Abstract
Background
In order to develop improved vaccines for patients with recurrent prostate cancer (PCa), we tested the feasibility of using type-1-polarized dendritic cells (DC1s) to cross-present antigens from allogeneic PCa cells and to induce functional CD8+ T cell responses against PCa cells and against defined MHC class I-restricted PCa-relevant epitopes.
Methods
Monocyte-derived DCs from PCa patients were matured using the “standard” cytokine cocktail (IL-1β/TNFα/IL-6/PGE2) or using the αDC1-polarizing cocktail (IL-1β/TNFα/IFNα/IFNγ/poly-I:C), loaded with UV-irradiated LNCaP cells, and used to sensitize autologous CD8+ T cells.
Results
αDC1s from PCa patients secreted 10-30 times higher levels of IL-12p70 than sDCs. Importantly this elevated capacity for IL-12p70 secretion was not inhibited by loading with apoptotic tumor cells. Comparing to standard DCs, αDC1s induced higher numbers of CD8+ T cells capable of recognizing both the original PCa cells as well as another PCa cell line, DU145, in MHC class I-restricted fashion. Furthermore, αDC1s induced higher numbers of CD8+ T cells recognizing defined prostate cancer-specific class I-restricted peptide epitopes of prostate-specific antigen and prostatic acid phosphatase: PAP135-143(average 49-fold higher), PAP112-120 (average 24-fold), PSA141-150 (average 5.5-fold) and PSA146-154 (average 11-fold).
Conclusion
Type-1 polarization of GM-CSF/IL-4-generated DCs enhances their ability to present allogeneic tumor cells and to induce CD8+ T cells recognizing different prostate cancer cells and multiple defined prostate cancer-specific epitopes. These observations help to develop improved immunotherapies of prostate cancer for patients with different HLA types and lacking autologous tumor material.
Keywords: prostate cancer, vaccines, dendritic cells, IL-12, CTLs
Introduction
Prostate cancer (PCa) is the most common non-cutaneous cancer and the second leading cause of cancer-related death in men in the US, with over 200,000 new patients diagnosed each year and over 30,000 succumbing to metastatic disease (1,2). Standard treatment of localized, organ-confined prostate cancer includes surgery or radiation therapy and is effective in the short term, but up to one third of patients relapse (3-6). Current systemic therapy of recurrent disease, involves androgen ablation and chemotherapy (7), is associated with significant morbidity (8), and most patients eventually developing resistance to both these treatment modalities. Prostate-specific vaccine therapy, if effective and if initiated early (i.e., at the time of initial relapse), offers the prospect of prolonged survival with minimal morbidity. Furthermore, the limited tumor burden in men who develop a PSA relapse following prostatectomy makes this group of patients particularly good candidates for immunotherapy. Among a vast array of novel therapies being developed for advanced PCa a number of immunotherapeutic approaches are being tested in clinics. Dendritic cell (DC)-based therapies which utilize whole tumor cells (9) or tumor-related peptides (10-13), proteins or DNA (14-20), as the source of tumor-related antigens, offer the advantage of targeting many prostate cancer-overexpressed antigens, including prostate specific antigen (PSA), and prostatic acid phosphatase (PAP) (9,18,21-23). Vaccines targeting PAP and PSA have generated immense enthusiasm, given the recent evidence of clinical efficacy, including a survival advantage in the setting of advanced PCa (16,17,24-27).
We have previously demonstrated that maturation of DCs in the presence of inflammatory mediators mimicking the conditions of acute viral infections strongly elevates the cytokine-producing and immunostimulatory function of DCs (28,29). The resulting alpha-type-1 polarized DCs (αDC1s) loaded with defined HLA-A2-restricted antigenic peptides induce on average 20-fold higher levels of functional CD8+ CTLs against such defined melanoma-specific epitopes, compared to sDCs (29). Furthermore, our observations that αDC1s loaded with autologous tumor material induce MHC class I-restricted CD8+ CTLs capable of recognizing the same autologous tumor cells open the possibility of applying αDC1s to the treatment of patients without clearly-identified tumor rejection antigens but with available sterile tumor cells, such as CLL (30).
In the current study we tested the feasibility of employing αDC1-based vaccines for the treatment of cancer patients without available autologous tumor tissue, such as prostate cancer patients whose prostates have been previously removed, and who now present with PSA-only disease. Our data demonstrate that αDC1s generated from the blood of such prostate cancer patients are highly-effective in cross-presenting allogeneic PCa cells and inducing functional CD8+ T cells capable of targeting multiple defined HLA class I-restricted epitopes of PAP and PSA.
Materials and Methods
Patients and blood samples
Peripheral blood (PB) was collected from prostate cancer patients under an IRB-approved protocol, following an informed consent.
Generation of DCs (Supplemental Figure 1)
Peripheral blood mononuclear cells (PBMCs) were isolated from the blood of prostate cancer patients by density gradient centrifugation with Lymphocyte Separation Medium (Cellgro Mediatech). Monocyte and CD8+ lymphocyte fractions were further isolated using CD14 and CD8 Microbeads, respectively (Miltenyi Biotech). Monocytes were cultured in Cellgenix medium (Cellgenix) containing rhu GM-CSF and IL-4 (both 1,000 IU/mL) (Schering-Plough) for 6 days in 24-well plates at 5 × 105 cells per well. On day 6, DCs were induced to mature using either conventional cytokine cocktail composed of IL-1β (25ng/mL) (Miltenyi Biotech), TNFα (50 ng/mL) (Miltenyi Biotech), IL-6 (1,000 units/mL) (Thermo Scientific), and PGE2 (10−6 mol/L) (Sigma-Aldrich) for sDCs or with an αDC1-polarizing cocktail composed of IL-1β (25 ng/mL), TNFα (50 ng/mL), IFNα (3,000 units/mL) (Intron A-IFN-α-2b; Schering-Plough), IFN-γ (1,000 units/mL) (Miltenyi Biotech) and poly-I:C (20 μg/mL) (Sigma-Aldrich) for αDC1s. Maturing DCs were pulsed with UVB- and gamma-irradiated LNCaP cells at a ratio of 3:1 (DC:tumor) at 20 minutes after the addition of maturation-inducing cytokines. Differentially-matured and antigen-loaded DCs were harvested on day 8. Alternatively, sDC and αDC1 were matured with cytokines as described above without tumor pulsing, and on day 8 they were loaded with MHC class I prostate cancer related peptides: PSA 1 (141-150; FLTPKLQCV), PSA 2 (146-154; KLQCVDLHV), PAP-3 (135-143; ILLWQPIPV), and PAP-5 (112-120; TLMSAMTNL) at 10 μg/ml for 2 hr at 37° C.
