Abstract
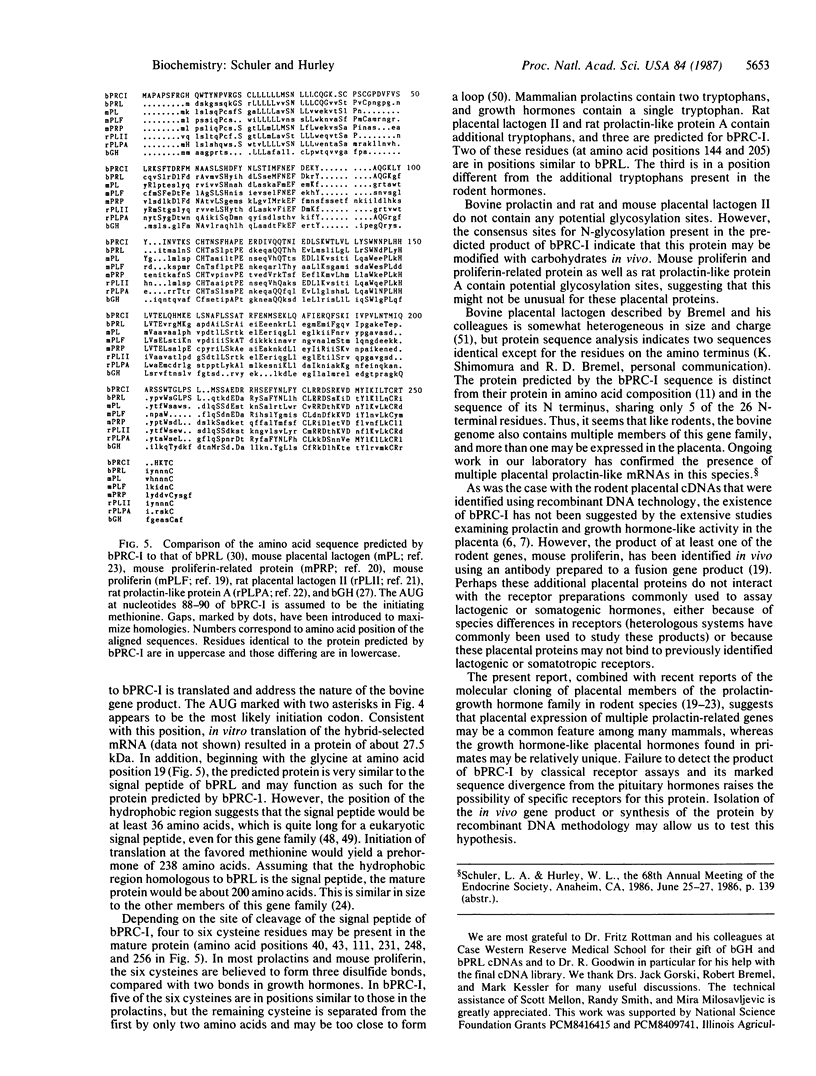
Bovine (Bos taurus) prolactin-related cDNA I (bPRC-I), distinct from the isolated bovine placental lactogen, was derived from bovine fetal placental mRNA by molecular cloning. The nucleotide sequence is 63% homologous to bovine prolactin cDNA and only 45% to bovine growth hormone. The region of bPRC-I corresponding to the 5' portion of the signal peptide and 5' untranslated region of bovine prolactin mRNA is markedly different from prolactin. The predicted protein is 39% homologous to bovine prolactin and about 30% to the related placental hormones in rodents. This identification of a prolactin-related gene in the cow in addition to those reported in rodents suggests that multiple prolactin-related genes expressed in the placenta may be a general phenomenon in nonprimates. The role of these related hormones during gestation remains to be investigated.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arima Y., Bremel R. D. Purification and characterization of bovine placental lactogen. Endocrinology. 1983 Dec;113(6):2186–2194. doi: 10.1210/endo-113-6-2186. [DOI] [PubMed] [Google Scholar]
- Becka S., Bílek J., Slaba J., Skarda J., Mikulás I. Some properties of the goat placental lactogen. Experientia. 1977 Jun 15;33(6):771–772. doi: 10.1007/BF01944183. [DOI] [PubMed] [Google Scholar]
- Benton W. D., Davis R. W. Screening lambdagt recombinant clones by hybridization to single plaques in situ. Science. 1977 Apr 8;196(4286):180–182. doi: 10.1126/science.322279. [DOI] [PubMed] [Google Scholar]
- Berger S. L., Wallace D. M., Siegal G. P., Hitchcock M. J., Birkenmeier C. S., Reber S. B. Preparation of interferon messenger RNAs with the use of ribonucleoside--vanadyl complexes. Methods Enzymol. 1981;79(Pt B):59–68. doi: 10.1016/s0076-6879(81)79013-x. [DOI] [PubMed] [Google Scholar]
- Byatt J. C., Shimomura K., Duello T. M., Bremel R. D. Isolation and characterization of multiple forms of bovine placental lactogen from secretory granules of the fetal cotyledon. Endocrinology. 1986 Sep;119(3):1343–1350. doi: 10.1210/endo-119-3-1343. [DOI] [PubMed] [Google Scholar]
- Colosi P., Marr G., Lopez J., Haro L., Ogren L., Talamantes F. Isolation, purification, and characterization of mouse placental lactogen. Proc Natl Acad Sci U S A. 1982 Feb;79(3):771–775. doi: 10.1073/pnas.79.3.771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duckworth M. L., Kirk K. L., Friesen H. G. Isolation and identification of a cDNA clone of rat placental lactogen II. J Biol Chem. 1986 Aug 15;261(23):10871–10878. [PubMed] [Google Scholar]
- Duckworth M. L., Peden L. M., Friesen H. G. Isolation of a novel prolactin-like cDNA clone from developing rat placenta. J Biol Chem. 1986 Aug 15;261(23):10879–10884. [PubMed] [Google Scholar]
- Eakle K. A., Arima Y., Swanson P., Grimek H., Bremel R. D. A 32,000-molecular weight protein from bovine placenta with placental lactogen-like activity in radioreceptor assays. Endocrinology. 1982 May;110(5):1758–1765. doi: 10.1210/endo-110-5-1758. [DOI] [PubMed] [Google Scholar]
- Glisin V., Crkvenjakov R., Byus C. Ribonucleic acid isolated by cesium chloride centrifugation. Biochemistry. 1974 Jun 4;13(12):2633–2637. doi: 10.1021/bi00709a025. [DOI] [PubMed] [Google Scholar]
