version of HIPK2 as a bait in a yeast-two-hybrid screen, we isolated several copies of Han11 as an interaction partner (Supplementary Figure S1). To confirm this finding by an independent experimental approach, cells were transfected to express epitope-tagged versions of Han11 and HIPK2. After preparation of cell lysates, coimmunoprecipitations were performed with epitope-specific antibodies. These experiments allowed to detect Han11 in immunoprecipitates of HIPK2 and vice versa (Figure 1A), indicating an interaction between both proteins. To test whether this interaction also occurs in intact cells prior to their lysis, cells expressing a hexahistidine-tagged version of HIPK2 were treated with the membrane permeable cross-linking agent dimethyl dithiobispropionimidate (DTBP) which allows covalent cross-linking of proteins which are immediately binding and thus occur in very close proximity. After denaturing lysis and purification of His-tagged HIPK2 on Ni-NTA columns, immunoblotting allowed to detect binding of a fraction of the endogenous Han11 protein to His-tagged HIPK2 (Figure 1B). To investigate whether binding between HIPK2 and Han11 is direct, both proteins were produced by in vitro translation in a cell-free system. Coimmunoprecipitation experiments showed a direct binding (Figure 1C), revealing HIPK2 as a new interaction partner for this WD40-repeat protein. Further, coimmunoprecipitation experiments revealed binding of Han11 to the HIPK2 kinase domain, regardless whether the proteins were expressed in cells (Supplementary Figure S2) or produced by in vitro translation (Supplementary Figure S3). Given the reported interactions between Han11 and DYRK1a/DYRK1b (Skurat and Dietrich, 2004) and also MEKK1 (Bouwmeester et al, 2004) in intact cells, we tested whether also these interactions are direct or alternatively depend on further proteins contained in cell lysates. Han11 and the three kinases together with the more distantly related DYRK2 protein as a control were produced by in vitro translation and then tested by coimmunoprecipitation for direct binding. MEKK1, DYRK1a and DYRK1b showed direct association with Han11 (Figure 1D–F), whereas DYRK2 showed no interaction with Han11 (Supplementary Figure S4). These binding data are schematically summarized in Figure 1G. A subgroup of scaffolding proteins helps to localize and anchor the signalling molecule within distinct cellular loci (Shaw and Filbert, 2009), thus prompting us to investigate the localization of Han11 and its interacting kinases. Immunofluorescence studies showed the occurrence of the endogenous Han11 protein throughout the cell. This localization was faithfully recapitulated for GFP- and Flag-tagged versions of Han11 (Figure 2A). The interacting kinases showed distinct localization patterns (Figure 2B) corresponding to their reported localizations (Hofmann et al, 2002; Schlesinger et al, 2002; Alvarez et al, 2003). Although HIPK2 was mostly found in the typical nuclear speckles, DYRK1a showed a more dispersed localization within the nucleus in the nucleoplasm and in microspeckles. DYRK1b was distributed throughout the entire cell, whereas MEKK1 localized to cytoplasmic structures. Coexpression of HIPK2 with Han11 showed the efficient recruitment of Han11 to nuclear HIPK2 speckles (Figure 2C), dependent on the HIPK2 C-terminus (Supplementary Figure S5). Moreover, expression of Han11 together with DYRK1a resulted in strongly overlapping localizations (Figure 2C), showing that Han11 is not functioning as an anchor protein that assembles kinases in a specific cellular compartment, but rather functions as a subordinate and flexible adapter that follows the localization of its interacting kinase.
Figure 1.
Han11 binds to HIPK2, MEKK1 and DYRK1a/b. (A) HA-tagged HIPK2 and Flag-tagged Han11 were expressed in 293T cells as shown. Equal amounts of protein contained in cell lysates were tested for adequate protein expression (input) or were used for coimmunoprecipitation experiments (IP) with the indicated antibodies. The eluted proteins were detected by immunoblotting as shown. (B) Cells expressing Flag-tagged HIPK2 with or without a hexahistidine tag were treated with the cell permeable agent DTBP to cross-link directly neighbouring proteins. After purification of His(6)-tagged HIPK2 on Ni-NTA agarose columns, the input material (INP) and the interacting endogenous Han11 protein was detected by immunoblotting. (C) Tagged versions of HIPK2 and Han11 were produced by in vitro translation. The quality of the proteins was ensured by immunoblotting of the input control (lower), direct interactions were tested by coimmunoprecipitation (upper) as shown. HA-tagged Han11 and epitope-tagged versions of the indicated kinases were produced by in vitro translation. Coimmunoprecipitation experiments were conducted to investigate the direct binding of Han11 to MEKK1 (D), DYRK1a (E) and DYRK1b (F). The interacting proteins were detected by western blotting. (G) Summary of these experiments showing direct interactions between Han11 and MEKK1, DYRK1a and DYRK1b, the C-termini of the kinases are indicated.
Figure 2.
Han11 is recruited to its interacting kinases. (A) Indirect immunofluorescence pictures showing the localization of endogenous and epitope-tagged Han11 in U2OS cells, respectively. The nucleus was revealed by staining with Hoechst (blue). (B) GFP-tagged HIPK2, DYRK1a and DYRK1b as well as HA-tagged MEKK1 were expressed in U2OS cells. Proteins were visualized either by indirect immunofluorescence or by the fluorescence emitted from GFP. (C) GFP-tagged Han11 was coexpressed with tagged versions of DYRK1a, DYRK1b, HIPK2 or MEKK1 in U2OS cells as indicated. Proteins were visualized by immunofluorescence staining or by GFP fluorescence. Chromosomal DNA was stained with Hoechst, and the merged images indicate colocalization in yellow colour.
Han11 connects MEKK1 to the DYRK1/HIPK2 module
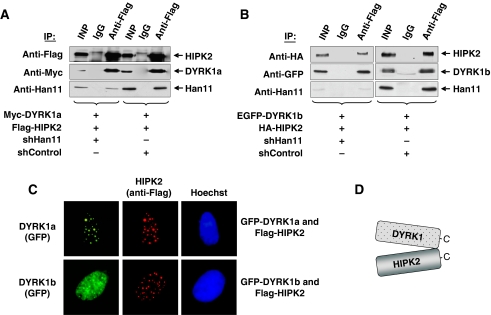
The binding of four different kinases to Han11 raises the question for potential direct interactions between these kinases. To address this issue, cells were transfected to express HIPK2 and DYRK1a either in the presence of endogenous Han11 or after expression of an shRNA downregulating this WD40-repeat protein. Coimmunoprecipitation experiments showed efficient binding of DYRK1a to HIPK2 independent from the level of Han11 (Figure 3A). A similar experimental approach revealed the Han11-independent interaction between DYRK1b and HIPK2 (Figure 3B). Accordingly, immunofluorescence experiments revealed substantially overlapping localization between HIPK2 and both DYRK1 kinases (Figure 3C), suggesting a direct interaction between these kinases as displayed in Figure 3D.
Figure 3.