Preparation of irradiated LNCaP cells as antigen source
The clinical grade LNCaP cells (HLA class I-low, see Supplemental Figure 2), selected in order to limit the extent of allo-specific component of the immune response against cancer cells), were obtained from ATCC, and maintained in RPMI medium supplemented with 10% heat inactivated fetal bovine serum (Gemini Bio-Products), penicillin, streptomycin and L-Glutamine (all from Gibco, Invitrogen). LNCaP cells were harvested using 1mM EDTA (Gibco, Invitrogen) in PBS and were treated with UVB-irradiation (120 mJ/cm2) and gamma-irradiation (30 Gy). The irradiated LNCaP cells were washed, tested for apoptosis using nonyl acridine orange (NAO) staining (30,31), and added to DC cultures 20 min after the addition of the maturation-inducing cytokines.
Immunophenotyping
Flow cytometry analyses were performed using Beckman Coulter Epics XL, after labeling cells with CD80-fluorescein isothiocyanate (FITC)(Becton Dickinson), CD83-FITC (Beckman Coulter), CD86-FITC (Becton Dickinson), CD11c-FITC (e-Bioscience) and CCR7-FITC (R&D) for DCs, CD8-FITC (Beckman Coulter), CD3-PE (e-Bioscience) for T cells, CD14-FITC (Becton Dickinson) for monocytes, and appropriate isotype control antibodies. In order to facilitate the analysis of the maturation-associated changes in the levels of expression of different costimulatory molecules and maturation markers on DCs from 10 different patients, and to eliminate the differences in autofluorescence and nonspecific antibody binding between different DC preparations (30), the data in Fig. 1C has been expressed as the means of delta MFI (a ratio of specific marker fluorescence to fluorescence observed with isotype control MFI), in addition to showing representative data from a single patient in Fig. 1B.
Fig. 1. Feasibility of generating tumor-loaded functional αDC1s from prostate cancer patients.
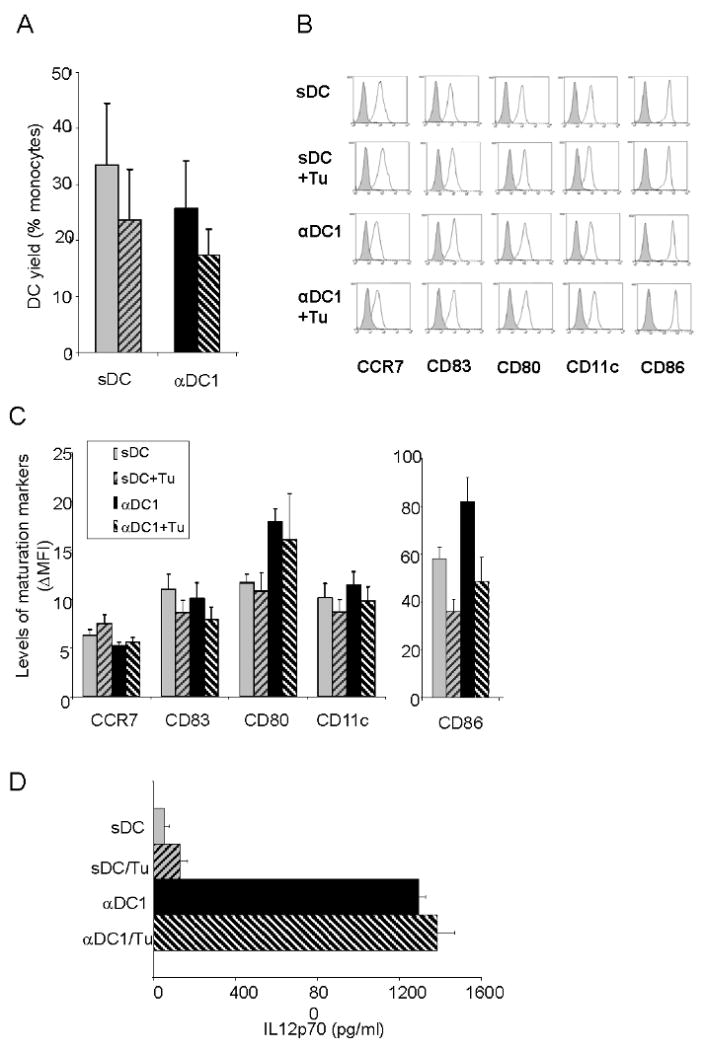
A. Recovery of DCs is not affected by tumor loading. Monocytes isolated from PCa patients' blood were plated at 5×105 cells per well in 24 well plates and cultured for 6 days in the presence of GM-CSF and IL4. On day 6, DC were induced to mature into polarized αDC1 (with TNFα, IL1β, IFNα, IFNγ, and Poly-I:C) or sDC (with TNFα, IL1β, IL6, and PGE2) in the presence or absence of UV-irradiated apoptotic LNCaP cells. αDC1s and sDCs were harvested on day 8, washed and counted under trypan blue. DC recovery was calculated as % of plated monocytes (n=10). B-C. Intact mature phenotype of the αDC1s and sDCs loaded with UVB-irradiated LNCaP cells. αDC1s and sDCs were harvested on day 8 and analyzed by flow cytometry for the expression of maturation and migratory markers. B. Representative data from one patient. C. Cumulative data from 10 donors expressed as mean of delta MFIs (increase in specific fluorescence over isotype controls) ±SD; n=10. Note that the expression levels of CD86 and CD80 on αDC1s were higher than those on sDCs (P < 0.05), while the expression levels of CD11c, CD83 and CCR7 were comparable. In addition, there was no significant impact of the tumor loading in the expression of these molecules in DCs by the pulsing of tumor antigens. C. Elevated L-12 production by αDC1s is not affected by loading with PCa cells. αDC1s and sDCs from 10 PCa patients were harvested on day 8 and stimulated with CD40L for 24 hr. The αDC1s showed significantly higher production of IL-12p70 than sDCs (P < 0.001). Loading of tumor antigen onto αDC1s did not significantly changed IL-12 secretion by these cells (n=10 different patients).