- Gluckman P. D., Kaplan S. L., Rudolph A. M., Grumbach M. M. Hormone ontogeny in the ovine fetus. II. Ovine chorionic somatomammotropin in mid- and late gestation in the fetal and maternal circulations. Endocrinology. 1979 Jun;104(6):1828–1833. doi: 10.1210/endo-104-6-1828. [DOI] [PubMed] [Google Scholar]
- Hanahan D., Meselson M. Plasmid screening at high colony density. Gene. 1980 Jun;10(1):63–67. doi: 10.1016/0378-1119(80)90144-4. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Hoffman L. M., Fritsch M. K., Gorski J. Probable nuclear precursors of preprolactin mRNA in rat pituitary cells. J Biol Chem. 1981 Mar 25;256(6):2597–2600. [PubMed] [Google Scholar]
- Hurley T. W., Handwerger S., Fellows R. E. Isolation and structural characterization of ovine placental lactogen. Biochemistry. 1977 Dec 13;16(25):5598–5604. doi: 10.1021/bi00644a033. [DOI] [PubMed] [Google Scholar]
- JOSIMOVICH J. B., MACLAREN J. A. Presence in the human placenta and term serum of a highly lactogenic substance immunologically related to pituitary growth hormone. Endocrinology. 1962 Aug;71:209–220. doi: 10.1210/endo-71-2-209. [DOI] [PubMed] [Google Scholar]
- Jackson L. L., Colosi P., Talamantes F., Linzer D. I. Molecular cloning of mouse placental lactogen cDNA. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8496–8500. doi: 10.1073/pnas.83.22.8496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayatilak P. G., Glaser L. A., Basuray R., Kelly P. A., Gibori G. Identification and partial characterization of a prolactin-like hormone produced by rat decidual tissue. Proc Natl Acad Sci U S A. 1985 Jan;82(1):217–221. doi: 10.1073/pnas.82.1.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly P. A., Tsushima T., Shiu R. P., Friesen H. G. Lactogenic and growth hormone-like activities in pregnancy determined by radioreceptor assays. Endocrinology. 1976 Sep;99(3):765–774. doi: 10.1210/endo-99-3-765. [DOI] [PubMed] [Google Scholar]
- Leszczynski J. F., Rose G. D. Loops in globular proteins: a novel category of secondary structure. Science. 1986 Nov 14;234(4778):849–855. doi: 10.1126/science.3775366. [DOI] [PubMed] [Google Scholar]
- Linzer D. I., Lee S. J., Ogren L., Talamantes F., Nathans D. Identification of proliferin mRNA and protein in mouse placenta. Proc Natl Acad Sci U S A. 1985 Jul;82(13):4356–4359. doi: 10.1073/pnas.82.13.4356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linzer D. I., Nathans D. A new member of the prolactin-growth hormone gene family expressed in mouse placenta. EMBO J. 1985 Jun;4(6):1419–1423. doi: 10.1002/j.1460-2075.1985.tb03796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller W. L., Eberhardt N. L. Structure and evolution of the growth hormone gene family. Endocr Rev. 1983 Spring;4(2):97–130. doi: 10.1210/edrv-4-2-97. [DOI] [PubMed] [Google Scholar]
- Murthy G. S., Schellenberg C., Friesen H. G. Purification and characterization of bovine placental lactogen. Endocrinology. 1982 Dec;111(6):2117–2124. doi: 10.1210/endo-111-6-2117. [DOI] [PubMed] [Google Scholar]
- Niall H. D., Hogan M. L., Sauer R., Rosenblum I. Y., Greenwood F. C. Sequences of pituitary and placental lactogenic and growth hormones: evolution from a primordial peptide by gene reduplication. Proc Natl Acad Sci U S A. 1971 Apr;68(4):866–870. doi: 10.1073/pnas.68.4.866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicoll C. S. Prolactin and growth hormone: specialists on one hand and mutual mimics on the other. Perspect Biol Med. 1982 Spring;25(3):369–381. doi: 10.1353/pbm.1982.0025. [DOI] [PubMed] [Google Scholar]