DYRK1a/b and HIPK2 interact directly in the absence of Han11. (A) 293T cells were transfected with Han11-specific shRNA (shHan11) or with a control shRNA construct (shControl), which differs in two nucleotides from shHan11. One day after transfection, the cells were selected with puromycin for another 24 h. Then, the cells were retransfected to express Myc-DYRK1a, Flag-HIPK2 and the vectors directing the synthesis of the indicated shRNAs for another 30 h as indicated. Equal amounts of proteins were immunoprecipitated with anti-Flag or isotype-matched IgG control antibodies. Immunoblotting was used to detect the (co)immunoprecipitated proteins, 10% of the material was used for the input (INP) control. (B) The experiment was performed as in (A) with the exception that the interaction between expressed HIPK2 and DYRK1b was analysed. (C) Flag-tagged HIPK2 was coexpressed with GFP-tagged DYRK1a or DYRK1b in U2OS cells. HIPK2 was visualized by staining with anti-Flag and anti-Cy3 antibodies, whereas the nuclei were stained with Hoechst. (D) Model of the direct, Han11-independent interaction between HIPK2 and both DYRK1 isoforms.
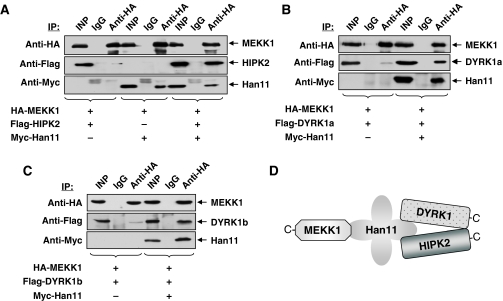
MEKK1 showed only very weak binding to the other three kinases (data not shown), raising the possibility that Han11 might be a limiting factor. To address this question, these binding experiments were repeated in the absence or presence of coexpressed Han11. MEKK1 and HIPK2 were expressed either alone or together with Han11, followed by coimmunoprecipitation experiments. The low amounts of HIPK2 in immunoprecipitated MEKK1 was strongly increased upon coexpression of Han11 (Figure 4A), showing that Han11 is needed as a bridging factor for this interaction. Similar experimental approaches were used to test the interaction of MEKK1 with DYRK1a and DYRK1b. In each case, the weak interaction, which is attributable to endogenous Han11, was strongly augmented upon coexpression of the adapter protein (Figure 4B and C). These data are schematically summarized in Figure 4D, which integrates all data assembled to this point.
Figure 4.
MEKK1 needs Han11 for coupling to HIPK2/DYRK1. Cells were transfected to express HA-tagged MEKK1 together with Flag-tagged HIPK2 (A), DYRK1a (B) or DYRK1b (C) either alone or together with Han11. Equal amounts of proteins were immunoprecipitated with anti-HA or adequate control antibodies and tested for the occurrence of the (co)immunoprecipitated proteins and the input material as indicated. (D) Schematic summary of the protein/protein interaction experiments.
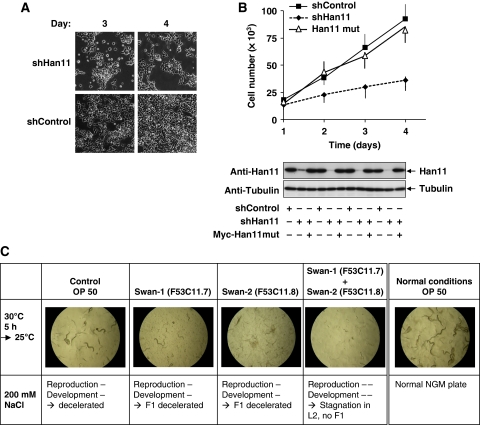
An essential role of Han11 for cell proliferation and the osmotic stress response
The strong evolutionary conservation of Han11 from plants to humans points to a fundamental role of this largely unexplored WD40-repeat protein. As we noted an impaired proliferation of cells transfected with a vector directing the production of a Han11-specific shRNA (Figure 5A), we followed up this finding in more detail. Cells were transfected to express a control shRNA or a Han11-specific shRNA either alone or together with a Han11 version that was point mutated to escape shRNA-mediated decay. A quantitative analysis of cell numbers showed that knockdown of endogenous Han11 resulted in a decreased ability to proliferate, whereas reconstitution of physiological levels of Han11 expression allowed to largely restore cell proliferation (Figure 5B). Cells with reduced Han11 levels showed no obvious changes in cell cycle distribution (data not shown), suggesting that Han11 is important for more general processes. Is there also a role of Han11 in a model system for an ancient organism? A database search identified Swan-1 and Swan-2 as Han11 orthologs in C. elegans (Supplementary Figure S6). To address the role of Swan-1 and Swan-2 under normal and stress conditions, knockdown experiments were performed (Table I). Only the combined downregulation of Swan-1 and Swan-2 resulted in an impaired reproduction and development of C. elegans. The induction of osmotic stress showed that the knockdown of Swan-1 was sufficient to stop larval development at the L3 stage. A combination of heat shock with osmotic stress revealed that downregulation of either Swan-1 or Swan-2 was sufficient to halt larval development at L3. The combined knockdown of both Han11 orthologs resulted in absent progeny under stress conditions, thus revealing an essential function of Swan-1 and Swan-2 for the stress response in C. elegans. Representative pictures from the worms under normal and stress conditions are shown in Figure 5C.
Figure 5.
Effects of An11 downregulation in physiological models. (A) Equal numbers of 293T cells were transfected with vectors encoding shHan11 or shControl and selected with puromycin. Three and 4 days later, phase contrast pictures were taken with a microscope to document different cell numbers. (B) 293T cells were transfected with Han11-specific or control shRNAs as shown together with Myc-Han11 that was point mutated to become shRNA resistant (Myc-Han11mut). After 3 days of selection in puromycin, equal numbers of cells were seeded into dishes and further grown for the indicated time periods. Cell numbers were quantified in a FACSCalibur (upper), and Han11 expression was monitored by immunoblotting (lower). Error bars show s.d. from three different experiments. (C) Synchronized C. elegans L1 larvae were grown on agar plates with the E. coli control strain OP50 or E. coli strains expressing the indicated siRNAs specific for Swan-1 and Swan-2 as food source. Animals were either kept under normal laboratory conditions (right) or stressed with heat shock and further growth on high-salt plates to trigger osmotic stress. Representative pictures from the worms are displayed, and all siRNA experiments were performed in four independent experiments for each of the strains.
Table 1. Synchronized C. elegans L1 larvae were grown on agar plates with the E. coli control strain OP50 or the E. coli strains expressing siRNAs targeting Swan-1 (F53C11.7) or Swan-2 (F53C11.8) either alone or in combination.