Interleukin-12p70 Production
DCs harvested on day 8 were plated in 96-well plates (at 2 × 104 cells/well) and stimulated with CD40L-transfected J558 cells as an analog of CD40L-expressing Th cells at 5 × 104 cells/well for 24 hours to measure the ability of the differentially-matured DCs to secrete IL-12p70 upon the follow-up stimulation in neutral conditions. The levels of IL-12p70 in the culture supernatants were measured using ELISA (Endogen).
Induction of prostate cancer-specific CD8+ T cell responses
Autologous CD8+ T cells (purity > 90%) from PCa patients were positively isolated from PBMCs using MACS (Miltenyi Biotech). CD8+ T cells (5 × 105 cells) were sensitized by autologous αDC1s or sDCs (7.5 × 104 cells) loaded with apoptotic LNCaP cells (at 3:1 ratio) in the presence of CD40L transfected J558 cells (29). Low MHC class I-expressing (Supplemental Figure 2) LNCap cell line was used to reduce the allo-component of the responses. At day 3, rhuIL-2 (50 units/mL, Chiron) and IL-7 (10 ng/mL, Peprotech) were added. The expanding CD8+ T cell lines were harvested on day 14 and tested for reactivity against LNCaP or DU145 cells or against T2 cells loaded with the defined HLA-A2-restricted peptides, using IFN-γ ELISPOT. The original (LNCaP) or third-party (DU145) PCa cells were used as tumor targets and anti-MHC class I blocking antibody (W6/32) was used to determine MHC class I restriction. To identify the induction of CD8+ T cells specific for unique PCa-specific Ags by LNCap-loaded DCs, we used the blood from the patients expressing HLA-A2 as one of the MHC class I alleles, and analyzed the responses to defined HLA-A2-restricted prostate-specific peptides.
Statistical evaluation
The differences between the individual groups were evaluated using Student's t test. The ability of the individual vaccines to induce multi-epitope responses to different PCa-associated antigens was defined by the results of a one tailed permutation test comparing test wells to control wells. If a one tailed permutation test gave p >.05 the response was defined as zero. Otherwise the response to peptide was judged to be greater than the control well response and was calculated as the average between the 3 test wells and the average of the 3 control well. The association between type of DC and the proportion of multi-epitope responses to PCa-associated antigens was evaluated by Fisher's Exact test.
Results
Polarized αDC1s from prostate cancer patients show fully mature phenotype and high ability to produce IL-12
The effectiveness of generation of DCs from monocyte population was within the range of 25% to 30% (compared to the original numbers of monocytes), with a trend towards slightly higher recovery of sDC compared to αDC1 (Fig. 1A). Loading of DC with apoptotic allogeneic tumor LNCaP tended to lower the recovery of both sDC and αDC1 as compared to non-tumor-exposed DC, but none of the above differences were statistically significant (Fig. 1A).
αDC1s and sDCs harvested at the end (day 8) of cultures showed a typical mature phenotype (Fig. 1B-C) and DC-like morphology (data not shown). The levels of expression of the costimulatory molecules CD86, and CD80 on αDC1s was significantly higher than on sDCs (P < 0.05; n =10 patients), while the levels of expression of CD11c, CD83 and CCR7 was comparable. As expected, the expression of all the above maturation markers was significantly elevated on both sDCs and αDC1s compared to immature DCs (data not shown)
In contrast to the previously-reported negative impact of tumor cells upon the early stages of DC differentiation in vivo or in vitro (32-34) the exposure of DCs to LNCaP cells at this later stage of their maturation did not significantly affect the expression of the above costimulatory factors on maturing sDCs and αDC1s or their recovery. (Fig. 1A and Fig. 1B-C).
Elevated production of IL-12p70 by αDC1s matured in the presence of LNCaP cells
Multiple pathways of induction of DC maturation are accompanied by transient production of IL-12p70 (28), followed, however, by irreversible exhaustion of their ability to produce IL-12p70 (35,36), which limits their applicability as cancer vaccines. In contrast, we observed that DC maturation in the presence of IFNγ is uniquely associated with the elevation, rather than “exhaustion” of the DCs ability to produce IL-12 during their subsequent stimulation with CD40L or the interaction with T cells (28,29). In order to determine whether the induction of functional type-1 polarized DC1s can proceed in the presence of tumor material from LNCaP cells, we measured the level of IL-12p70 production after subsequent stimulation of the cells with CD40L.