- Proudfoot N. J., Brownlee G. G. 3' non-coding region sequences in eukaryotic messenger RNA. Nature. 1976 Sep 16;263(5574):211–214. doi: 10.1038/263211a0. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Robertson M. C., Friesen H. G. Two forms of rat placental lactogen revealed by radioimmunoassay. Endocrinology. 1981 Jun;108(6):2388–2390. doi: 10.1210/endo-108-6-2388. [DOI] [PubMed] [Google Scholar]
- Roychoudhury R., Jay E., Wu R. Terminal labeling and addition of homopolymer tracts to duplex DNA fragments by terminal deoxynucleotidyl transferase. Nucleic Acids Res. 1976 Jan;3(1):101–116. doi: 10.1093/nar/3.1.101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala-Trepat J. M., Savage M. J., Bonner J. Isolation and characterization of poly(adenylic acid)-containing messenger ribonucleic acid from rat liver polysomes. Biochim Biophys Acta. 1978 Jun 22;519(1):173–193. doi: 10.1016/0005-2787(78)90071-0. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasavage N. L., Nilson J. H., Horowitz S., Rottman F. M. Nucleotide sequence of bovine prolactin messenger RNA. Evidence for sequence polymorphism. J Biol Chem. 1982 Jan 25;257(2):678–681. [PubMed] [Google Scholar]
- Schellenberg C., Friesen H. G. The bioassay of bovine placental lactogen. Endocrinology. 1982 Dec;111(6):2125–2128. doi: 10.1210/endo-111-6-2125. [DOI] [PubMed] [Google Scholar]
- Simpson E. R., MacDonald P. C. Endocrine physiology of the placenta. Annu Rev Physiol. 1981;43:163–188. doi: 10.1146/annurev.ph.43.030181.001115. [DOI] [PubMed] [Google Scholar]
- Southard J. N., Thordarson G., Talamantes F. Purification and partial characterization of hamster placental lactogen. Endocrinology. 1986 Aug;119(2):508–514. doi: 10.1210/endo-119-2-508. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Talamantes F., Jr Comparative study of the occurrence of placental prolactin among mammals. Gen Comp Endocrinol. 1975 Sep;27(1):115–121. doi: 10.1016/0016-6480(75)90059-3. [DOI] [PubMed] [Google Scholar]
- Talamantes F., Ogren L., Markoff E., Woodard S., Madrid J. Phylogenetic distribution, regulation of secretion, and prolactin-like effects of placental lactogens. Fed Proc. 1980 Jun;39(8):2582–2587. [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson M. E. Compilation of published signal sequences. Nucleic Acids Res. 1984 Jul 11;12(13):5145–5164. doi: 10.1093/nar/12.13.5145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woychik R. P., Camper S. A., Lyons R. H., Horowitz S., Goodwin E. C., Rottman F. M. Cloning and nucleotide sequencing of the bovine growth hormone gene. Nucleic Acids Res. 1982 Nov 25;10(22):7197–7210. doi: 10.1093/nar/10.22.7197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanisch-Perron C., Vieira J., Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33(1):103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Patterns of amino acids near signal-sequence cleavage sites. Eur J Biochem. 1983 Jun 1;133(1):17–21. doi: 10.1111/j.1432-1033.1983.tb07424.x. [DOI] [PubMed] [Google Scholar]