| Control OP 50 | Swan-1 (F53C11.7) | Swan-2 (F53C11.8) | Swan-1 (F53C11.7)+swan-2 (F53C11.8) | |
|---|---|---|---|---|
| Cultivation: 17°C | ||||
| NGM plates | Reproduction ++Development ++ | Reproduction ++Development ++ | Reproduction ++Development ++ | Reproduction +Development + |
| NGM plates + 100 mM NaCl | Reproduction −Development + | Reproduction +Development + | Reproduction −Development + | Reproduction −Development −F1 arrest in L2 |
| NGM plates + 200 mM NaCl | Reproduction +Development + | Reproduction +Development +F1 arrest in L3 | Reproduction −Development + | Reproduction −Development −F1 arrest in L2/L3 |
| Stress: 30°C, 5 h → Cultivation: 17°C | ||||
| NGM plates | Reproduction ++Development + | Reproduction +Development + | Reproduction +Development + | Reproduction −Development − |
| NGM plates + 100 mM NaCl | Reproduction +Development + | Reproduction −Development −F1 arrest in L2/L3 | Reproduction −Development −F1 arrest in L2/L3 | Reproduction −−Development −− → Stagnation in L2/L3, no F1 |
| NGM plates + 200 mM NaCl | Reproduction +Development + | Reproduction −Development −F1 arrest in L2/L3 | Reproduction −Development −F1 arrest in L2/L3 | Reproduction −−Development −− → Stagnation in L3, no F1 |
| Stress: 30°C, 5 h → Cultivation: 25°C | ||||
| NGM plates | Reproduction −Development − | Reproduction −Development − | Reproduction −Development − | Reproduction −−Development −− → Stagnation in L2, no F1 |
| NGM plates + 100 mM NaCl | Reproduction −Development − | Reproduction −Development − | Reproduction −Development − | Reproduction −−Development −− → Stagnation in adult−stage, no F1 |
| NGM plates + 200 mM NaCl | Reproduction −Development − | Reproduction −Development − | Reproduction −Development − | Reproduction −−Development −− → Stagnation in L2, no F1 |
| Animals were either kept under laboratory conditions or exposed to heat stress (5 h, 30°C), followed by further cultivation at 17 or 25°C as shown. Animals were further cultured in normal medium or media containing increasing NaCl concentrations to trigger the osmotic stress response. Reproduction and development were determined microscopically and scored as shown. | ||||
| ++, normal; +, slightly decelerated; −, decelerated; −−, stagnation. | ||||
Control of HIPK2 functions by the Han11 adapter protein
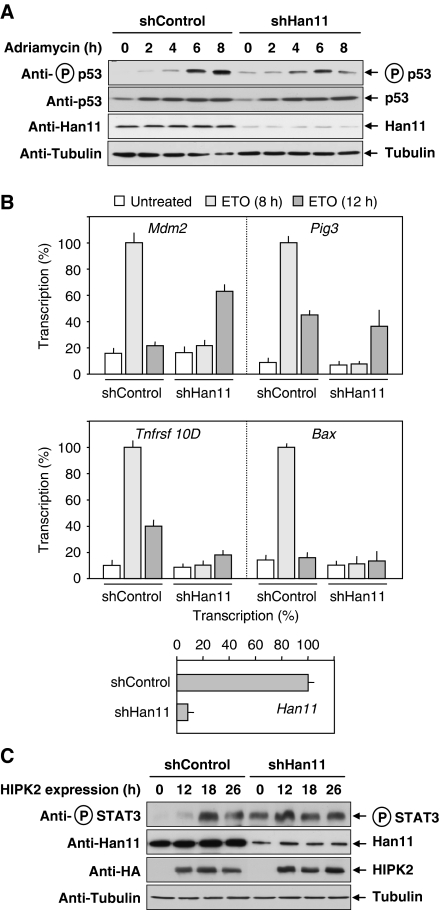
To test the role of Han11 for HIPK2 functions, cells were transfected with vectors encoding a Han11-specific shRNA or a mutated control and selected with puromycin to generate stable cell pools. Cells were exposed for different periods to the DNA-damaging chemotherapeutic agent adriamycin and analysed for p53 serine 46 phosphorylation, which is largely dependent on HIPK2 (Rinaldo et al, 2008; Puca et al, 2010). Although control cells showed no basal p53 phosporylation and a maximal p53 phosphorylation after 8 h, the knockdown of Han11 resulted in an elevated basal p53 phosphorylation and a blunted induction of the response, which was maximal already 6 h after stimulation (Figure 6A). It was then interesting to investigate the contribution of Han11 to the induction of DNA damage-triggered expression of genes that were reported to be HIPK2 dependent (Puca et al, 2008). Cell pools expressing Han11-specific shRNAs or controls were stimulated for different periods with the DNA-damaging agent etoposide, followed by quantitative analysis of gene expression by real-time PCR (Figure 6B). The downregulation of Han11 changed either the kinetics or the amplitude of HIPK2-dependent gene expression. The Han11 dependence of inducible Mdm2 and Pig3 expression was only evident after 8 h of stimulation, whereas stimulation for 12 h was similar or even better than in control cells expressing Han11. In contrast, etoposide-triggered transcription of the genes encoding Bax and the TNF receptor family member TNFRST 10D was severely compromised upon Han11 downregulation. To reveal a role of Han11 for a p53-independent function of HIPK2, we determined its impact on the kinetics of HIPK2-triggered phosphorylation of STAT3 at serine 727 (Matsuo et al, 2001). Control cells or knockdown cells were transfected to express HIPK2 and analysed at different time points for phosphorylation of the endogenous STAT3 protein using a phosphospecific antibody. Han11 knockdown resulted in strongly elevated basal STAT3 phosphorylation and a changed kinetics of STAT3 serine 727 phosphorylation without a significant impact on the amplitude (Figure 6C).
Figure 6.
Han11 controls HIPK2-mediated functions. (A) Cells were transfected to express a Han11-specific shRNA or a point-mutated control. After selection for 1 week in puromycin, equal numbers of cells were replated and stimulated with adriamycin (0.5 μg/ml) for the indicated periods. Equal amounts of protein contained in cell lysates were analysed for phosphorylation and expression of p53 and for Han11 expression as shown. (B) Han11 was knocked down in HCT116 cells after lentiviral delivery of shRNAs. After selection of stable cell pools with puromycin, cells were replated and stimulated with etoposide (3 μM) for 8 and 12 h as shown, followed by quantitative analysis of transcription of the indicated HIPK2-dependent genes and of Han11 to control efficient knockdown. Error bars show s.d. from two independent experiments performed in triplicates. (C) 293T cells were transfected to express the indicated shRNAs, followed by puromycin selection of stably transfected cells for 1 week. After reseeding of equal cell numbers on 6 cm dishes, cells were transfected to express HA-tagged HIPK2 and harvested after 12, 18 and 26 h after transfection. Cell extracts were analysed for phosphorylation of the endogenous STAT3 protein and HIPK2 expression with the indicated antibodies.