As shown in Fig. 1C, αDC1s showed strongly elevated production of IL-12p70 than sDCs in response to subsequent CD40L-mediated stimulation in neutral conditions, in the absence of DC maturation-inducing factors (p < 0.01). In DC preparations from 10 different prostate cancer patients we observed that IL-12p70 production was 10-30 times higher in αDC1s compared to sDCs. Importantly, the exposure of maturing DCs to tumor cells at the stage of DC maturation did not result in any suppression of IL-12 production.
These data demonstrate the feasibility of generating mature non-exhausted, αDC1s from PCa patients that are capable of high IL-12-production following their subsequent activation. Furthermore, they demonstrate that prostate cancer cells do not significantly affect the phenotype and IL-12-producing function of maturing αDC1s. These findings indicate that the immune deficit observed in DCs from PCa patients may be overcome by generating polarized DCs ex vivo, which postpones the DC exposure to PCa cell-related factors until the stage of DC maturation.
αDC1s loaded with allogeneic prostate cancer cells induce high numbers of tumor-specific CD8+ T cells able of recognizing the original and third-party PCa cells
In order to compare the abilities of the tumor-loaded polarized αDC1 and standard DCs to act as inducers of anti-prostate cancer-specific responses vaccines, we used our established in vitro sensitization (IVS) model previously used to demonstrate the ability of αDC1s to present peptide antigens (29), to evaluate the induction of CD8+ T cells able of recognizing the original PCa cells used as the antigen source and third party PCa cells. Consistent with the differences in the ability of both DC types to produce IL-12 (see Fig 1C) and with the recently-reported key role of αDC1-produced IL-12 in inducing functional CD8+ T cells (37), tumor-loaded αDC1s consistently showed highly-elevated activity in inducing functional IFNγ-producing CD8+ T cells against both LNCap cells (Fig. 2A; also see Supplemental Figure 3) and DU145 cells (Fig. 2B). In both cases, the responses were inhibited in the presence MHC class I-blocking antibody. These data show that allogeneic tumor-loaded DCs, known to activate T cells from healthy donors (9) can be effective inducers of tumor-specific responses of CD8+ T cells from PCa patients and demonstrate that the effectiveness of DC vaccines is strongly affected by the conditions of DC maturation.
Fig. 2. LNCap-loaded αDC1s are effective inducers of functional CD8+ T cells able of recognizing different prostate cancer cells.
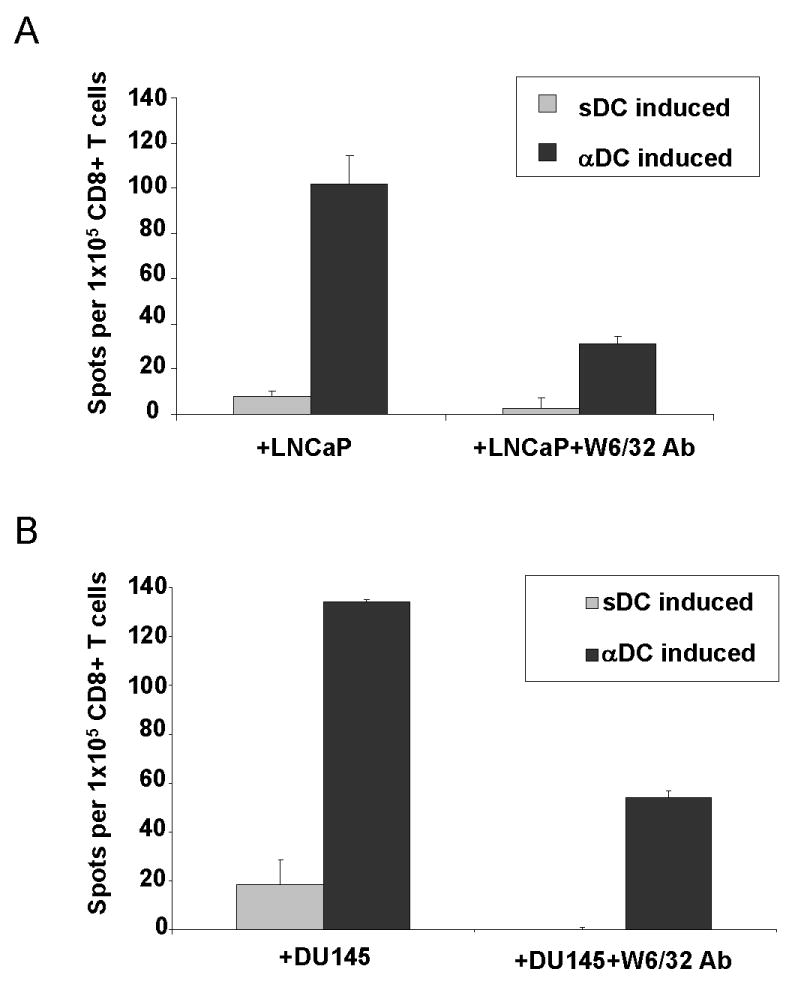
CD8+ T cells from PCa patients were sensitized with autologous DCs (αDC1s or sDCs) loaded with allogeneic tumor cells (LNCaP). After a single round of in vitro sensitization, the differentially-sensitized CD8+ T cells were used as responder cells against (A) the original LNCap cells or (B) against unrelated DU145 cells. W6/32 pan-HLA class I-neutralizing antibody was used to demonstrate the MHC class I dependence of the T cell recognition. The data is shown as a mean +/- SD of triplicate cultures from a representative experiment of eight (including four experiments involving MHC blockade) involving DCs and CD8+ T cells from different PCa patients. The background values obtained in the absence of target cells (typically within 5- 20%) have been subtracted.