Han11 is a MEKK1-regulating scaffold protein
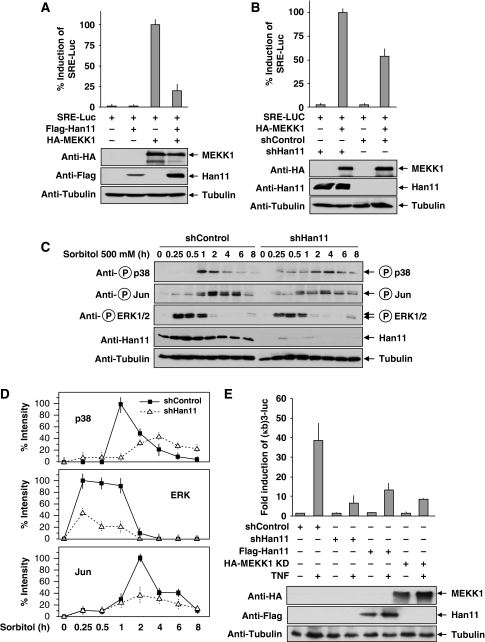
Besides the ability to bind to more than one signalling molecule, another defining hallmark of an adapter is its ability to interfere with signalling upon overexpression (Levchenko et al, 2000; Dhanasekaran et al, 2007). This inhibitory effect is attributable to sequestration of individual components, which results in a series of non-functional monovalent scaffolds. It was therefore interesting to test the impact of Han11 overexpression on MEKK1 signalling. Cells were transfected with a luciferase reporter gene controlled by three serum response elements (SREs) and combinations of MEKK1 and Han11. Luciferase assays showed that MEKK1-triggered MAP kinase signalling pathways augmenting SRE-dependent gene expression were strongly reduced upon overexpression of Han11 (Figure 7A). Similarly, downregulation of Han11 by shRNA blunted the ability of MEKK1 to trigger SRE-dependent gene expression (Figure 7B). As MEKK1 critically controls the activation of JNK, p38 and ERK pathways in response to osmotic stress (Winter-Vann and Johnson, 2007), we investigated the contribution of Han11 for this process. Stable cell pools transfected with vectors encoding a Han11-specific shRNA or an adequate control were generated. Osmotic stress was induced by sorbitol treatment for various periods, and activation of the MAPK signalling pathways was monitored with phosphospecific antibodies that recognize the phosphorylated JNK substrate Jun or the activated kinases p38 and ERK1/2 (Figure 7C). The downmodulation of Han11 resulted in a different kinetics and amplitude for activation of all three MAPKs investigated. Although control cells showed no p38 phosphorylation after 30 min and a sharp peak after 60 min, cells with downmodulated Han11 displayed a diminished maximal response and increased kinase activities during the late time points. Similarly, the kinetics and amplitude of Jun and ERK1/2 phosphorylation was markedly changed upon Han11 knockdown, as also evident by quantitative analysis of several experiments (Figure 7D). To test whether Han11 regulates MAPK signalling also in response to other stimuli, serum-starved cells were stimulated for different periods with FCS to trigger ERK activation. The knockdown of Han11 resulted in a blunted ERK induction and an elevated basal ERK phosphorylation (Supplementary Figure S7), suggesting that this scaffold protein is important for several MEKK1-mediated signalling pathways. As p38 MAP kinase activity is required for the maximal induction of NF-κB transcriptional activity (Beyaert et al, 1996; Vermeulen et al, 2003), the impact of Han11 over- or underexpression on NF-κB activity was measured. Cells were transiently transfected with a luciferase reporter gene under the control of three NF-κB binding sites along with expression vectors for a Han11 shRNA, Han11 or a kinase inactive point mutant of MEKK1 as a control. TNF-induced reporter gene activity was strongly impaired upon interference with Han11 levels (Figure 7E), supporting a role for Han11 in the regulation of MAPK-dependent signalling events.
Figure 7.
Han11 controls MEKK1-dependent signal outputs. (A) Cells were transfected with an SRE-dependent firefly luciferase reporter gene along with expression vectors for MEKK1 and Han11 at the indicated combinations and further grown in a medium containing 0.5% FCS to minimize SRE-dependent gene expression. After 24 h, cells were lysed and analysed for luciferase activity (upper) and protein expression (lower). In order to facilitate comparison, maximal gene activation was arbitrarily set as 100%. (B) Cells were transfected with the indicated shRNA vectors, and transfected cells were selected by puromycin treatment for 24 h. Then, the cells were retransfected with the SRE-dependent luciferase reporter gene and the indicated vectors encoding MEKK1 and a Han11-specific shRNA, followed by incubation in medium containing 0.5% FCS and puromycin for 1 day. Cells were lysed and further processed as described for (A). (C) HEK293 cells were transfected with shHan11 or shControl and selected with puromycin for several days. Equal cell numbers were reseeded in dishes and treated with sorbitol to trigger the osmotic stress response for different periods as shown. Equal amounts of proteins were further used for immunoblotting with the indicated phosphospecific and control antibodies. (D) The experiment from (C) was repeated four times and band intensities were quantified by densitometry. Maximal band intensity was set as 100%. Error bars show s.d. (E) Cells were transfected with an NF-κB-dependent luciferase reporter gene along with the indicated expression vectors for cDNAs and shRNAs. After 1 day, cells were stimulated for 8 h with TNF as shown and further analysed for luciferase activity (upper) and protein expression (lower). Luciferase activity values are expressed as fold induction relative to cells transfected only with the reporter gene. Error bars depict s.d from three different experiments in all luciferase experiments.
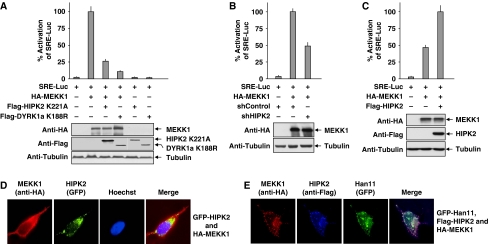
Scaffold proteins can coordinate branch points in signalling cascades and also provide directionality to signal transmission (Bashor et al, 2008). Given the broad impact of MEKK1 for the control of several downstream distinct signalling pathways (Uhlik et al, 2004), it was interesting to investigate whether the other Han11-associated kinases receive input signals from MEKK1. To address this possibility, the impact of kinase inactive and thus dominant negative versions of HIPK2 and DYRK1a on MEKK1-triggered activation of SRE-Luc was tested. Both kinase inactive forms interfered with MEKK1-mediated activation of the reporter gene (Figure 8A), thus suggesting that they are part of the complex downstream meshwork receiving input from MEKK1. Along this line, the shRNA-mediated knockdown of HIPK2 decreased MEKK1-triggered expression of the SRE-controlled luciferase reporter gene (Figure 8B). Conversely, MEKK1-triggered reporter gene expression in HIPK2 knockout cells was increased upon reexpression of HIPK2 (Figure 8C). Consistent with the concept of HIPK2 as a kinase receiving signals from MEKK1, immunofluorescence experiments frequently showed that MEKK1 expression caused a redistribution of HIPK2 away from nuclear bodies to the entire cell (Figure 8D). Coexpression of Han11, which increases the interaction between HIPK2 and MEKK1 (see also Figure 4A), resulted in a complete recruitment of HIPK2 to MEKK1 (Figure 8E), supporting the idea that Han11 can function as a bridging protein that allows coupling of MEKK1-dependent effects on HIPK2.
Figure 8.
Han11 mediates directional signalling from MEKK1. (A) Cells were transfected with an SRE-dependent firefly luciferase reporter construct and expression vectors for MEKK1 and kinase inactive versions of HIPK2 and DYRK1a. After further incubation under low serum conditions, cells were lysed and analysed for luciferase activity and protein expression. Full activation was set as 100%. Error bars depict s.d from three different experiments. (B) 293T cells were transfected with vectors allowing expression of HIPK2-specific or control shRNAs. After 2 days, cells were retransfected with the SRE-luciferase reporter gene and the expression vectors for MEKK1 and the indicated shRNAs, followed by analysis of luciferase gene expression as in (A). (C) HIPK2-deficient mouse embryonic fibroblasts were transfected as shown and analysed 1 day later for MEKK1-induced expression of the SRE-dependent luciferase activity as in (A). (D) Cells transfected to express HA-tagged MEKK1 and GFP-tagged HIPK2 were analysed for the intracellular localization of both proteins by immunofluorescence analysis as shown. (E) Immunofluorescence analysis of cells transfected to express MEKK1, Han11 and HIPK2. The merge displays colocalization of all three proteins.