Prostate tumor-loaded αDC1s show highly-elevated ability to induce functional CD8+ T cells recognizing multiple PCa-relevant epitopes
In order to demonstrate ability of αDC1 and standard DCs to cross-present allogeneic LNCap cells and act as inducers prostate cancer-specific responses against defined PCa-specific epitopes, in additional set of experiments we used the DCs and CD8+ T cells from four HLA-A2+ PCa patient samples, and we analyzed the ability of the differentially-sensitized CD8+ T cells to recognize defined HLA-A2-restricted peptide epitopes of PAP and PSA. As shown in Fig. 3, in each of the four samples, tumor-loaded αDC1s consistently showed highly-elevated activity in inducing functional IFNγ-producing CD8+ T cells against different epitopes of the two PCa-associated antigens tested, inducing wider range and higher magnitude of the responses. In case of the responses to PAP135-143, the αDC1-sensitized cultures showed an average of 49-fold advantage over sDC-primed cultures (range 2- to 177-fold). In case of PAP112-120-specific responses, we observed an average 24-fold advantage (range 9- to 59-fold). Similar, although smaller in magnitude advantage of αDC1s was observed in case of both PSA epitopes tested: PSA141-150 (Avg. 5.5-fold; range 1.5- to 9.5-fold) and PSA146-154 (Avg. 11-fold advantage; range 4.5- to 19-fold), with one additional donor not showing any PSA146-154-specific responses in either culture conditions (Fig. 3).
Fig. 3. LNCap-loaded αDC1s are effective inducers of CD8+ T cells specific for multiple prostate cancer-associated antigenic epitopes.
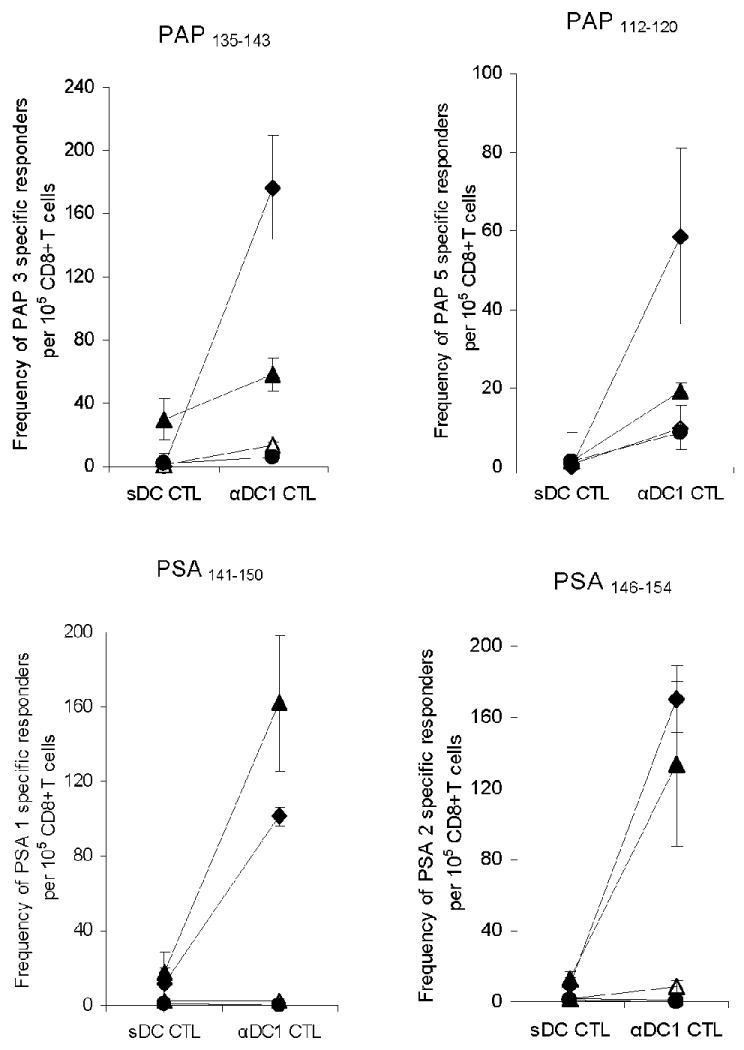
CD8+ T cells from four blood samples derived from three HLA-A2-expressing PCa patients were stimulated once with LNCap-loaded autologous DCs (αDC1s or sDCs). After two weeks, the differentially-sensitized CD8+ T cells were used as responder cells against the defined peptide epitopes of the prostate-specific antigen (PSA1141-150 and PSA2146-154), and prostatic acid phosphatase (PAP3135-143 and PAP5112-120). Each pair of symbols represents the average +/- SD of triplicate cultures from the same individual patient sensitized using sDCs or αDC1s. Testing the response to the four epitopes in each of 4 samples allowed 16 possible CD8+ T cells responses. Positive response was defined by a permutation test (see Methods). Tumor-loaded αDC1s induced 9 of the possible 16 responses to the individual PCa-associated epitopes (compared to the total of 2 of 16 possible responses induced by sDCs), consistently inducing their higher magnitude. Overall, the proportion of responses differed by the DC type (Fisher's Exact test, p = 0.023).
Discussion
In order to develop improved vaccines against prostate cancer capable of bypassing the deficit of endogenous DCs immunosuppression in prostate cancer patients with recurrent cancer, we tested the feasibility of generating functional DCs from the blood of prostate cancer patients, their loading with allogeneic tumor cells, and their effectiveness in inducing functional prostate cancer specific immune responses.