Discussion
Here, we identify a function of Han11 as a scaffold protein in kinase signalling. Han11 has a rather simple architecture, as it contains only four WD40 repeats, which presumably form a propeller-like structure and thereby would be ideally suited to bind and assemble protein kinases (van der Voorn and Ploegh, 1992; Garcia-Higuera et al, 1998). Also other scaffold proteins contain WD40 repeats, as exemplified by MORG1, which can associate with Raf-1, B-Raf, MEK1, MEK2, ERK1 and ERK2 (Vomastek et al, 2004). In this context, it would be interesting to study whether different WD40 repeat containing scaffold proteins can mutually compete for binding to substrate proteins and thus increase the combinatorial and regulatory repertoire. Here, we discover HIPK2 as a further Han11-binding kinase, and it will be of interest in future studies if additional kinases assembling with this scaffold protein can be identified. The kinase domains of the interactors could be ideal candidates for the docking to Han11, as it is the case for HIPK2. On the other hand, the MEKK1 kinase domain (MEKK1Δ) failed to bind to Han11 (Supplementary Figure S8A and B), so that also other structures seem to mediate the interaction between Han11 and its interacting kinases. Besides its ability to interact with several kinases, Han11 fulfills several additional criteria that allow its definition as a kinase scaffold protein: Han11 lowers the threshold, amplitude and kinetics of HIPK2- and MEKK1-triggered signalling pathways. It will be interesting to study whether Han11-associated kinases are enzymatically active in substrate phosphorylation when bound to the adapter protein. The elevated kinase activities after downregulation of Han11 in unstimulated cells is compatible with the view that substrate phosphorylation is occurring independent from association to Han11, and accordingly, we found no evidence for binding of HIPK2 substrate proteins to Han11 (Supplementary Figure S9A and B).
A large number of input signals trigger the activation of MEKK1. These signals range from osmotic stress to CD40, TNF and growth factors (Kim et al, 2001; Matsuzawa et al, 2008). The MEKK1-dependent pathways can process this myriad of specific inputs into diverse biological outputs, such as the phosphorylation of its distinct downstream targets. The context-specific signalling of MEKK1 modules requires specific scaffold proteins that provide signalling conduits by assembling discrete sets of proteins into multiprotein complexes. In order to organize and isolate the diverse branch points from each other, MEKK1 does not only function as a scaffold protein by itself but also uses various different scaffolding complexes. These include JSAP1 that forms a complex with MEKK1, MKK4 and JNK (Ito et al, 1999), Axin (Zhang et al, 1999) and Han11, as revealed in this study. This complexity may in turn allow the regulation of adapter protein binding by the activity status of MEKK1. The kinase activity of MEKK1 was required to mediate ubiquitination of Han11 (Supplementary Figure S10). This raises the intriguing possibility that kinase-mediated regulation of adapter proteins allows mutual control of activity and accordingly also Han11-mediated cross-coupling to HIPK2 was dependent on the kinase activity of MEKK1 (Supplementary Figure S11), but the mechanisms and regulatory consequences of this mutual regulation for signal propagation await their elucidation in future studies.
The Han11 scaffold assembles kinases, which have partially overlapping functions. For example, MEKK1 and also DYRK1a suppresses NFAT signalling by retaining NFAT in the cytoplasm and thereby counteracting T-cell activation (Takahashi-Yanaga et al, 2006). But also MEKK1 and HIPK2 share some targets, as both have the ability to regulate the function of the acetyl transferase p300. Although MEKK1 stimulates p300-dependent transcription and needs p300 to mediate apoptosis, HIPK2 phosphorylates p300 at multiple sites (See et al, 2001; Schlesinger et al, 2002; Aikawa et al, 2006). It is also interesting to note that both, MEKK1 and HIPK2 can bind to the adapter protein Axin (Zhang et al, 1999; Rui et al, 2004). It will be of interest in future studies to investigate the potential contribution of MEKK1 to control the localization and activity of HIPK2. Downregulation of HIPK2 compromised MEKK1-triggered signalling only partially, suggesting that HIPK2 activity can be controlled by further upstream events. A previous study showed that Wnt-1 signalling activates HIPK2 via TAK1 (Kanei-Ishii et al, 2004), thus raising the possibility that several distinct upstream events contribute to the control of HIPK2 activity.
Han11 deficiency converted the mode of HIPK2 and MEKK1 activation from a digital/switch-like to a graded response. Overexpression of Han11 interferes with MEKK1-triggered signalling, which is in accordance with the mathematical models and experimental observations showing that low concentrations of a scaffold protein are optimal for the formation of a macromolecular complex, whereas high concentrations inhibit multiprotein complex formation (Levchenko et al, 2000; Morrison and Davis, 2003). This concept might also have biomedical implications, as dysregulated expression of scaffold proteins such as Han11 could affect oncogenic signalling cascades. A meta-analysis of gene array experiments in the public Oncomine microarray database revealed strongly decreased Han11 levels in invasive breast carcinomas (Supplementary Figure S12), raising the possibility that such cases of aberrant Han11 expression might result in dysregulated HIPK2 and MAPK signalling.
Materials and methods
Cells, reagents, plasmids and antibodies
Human osteosarcoma U2OS, 293, HIPK2-deficient mouse embryonic fibroblasts, HCT116 and 293T cells were maintained in DMEM supplemented with 10% FCS, 2 mM L-glutamine and 1% (v/v) penicillin/streptomycin at 37°C in a humidified atmosphere containing 5% CO2. Transient transfections were done with Rotifect according to the instructions of the manufacturer (Roth). The antibodies recognizing Flag (M2), tubulin (tub 2.1) (Sigma), Xpress (Invitrogen), Myc (9E10), GFP (7.1 and 13.1) and HA (3F10) (Roche) were purchased from the indicated suppliers. The phospho-p38 (Thr180/Tyr182), phospho-c-Jun (Ser63), phospho-STAT3 (Ser727), phospho-p53 (ser46) and control IgG antibodies were from Cell Signaling Technology, the phospho-ERK1/2 (#9101) antibody was purchased from New England Biolabs. Antibodies recognizing HIPK2 (Hofmann et al, 2002) and Han11 (Skurat and Dietrich, 2004) were previously described. The SRE-Luc reporter gene and the plasmids encoding Flag-HIPK2 and its derivatives (Hofmann et al, 2003), Flag-Han11 (Skurat and Dietrich, 2004) and pSUPER-HIPK2 (Gresko et al, 2009) were published. Epitope-tagged MEKK1 and its derivatives were from Addgene, HA-HIPK2, Flag-HIPK2(His)6, Myc-Han11 and a point-mutated version thereof, Flag-DYRK1a, Flag-DYRK1b, Myc-DYRK1a and Flag-DYRK1a K188R were cloned by standard procedures. The pSUPER-Puro and pSIREN-RetroQ-based vectors producing Han11-specific shRNAs were cloned by inserting an appropriate oligonucleotide targeting the Han11 sequence 5′-CGGAAGGAGATCTACAAG-3′ as described (Brummelkamp et al, 2002). The control vector was produced by targeting the sequence 5′-CGGTCGGAGATCTACAAG-3′ in which the nucleotides given in bold are different from the Han11-specific sequence. We are grateful to Dr W Becker (Aachen, Germany) for GFP-tagged DYRK1a and DYRK1b (Becker et al, 1998) and to A Rao (Boston) for Flag-DYRK2 (Gwack et al, 2006).
Real-time PCR
HCT116 cells were transduced with lentiviruses allowing the expression of Han11-specific shRNAs or the point-mutated controls according to standard protocols. One day later, cells were further grown in the presence of puromycin (1 μg/ml) for 10 days. Equal amounts of cells were then seeded in 6 cm dishes and treated with etoposide as specified in the figure legend. RNA was extracted with the RNAeasy kit (Qiagen), and samples were further processed as described (Renner et al, 2010). The sequences of the PCR primers are given as Supplementary data.
Immunofluorescence microscopy and determination of cell numbers
Localization of the epitope tagged or endogenous proteins in U2OS cells was measured by immunofluorescence as described (Renner et al, 2010). Cell numbers were determined after removal of the supernatant and washing of cells in PBS. The number of cells contained in 500 μl PBS was measured over a constant time of 120 s in a FACSCalibur.
Cross-linking experiments, cell lysis and coimmunoprecipitation experiments
Cells expressing hexahistidine-tagged proteins or the adequate controls were treated with freshly prepared DTBP solution (final concentration 0.5 mM in PBS) for 30 min at room temperature. After aspirating off this solution, cells were washed twice in PBS containing 200 mM Tris/HCl pH 7.5 and further processed as described (Roscic et al, 2006). Cells were lysed in IP lysis buffer (50 mM HEPES pH 7.5, 1% (v/v) Triton X-100, 50 mM NaCl, 5 mM EGTA, 2 mM sodium vanadate, 10 mM NaF, 0.2 μg/μl Aprotinin, 0.2 μg/μl Leupeptin, 1 mM phenylmethylsulfonylfluoride). Nuclear DNA of the lysates was sheared by two sonication steps for 5–10 s, and extracts were further used for immunoblotting or coimmunoprecipitation experiments as described (Renner et al, 2010).
Luciferase assays and western blotting
Luciferase assays were performed using the Promega kit according to the manufacturer's instructions. Western blotting was done with the semi-dry method, and secondary antibodies were detected using the enhanced chemiluminescence system.
C. elegans culture
C. elegans (wild-type strain N2, var. Bristol) was maintained on agar plates with Escherichia coli OP50 as a food source. Nematodes were separated from bacterial contaminants by density centrifugation on sucrose. Eggs were obtained by sodium hypochlorite treatment. Stage 1 larvae (L1) were transferred to nematode growth medium in 35 mm diameter agar plates (containing 25 mg/ml carbenicillin, 1 mM isopropyl-β-D-thiogalactopyranoside IPTG, 200 mM NaCl) together with control E. coli strains or the E. coli strains expressing siRNAs targeting the genes swan-1 (F53C11.7) and/or swan-2 (F53C11.8) (MRC Geneservice, Cambridge, UK) (Fraser et al, 2000). Stress experiments were performed upon cultivation of worms for 5 h at 30°C followed by further cultivation at room temperature under various conditions that are specified in Table I. Further development of worms was analysed microscopically.
Supplementary Material
Acknowledgments
We are indepted to Dr Issay Kitabayashi (Tokyo) for providing HIPK2-deficient cells. We thank Dres. A Rao (Boston, USA) and W Becker (Aachen, Germany) for generously providing plasmids and to Dr M Kracht (Giessen) for helpful comments on the manuscript. This work was supported by grants from the Deutscher akademischer Austauschdienst (A/08/98404), Deutsche Forschungsgemeinschaft projects SCHM 1417/4-2, SCHM 1417/7-1, SCHM 1417/8-1, GRK 1566/1, SFB/TRR 81, the Excellence Cluster Cardio-Pulmonary System (ECCPS) and the LOEWE/UGMLC program.
Footnotes
The authors declare that they have no conflict of interest.
References
- Aikawa Y, Nguyen LA, Isono K, Takakura N, Tagata Y, Schmitz ML, Koseki H, Kitabayashi I (2006) Roles of HIPK1 and HIPK2 in AML1- and p300-dependent transcription, hematopoiesis and blood vessel formation. EMBO J 25: 3955–3965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albert R, Oltvai ZN (2007) Shaping specificity in signaling networks. Nat Genet 39: 286–287 [DOI] [PubMed] [Google Scholar]
- Altafaj X, Dierssen M, Baamonde C, Marti E, Visa J, Guimera J, Oset M, Gonzalez JR, Florez J, Fillat C, Estivill X (2001) Neurodevelopmental delay, motor abnormalities and cognitive deficits in transgenic mice overexpressing Dyrk1A (minibrain), a murine model of Down's syndrome. Hum Mol Genet 10: 1915–1923 [DOI] [PubMed] [Google Scholar]
- Alvarez M, Estivill X, de la Luna S (2003) DYRK1A accumulates in splicing speckles through a novel targeting signal and induces speckle disassembly. J Cell Sci 116: 3099–3107 [DOI] [PubMed] [Google Scholar]
- Bashor CJ, Helman NC, Yan S, Lim WA (2008) Using engineered scaffold interactions to reshape MAP kinase pathway signaling dynamics. Science 319: 1539–1543 [DOI] [PubMed] [Google Scholar]
- Becker W, Joost HG (1999) Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity. Prog Nucleic Acid Res Mol Biol 62: 1–17 [DOI] [PubMed] [Google Scholar]
- Becker W, Weber Y, Wetzel K, Eirmbter K, Tejedor FJ, Joost HG (1998) Sequence characteristics, subcellular localization, and substrate specificity of DYRK-related kinases, a novel family of dual specificity protein kinases. J Biol Chem 273: 25893–25902 [DOI] [PubMed] [Google Scholar]
- Beyaert R, Cuenda A, Vanden Berghe W, Plaisance S, Lee JC, Haegeman G, Cohen P, Fiers W (1996) The p38/RK mitogen-activated protein kinase pathway regulates interleukin-6 synthesis response to tumor necrosis factor. EMBO J 15: 1914–1923 [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya RP, Remenyi A, Good MC, Bashor CJ, Falick AM, Lim WA (2006) The Ste5 scaffold allosterically modulates signaling output of the yeast mating pathway. Science 311: 822–826 [DOI] [PubMed] [Google Scholar]
- Bouwmeester T, Bauch A, Ruffner H, Angrand PO, Bergamini G, Croughton K, Cruciat C, Eberhard D, Gagneur J, Ghidelli S, Hopf C, Huhse B, Mangano R, Michon AM, Schirle M, Schlegl J, Schwab M, Stein MA, Bauer A, Casari G et al. (2004) A physical and functional map of the human TNF-alpha/NF-kappa B signal transduction pathway. Nat Cell Biol 6: 97–105 [DOI] [PubMed] [Google Scholar]
- Brummelkamp TR, Bernards R, Agami R (2002) A system for stable expression of short interfering RNAs in mammalian cells. Science 296: 550–553 [DOI] [PubMed] [Google Scholar]