Compared to standard DCs, αDC1s of patients with prostate cancer showed higher expression of several costimulatory molecules and 10-30 times greater secretion of IL-12p70 (Fig 1), the key factor responsible for elevated ability of αDC1s to induce CTL pathway of differentiation of CD8+ T cells (37). Consistent with the undisturbed IL-12-produing function of PCa-loaded αDC1s, they were much more effective than standard DCs in inducing functional tumor-specific CD8+ T cell responses against PAP and PSA (Figure 3), the two PCa-related antigens targeted in three recent successful clinical trials of different PCa vaccines (26,27). Importantly, in addition to their ability to recognize the defined PCa-specific epitopes, αDC1-primed cells were also highly effective in recognizing whole tumor cells, both the original cells used as a source of PCa-specific antigens (LNCap) and third-party PCa cells (DU145).
While our previous experiments demonstrated that αDC1s (loaded with defined HLA-A2-restricted peptides) are effective inducers of CTL responses against such antigenically well-defined tumors as melanoma (29), and (when loaded with sterile autologous tumor material) against autologous CLL cells (30), the current data paves the way for the application of αDC1-based strategies of immunotherapy of tumors with poorly-defined rejection antigens, for patients with micrometastatic disease (lack of available autologous tumor material), who constitute the group of patients who are most likely to benefit from vaccination. Our demonstration that the αDC1s loaded with the allogeneic tumor-cells are effective inducer of CD8+ T cells recognizing both the original and unrelated PCa tumor cells provides the rationale for the clinical application of such vaccines in the general cohort of PCa patients with recurrent disease, independent of their HLA type.
Whole-cell vaccination with allogeneic human prostate cancer cell lines (PC-3 and LNCaP) modified to secrete granulocyte-macrophage colony-stimulating factor (such as GVAX) has been investigated for over 5 years in patients with metastatic castrate-resistant prostate cancer. Although the signs of clinical efficacy were observed in small phase I and II clinical trials, in the past 3 years several high-profile phase III trials failed to meet the predicted endpoints (38,39). Our study differs from the above in several important aspects: We are priming DCs ex vivo with apoptotic bodies from allogeneic cell lines (as opposed to injecting live tumor cells in vivo) and we are using autologous dendritic cells as the source of the signals attracting and activating PAP-specific and PSA-specific T cells. Moreover, in addition to inducing higher numbers of tumor-specific T cells, the use of polarized DC1s, with selectively-elevated ability to attract effector (rather than regulatory T cells) (40) and to induce the IL-12-dependent effector (CTL) pathway of differentiation in CD8+ T cells (37), may help to induce tumor-specific immunity of a more desirable pattern than either whole tumor cells or standard nonpolarized DCs (37,40).
Important for the feasibility of utilizing prostate cancer-loaded αDC1s as cancer vaccines or as ex-vivo inducers of PCa-specific CD8+ T cells for adoptive immunotherapy, we have observed that high IL-12-producing function and CD8+ T cell-inducing function of DCs is not compromised by the exposure to prostate cancer cells at the stage of DC maturation. In light of the previously-reported contribution of the dysfunction of endogenous DCs to the overall immunosuppression observed in prostate cancer patients (41), and the recent evidence from mouse models suggesting that increased DC function may translate into improved vaccination outcomes (42), the current data suggest the possibility of restoring immunosurveillance in this group of patients using ex-vivo generated polarized DCs.
In conclusion, this study demonstrates that high numbers of αDC1s can be successfully generated from the blood of prostate cancer patients and used as effective inducers of PCa-specific CD8+ T cells. High immunologic activity of such cells suggest their possible use as therapeutic vaccines against PCa or ex vivo-inducers of prostate cancer specific CTLs for adoptive transfer, in order to overcome the prostate cancer-associated immune dysfunction.
Supplementary Material
Acknowledgments
This work was supported by the NIH grants CA095128, CA101944, CA114931 (to PK) and CA10373005 (to GSC). The authors thank Dr. Julie Urban for stimulating discussions and critical review of the manuscript and Tina Kilgore and Sharon Coutch for their administrative assistance.
References
- 1.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55(1):10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59(4):225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Coen JJ, Zietman AL, Thakral H, Shipley WU. Radical radiation for localized prostate cancer: local persistence of disease results in a late wave of metastases. J Clin Oncol. 2002;20(15):3199–3205. doi: 10.1200/JCO.2002.01.086. [DOI] [PubMed] [Google Scholar]
- 4.Roehl KA, Han M, Ramos CG, Antenor JA, Catalona WJ. Cancer progression and survival rates following anatomical radical retropubic prostatectomy in 3,478 consecutive patients: long-term results. J Urol. 2004;172(3):910–914. doi: 10.1097/01.ju.0000134888.22332.bb. [DOI] [PubMed] [Google Scholar]