- Calzado MA, Renner F, Roscic A, Schmitz ML (2007) HIPK2: a versatile switchboard regulating the transcription machinery and cell death. Cell Cycle 6: 139–143 [DOI] [PubMed] [Google Scholar]
- Cuevas BD, Abell AN, Johnson GL (2007) Role of mitogen-activated protein kinase kinase kinases in signal integration. Oncogene 26: 3159–3171 [DOI] [PubMed] [Google Scholar]
- D'Orazi G, Cecchinelli B, Bruno T, Manni I, Higashimoto Y, Saito S, Gostissa M, Coen S, Marchetti A, Del Sal G, Piaggio G, Fanciulli M, Appella E, Soddu S (2002) Homeodomain-interacting protein kinase-2 phosphorylates p53 at Ser 46 and mediates apoptosis. Nat Cell Biol 4: 11–19 [DOI] [PubMed] [Google Scholar]
- de Vetten N, Quattrocchio F, Mol J, Koes R (1997) The an11 locus controlling flower pigmentation in petunia encodes a novel WD-repeat protein conserved in yeast, plants, and animals. Genes Dev 11: 1422–1434 [DOI] [PubMed] [Google Scholar]
- Dhanasekaran DN, Kashef K, Lee CM, Xu H, Reddy EP (2007) Scaffold proteins of MAP-kinase modules. Oncogene 26: 3185–3202 [DOI] [PubMed] [Google Scholar]
- Dowjat WK, Adayev T, Kuchna I, Nowicki K, Palminiello S, Hwang YW, Wegiel J (2007) Trisomy-driven overexpression of DYRK1A kinase in the brain of subjects with Down syndrome. Neurosci Lett 413: 77–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AG, Kamath RS, Zipperlen P, Martinez-Campos M, Sohrmann M, Ahringer J (2000) Functional genomic analysis of C. elegans chromosome I by systematic RNA interference. Nature 408: 325–330 [DOI] [PubMed] [Google Scholar]
- Gallagher E, Enzler T, Matsuzawa A, Anzelon-Mills A, Otero D, Holzer R, Janssen E, Gao M, Karin M (2007) Kinase MEKK1 is required for CD40-dependent activation of the kinases Jnk and p38, germinal center formation, B cell proliferation and antibody production. Nat Immunol 8: 57–63 [DOI] [PubMed] [Google Scholar]
- Garcia-Higuera I, Gaitatzes C, Smith TF, Neer EJ (1998) Folding a WD repeat propeller. Role of highly conserved aspartic acid residues in the G protein beta subunit and Sec13. J Biol Chem 273: 9041–9049 [DOI] [PubMed] [Google Scholar]
- Gresko E, Ritterhoff S, Sevilla-Perez J, Roscic A, Frobius K, Kotevic I, Vichalkovski A, Hess D, Hemmings BA, Schmitz ML (2009) PML tumor suppressor is regulated by HIPK2-mediated phosphorylation in response to DNA damage. Oncogene 28: 698–708 [DOI] [PubMed] [Google Scholar]
- Gwack Y, Sharma S, Nardone J, Tanasa B, Iuga A, Srikanth S, Okamura H, Bolton D, Feske S, Hogan PG, Rao A (2006) A genome-wide Drosophila RNAi screen identifies DYRK-family kinases as regulators of NFAT. Nature 441: 646–650 [DOI] [PubMed] [Google Scholar]
- Hattangadi SM, Burke KA, Lodish HF (2010) Homeodomain-interacting protein kinase 2 plays an important role in normal terminal erythroid differentiation. Blood 115: 4853–4861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann TG, Mincheva A, Lichter P, Droge W, Schmitz ML (2000) Human homeodomain-interacting protein kinase-2 (HIPK2) is a member of the DYRK family of protein kinases and maps to chromosome 7q32–q34. Biochimie 82: 1123–1127 [DOI] [PubMed] [Google Scholar]
- Hofmann TG, Moller A, Sirma H, Zentgraf H, Taya Y, Droge W, Will H, Schmitz ML (2002) Regulation of p53 activity by its interaction with homeodomain-interacting protein kinase-2. Nat Cell Biol 4: 1–10 [DOI] [PubMed] [Google Scholar]
- Hofmann TG, Stollberg N, Schmitz ML, Will H (2003) HIPK2 regulates transforming growth factor-beta-induced c-Jun NH(2)-terminal kinase activation and apoptosis in human hepatoma cells. Cancer Res 63: 8271–8277 [PubMed] [Google Scholar]
- Hudson AM, Cooley L (2008) Phylogenetic, structural and functional relationships between WD- and Kelch-repeat proteins. Subcell Biochem 48: 6–19 [DOI] [PubMed] [Google Scholar]
- Ito M, Yoshioka K, Akechi M, Yamashita S, Takamatsu N, Sugiyama K, Hibi M, Nakabeppu Y, Shiba T, Yamamoto KI (1999) JSAP1, a novel jun N-terminal protein kinase (JNK)-binding protein that functions as a Scaffold factor in the JNK signaling pathway. Mol Cell Biol 19: 7539–7548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanei-Ishii C, Ninomiya-Tsuji J, Tanikawa J, Nomura T, Ishitani T, Kishida S, Kokura K, Kurahashi T, Ichikawa-Iwata E, Kim Y, Matsumoto K, Ishii S (2004) Wnt-1 signal induces phosphorylation and degradation of c-Myb protein via TAK1, HIPK2, and NLK. Genes Dev 18: 816–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karandikar M, Xu S, Cobb MH (2000) MEKK1 binds raf-1 and the ERK2 cascade components. J Biol Chem 275: 40120–40127 [DOI] [PubMed] [Google Scholar]
- Kim JW, Joe CO, Choi EJ (2001) Role of receptor-interacting protein in tumor necrosis factor-alpha-dependent MEKK1 activation. J Biol Chem 276: 27064–27070 [DOI] [PubMed] [Google Scholar]
- Kim YH, Choi CY, Lee SJ, Conti MA, Kim Y (1998) Homeodomain-interacting protein kinases, a novel family of co-repressors for homeodomain transcription factors. J Biol Chem 273: 25875–25879 [DOI] [PubMed] [Google Scholar]
- Kimura R, Kamino K, Yamamoto M, Nuripa A, Kida T, Kazui H, Hashimoto R, Tanaka T, Kudo T, Yamagata H, Tabara Y, Miki T, Akatsu H, Kosaka K, Funakoshi E, Nishitomi K, Sakaguchi G, Kato A, Hattori H, Uema T et al. (2007) The DYRK1A gene, encoded in chromosome 21 Down syndrome critical region, bridges between beta-amyloid production and tau phosphorylation in Alzheimer disease. Hum Mol Genet 16: 15–23 [DOI] [PubMed] [Google Scholar]
- Kolch W (2005) Coordinating ERK/MAPK signalling through scaffolds and inhibitors. Nat Rev Mol Cell Biol 6: 827–837 [DOI] [PubMed] [Google Scholar]
- Levchenko A, Bruck J, Sternberg PW (2000) Scaffold proteins may biphasically affect the levels of mitogen-activated protein kinase signaling and reduce its threshold properties. Proc Natl Acad Sci USA 97: 5818–5823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li XL, Arai Y, Harada H, Shima Y, Yoshida H, Rokudai S, Aikawa Y, Kimura A, Kitabayashi I (2007) Mutations of the HIPK2 gene in acute myeloid leukemia and myelodysplastic syndrome impair AML1- and p53-mediated transcription. Oncogene 26: 7231–7239 [DOI] [PubMed] [Google Scholar]
- Lu Z, Xu S, Joazeiro C, Cobb MH, Hunter T (2002) The PHD domain of MEKK1 acts as an E3 ubiquitin ligase and mediates ubiquitination and degradation of ERK1/2. Mol Cell 9: 945–956 [DOI] [PubMed] [Google Scholar]
- Manning G, Whyte DB, Martinez R, Hunter T, Sudarsanam S (2002) The protein kinase complement of the human genome. Science 298: 1912–1934 [DOI] [PubMed] [Google Scholar]
- Matsuo R, Ochiai W, Nakashima K, Taga T (2001) A new expression cloning strategy for isolation of substrate-specific kinases by using phosphorylation site-specific antibody. J Immunol Methods 247: 141–151 [DOI] [PubMed] [Google Scholar]
- Matsuzawa A, Tseng PH, Vallabhapurapu S, Luo JL, Zhang W, Wang H, Vignali DA, Gallagher E, Karin M (2008) Essential cytoplasmic translocation of a cytokine receptor-assembled signaling complex. Science 321: 663–668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercer SE, Ewton DZ, Deng X, Lim S, Mazur TR, Friedman E (2005) Mirk/Dyrk1B mediates survival during the differentiation of C2C12 myoblasts. J Biol Chem 280: 25788–25801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison DK, Davis RJ (2003) Regulation of MAP kinase signaling modules by scaffold proteins in mammals. Annu Rev Cell Dev Biol 19: 91–118 [DOI] [PubMed] [Google Scholar]
- Pimienta G, Pascual J (2007) Canonical and alternative MAPK signaling. Cell Cycle 6: 2628–2632 [DOI] [PubMed] [Google Scholar]
- Puca R, Nardinocchi L, Gal H, Rechavi G, Amariglio N, Domany E, Notterman DA, Scarsella M, Leonetti C, Sacchi A, Blandino G, Givol D, D'Orazi G (2008) Reversible dysfunction of wild-type p53 following homeodomain-interacting protein kinase-2 knockdown. Cancer Res 68: 3707–3714 [DOI] [PubMed] [Google Scholar]
- Puca R, Nardinocchi L, Givol D, D'Orazi G (2010) Regulation of p53 activity by HIPK2: molecular mechanisms and therapeutical implications in human cancer cells. Oncogene 29: 4378–4387 [DOI] [PubMed] [Google Scholar]
- Renner F, Moreno R, Schmitz ML (2010) SUMOylation-dependent localization of IKKepsilon in PML nuclear bodies is essential for protection against DNA-damage-triggered cell death. Mol Cell 37: 503–515 [DOI] [PubMed] [Google Scholar]
- Rinaldo C, Prodosmo A, Siepi F, Soddu S (2007) HIPK2: a multitalented partner for transcription factors in DNA damage response and development. Biochem Cell Biol 85: 411–418 [DOI] [PubMed] [Google Scholar]
- Rinaldo C, Siepi F, Prodosmo A, Soddu S (2008) HIPKs: Jack of all trades in basic nuclear activities. Biochim Biophys Acta 1783: 2124–2129 [DOI] [PubMed] [Google Scholar]
- Roscic A, Moller A, Calzado MA, Renner F, Wimmer VC, Gresko E, Ludi KS, Schmitz ML (2006) Phosphorylation-dependent control of Pc2 SUMO E3 ligase activity by its substrate protein HIPK2. Mol Cell 24: 77–89 [DOI] [PubMed] [Google Scholar]
- Rui Y, Xu Z, Lin S, Li Q, Rui H, Luo W, Zhou HM, Cheung PY, Wu Z, Ye Z, Li P, Han J, Lin SC (2004) Axin stimulates p53 functions by activation of HIPK2 kinase through multimeric complex formation. EMBO J 23: 4583–4594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell M, Lange-Carter CA, Johnson GL (1995) Direct interaction between Ras and the kinase domain of mitogen-activated protein kinase kinase kinase (MEKK1). J Biol Chem 270: 11757–11760 [DOI] [PubMed] [Google Scholar]
- Schlesinger TK, Bonvin C, Jarpe MB, Fanger GR, Cardinaux JR, Johnson GL, Widmann C (2002) Apoptosis stimulated by the 91-kDa caspase cleavage MEKK1 fragment requires translocation to soluble cellular compartments. J Biol Chem 277: 10283–10291 [DOI] [PubMed] [Google Scholar]
- See RH, Calvo D, Shi Y, Kawa H, Luke MP, Yuan Z (2001) Stimulation of p300-mediated transcription by the kinase MEKK1. J Biol Chem 276: 16310–16317 [DOI] [PubMed] [Google Scholar]
- Shaw AS, Filbert EL (2009) Scaffold proteins and immune-cell signalling. Nat Rev Immunol 9: 47–56 [DOI] [PubMed] [Google Scholar]
- Skurat AV, Dietrich AD (2004) Phosphorylation of Ser640 in muscle glycogen synthase by DYRK family protein kinases. J Biol Chem 279: 2490–2498 [DOI] [PubMed] [Google Scholar]
- Smith FD, Langeberg LK, Scott JD (2006) The where's and when's of kinase anchoring. Trends Biochem Sci 31: 316–323 [DOI] [PubMed] [Google Scholar]
- Takahashi-Yanaga F, Mori J, Matsuzaki E, Watanabe Y, Hirata M, Miwa Y, Morimoto S, Sasaguri T (2006) Involvement of GSK-3beta and DYRK1B in differentiation-inducing factor-3-induced phosphorylation of cyclin D1 in HeLa cells. J Biol Chem 281: 38489–38497 [DOI] [PubMed] [Google Scholar]
- Uhlik MT, Abell AN, Cuevas BD, Nakamura K, Johnson GL (2004) Wiring diagrams of MAPK regulation by MEKK1, 2, and 3. Biochem Cell Biol 82: 658–663 [DOI] [PubMed] [Google Scholar]
- van der Voorn L, Ploegh HL (1992) The WD-40 repeat. FEBS Lett 307: 131–134 [DOI] [PubMed] [Google Scholar]
- Vermeulen L, De Wilde G, Van Damme P, Vanden Berghe W, Haegeman G (2003) Transcriptional activation of the NF-kappaB p65 subunit by mitogen- and stress-activated protein kinase-1 (MSK1). EMBO J 22: 1313–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vomastek T, Schaeffer HJ, Tarcsafalvi A, Smolkin ME, Bissonette EA, Weber MJ (2004) Modular construction of a signaling scaffold: MORG1 interacts with components of the ERK cascade and links ERK signaling to specific agonists. Proc Natl Acad Sci USA 101: 6981–6986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei G, Ku S, Ma GK, Saito S, Tang AA, Zhang J, Mao JH, Appella E, Balmain A, Huang EJ (2007) HIPK2 represses beta-catenin-mediated transcription, epidermal stem cell expansion, and skin tumorigenesis. Proc Natl Acad Sci USA 104: 13040–13045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter-Vann AM, Johnson GL (2007) Integrated activation of MAP3Ks balances cell fate in response to stress. J Cell Biochem 102: 848–858 [DOI] [PubMed] [Google Scholar]
- Yu J, Deshmukh H, Gutmann RJ, Emnett RJ, Rodriguez FJ, Watson MA, Nagarajan R, Gutmann DH (2009) Alterations of BRAF and HIPK2 loci predominate in sporadic pilocytic astrocytoma. Neurology 73: 1526–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yujiri T, Sather S, Fanger GR, Johnson GL (1998) Role of MEKK1 in cell survival and activation of JNK and ERK pathways defined by targeted gene disruption. Science 282: 1911–1914 [DOI] [PubMed] [Google Scholar]
- Zhang Y, Neo SY, Wang X, Han J, Lin SC (1999) Axin forms a complex with MEKK1 and activates c-Jun NH(2)-terminal kinase/stress-activated protein kinase through domains distinct from Wnt signaling. J Biol Chem 274: 35247–35254 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.