- 5.Catalona WJ, Smith DS. Cancer recurrence and survival rates after anatomic radical retropubic prostatectomy for prostate cancer: intermediate-term results. J Urol. 1998;160(6 Pt 2):2428–2434. doi: 10.1097/00005392-199812020-00012. [DOI] [PubMed] [Google Scholar]
- 6.Dillioglugil O, Leibman BD, Kattan MW, Seale-Hawkins C, Wheeler TM, Scardino PT. Hazard rates for progression after radical prostatectomy for clinically localized prostate cancer. Urology. 1997;50(1):93–99. doi: 10.1016/S0090-4295(97)00106-4. [DOI] [PubMed] [Google Scholar]
- 7.Miyamoto H, Messing EM, Chang C. Androgen deprivation therapy for prostate cancer: current status and future prospects. Prostate. 2004;61(4):332–353. doi: 10.1002/pros.20115. [DOI] [PubMed] [Google Scholar]
- 8.Vogelzang N. New developments in the treatment of advanced prostate cancer. Seminars in Oncology. 1997;23(6) 14:1–5. [Google Scholar]
- 9.Nouri-Shirazi M, Banchereau J, Bell D, Burkeholder S, Kraus ET, Davoust J, Palucka KA. Dendritic cells capture killed tumor cells and present their antigens to elicit tumor-specific immune responses. J Immunol. 2000;165(7):3797–3803. doi: 10.4049/jimmunol.165.7.3797. [DOI] [PubMed] [Google Scholar]
- 10.Meidenbauer N, Harris DT, Spitler LE, Whiteside TL. Generation of PSA-reactive effector cells after vaccination with a PSA-based vaccine in patients with prostate cancer. Prostate. 2000;43(2):88–100. doi: 10.1002/(sici)1097-0045(20000501)43:2<88::aid-pros3>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 11.Murphy GP, Tjoa BA, Simmons SJ, Jarisch J, Bowes VA, Ragde H, Rogers M, Elgamal A, Kenny GM, Cobb OE, Ireton RC, Troychak MJ, Salgaller ML, Boynton AL. Infusion of dendritic cells pulsed with HLA-A2-specific prostate-specific membrane antigen peptides: a phase II prostate cancer vaccine trial involving patients with hormone-refractory metastatic disease. Prostate. 1999;38(1):73–78. doi: 10.1002/(sici)1097-0045(19990101)38:1<73::aid-pros9>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 12.Murphy GP, Tjoa BA, Simmons SJ, Ragde H, Rogers M, Elgamal A, Kenny GM, Troychak MJ, Salgaller ML, Boynton AL. Phase II prostate cancer vaccine trial: report of a study involving 37 patients with disease recurrence following primary treatment. Prostate. 1999;39(1):54–59. doi: 10.1002/(sici)1097-0045(19990401)39:1<54::aid-pros9>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 13.Tjoa BA, Simmons SJ, Bowes VA, Ragde H, Rogers M, Elgamal A, Kenny GM, Cobb OE, Ireton RC, Troychak MJ, Salgaller ML, Boynton AL, Murphy GP. Evaluation of phase I/II clinical trials in prostate cancer with dendritic cells and PSMA peptides. Prostate. 1998;36(1):39–44. doi: 10.1002/(sici)1097-0045(19980615)36:1<39::aid-pros6>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 14.Mincheff M, Tchakarov S, Zoubak S, Loukinov D, Botev C, Altankova I, Georgiev G, Petrov S, Meryman HT. Naked DNA and adenoviral immunizations for immunotherapy of prostate cancer: a phase I/II clinical trial. Eur Urol. 2000;38(2):208–217. doi: 10.1159/000020281. [DOI] [PubMed] [Google Scholar]
- 15.Pavlenko M, Roos AK, Lundqvist A, Palmborg A, Miller AM, Ozenci V, Bergman B, Egevad L, Hellstrom M, Kiessling R, Masucci G, Wersall P, Nilsson S, Pisa P. A phase I trial of DNA vaccination with a plasmid expressing prostate-specific antigen in patients with hormone-refractory prostate cancer. Br J Cancer. 2004;91(4):688–694. doi: 10.1038/sj.bjc.6602019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sipuleucel-T: APC 8015, APC-8015, prostate cancer vaccine--Dendreon. Drugs in R&D. 2006;7(3):197–201. doi: 10.2165/00126839-200607030-00006. [DOI] [PubMed] [Google Scholar]
- 17.Harzstark AL, Small EJ. Immunotherapy for prostate cancer using antigen-loaded antigen-presenting cells: APC8015 (Provenge) Expert opinion on biological therapy. 2007;7(8):1275–1280. doi: 10.1517/14712598.7.8.1275. [DOI] [PubMed] [Google Scholar]
- 18.Peshwa MV, Shi JD, Ruegg C, Laus R, van Schooten WC. Induction of prostate tumor-specific CD8+ cytotoxic T-lymphocytes in vitro using antigen-presenting cells pulsed with prostatic acid phosphatase peptide. Prostate. 1998;36(2):129–138. doi: 10.1002/(sici)1097-0045(19980701)36:2<129::aid-pros8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 19.Small EJ, Fratesi P, Reese DM, Strang G, Laus R, Peshwa MV, Valone FH. Immunotherapy of hormone-refractory prostate cancer with antigen-loaded dendritic cells. J Clin Oncol. 2000;18(23):3894–3903. doi: 10.1200/JCO.2000.18.23.3894. [DOI] [PubMed] [Google Scholar]
- 20.Valone FH, Small E, MacKenzie M, Burch P, Lacy M, Peshwa MV, Laus R. Dendritic cell-based treatment of cancer: closing in on a cellular therapy. Cancer J. 2001;7 2:S53–61. [PubMed] [Google Scholar]
- 21.Correale P, Walmsley K, Nieroda C, Zaremba S, Zhu M, Schlom J, Tsang KY. In vitro generation of human cytotoxic T lymphocytes specific for peptides derived from prostate-specific antigen. J Natl Cancer Inst. 1997;89(4):293–300. doi: 10.1093/jnci/89.4.293. [DOI] [PubMed] [Google Scholar]
- 22.Kobayashi K, Noguchi M, Itoh K, Harada M. Identification of a prostate-specific membrane antigen-derived peptide capable of eliciting both cellular and humoral immune responses in HLA-A24+ prostate cancer patients. Cancer Sci. 2003;94(7):622–627. doi: 10.1111/j.1349-7006.2003.tb01493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Machlenkin A, Paz A, Bar Haim E, Goldberger O, Finkel E, Tirosh B, Volovitz I, Vadai E, Lugassy G, Cytron S, Lemonnier F, Tzehoval E, Eisenbach L. Human CTL epitopes prostatic acid phosphatase-3 and six-transmembrane epithelial antigen of prostate-3 as candidates for prostate cancer immunotherapy. Cancer Res. 2005;65(14):6435–6442. doi: 10.1158/0008-5472.CAN-05-0133. [DOI] [PubMed] [Google Scholar]
- 24.Finke LH, Wentworth K, Blumenstein B, Rudolph NS, Levitsky H, Hoos A. Lessons from randomized phase III studies with active cancer immunotherapies--outcomes from the 2006 meeting of the Cancer Vaccine Consortium (CVC) Vaccine. 2007;25 2:B97–B109. doi: 10.1016/j.vaccine.2007.06.067. [DOI] [PubMed] [Google Scholar]
- 25.So-Rosillo R, Small EJ. Sipuleucel-T (APC8015) for prostate cancer. Expert review of anticancer therapy. 2006;6(9):1163–1167. doi: 10.1586/14737140.6.9.1163. [DOI] [PubMed] [Google Scholar]
- 26.Higano CS, Schellhammer PF, Small EJ, Burch PA, Nemunaitis J, Yuh L, Provost N, Frohlich MW. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer. 2009;115(16):3670–3679. doi: 10.1002/cncr.24429. [DOI] [PubMed] [Google Scholar]
- 27.Kantoff PW, Schuetz TJ, Blumenstein BA, Glode LM, Bilhartz DL, Wyand M, Manson K, Panicali DL, Laus R, Schlom J, Dahut WL, Arlen PM, Gulley JL, Godfrey WR. Overall Survival Analysis of a Phase II Randomized Controlled Trial of a Poxviral-Based PSA-Targeted Immunotherapy in Metastatic Castration-Resistant Prostate Cancer. J Clin Oncol. doi: 10.1200/JCO.2009.25.0597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vieira PL, de Jong EC, Wierenga EA, Kapsenberg ML, Kalinski P. Development of Th1-inducing capacity in myeloid dendritic cells requires environmental instruction. J Immunol. 2000;164(9):4507–4512. doi: 10.4049/jimmunol.164.9.4507. [DOI] [PubMed] [Google Scholar]
- 29.Mailliard RB, Wankowicz-Kalinska A, Cai Q, Wesa A, Hilkens CM, Kapsenberg ML, Kirkwood JM, Storkus WJ, Kalinski P. alpha-type-1 polarized dendritic cells: a novel immunization tool with optimized CTL-inducing activity. Cancer Res. 2004;64(17):5934–5937. doi: 10.1158/0008-5472.CAN-04-1261. [DOI] [PubMed] [Google Scholar]
- 30.Lee JJ, Foon KA, Mailliard RB, Muthuswamy R, Kalinski P. Type 1-polarized dendritic cells loaded with autologous tumor are a potent immunogen against chronic lymphocytic leukemia. Journal of leukocyte biology. 2008 doi: 10.1189/jlb.1107737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Watchmaker P, Urban J, Berk E, Nakamura Y, Mailliard RB, Watkins SC, Van Ham SM, Kalinski P. Memory CD8+ T cells protect dendritic cells from CTL killing. J Immunol. 2008;180:3857–3865. doi: 10.4049/jimmunol.180.6.3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Troy A, Davidson P, Atkinson C, Hart D. Phenotypic characterisation of the dendritic cell infiltrate in prostate cancer. J Urol. 1998;160(1):214–219. [PubMed] [Google Scholar]
- 33.Aalamian M, Pirtskhalaishvili G, Nunez A, Esche C, Shurin GV, Huland E, Huland H, Shurin MR. Human prostate cancer regulates generation and maturation of monocyte-derived dendritic cells. Prostate. 2001;46(1):68–75. doi: 10.1002/1097-0045(200101)46:1<68::aid-pros1010>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 34.Shurin GV, Aalamian M, Pirtskhalaishvili G, Bykovskaia S, Huland E, Huland H, Shurin MR. Human prostate cancer blocks the generation of dendritic cells from CD34+ hematopoietic progenitors. Eur Urol. 2001;39 4:37–40. doi: 10.1159/000052584. [DOI] [PubMed] [Google Scholar]
- 35.Kalinski P, Schuitemaker JH, Hilkens CM, Wierenga EA, Kapsenberg ML. Final maturation of dendritic cells is associated with impaired responsiveness to IFN-gamma and to bacterial IL-12 inducers: decreased ability of mature dendritic cells to produce IL-12 during the interaction with Th cells. J Immunol. 1999;162(6):3231–3236. [PubMed] [Google Scholar]
- 36.Langenkamp A, Messi M, Lanzavecchia A, Sallusto F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat Immunol. 2000;1(4):311–316. doi: 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- 37.Watchmaker PB, Berk E, Muthuswamy R, Mailliard RB, Urban JA, Kirkwood JM, Kalinski P. Independent regulation of chemokine responsiveness and cytolytic function versus CD8+ T cell expansion by dendritic cells. J Immunol. 2010;184(2):591–597. doi: 10.4049/jimmunol.0902062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Copier J, Dalgleish A. Whole-cell vaccines: A failure or a success waiting to happen? Curr Opin Mol Ther. 12(1):14–20. [PubMed] [Google Scholar]
- 39.Urba WJ, Nemunaitis J, Marshall F, Smith DC, Hege KM, Ma J, Nguyen M, Small EJ. Treatment of biochemical recurrence of prostate cancer with granulocyte-macrophage colony-stimulating factor secreting, allogeneic, cellular immunotherapy. J Urol. 2008;180(5):2011–2017. doi: 10.1016/j.juro.2008.07.048. discussion 2017-2018. [DOI] [PubMed] [Google Scholar]
- 40.Muthuswamy R, Urban J, Lee JJ, Reinhart TA, Bartlett D, Kalinski P. Ability of mature dendritic cells to interact with regulatory T cells is imprinted during maturation. Cancer Res. 2008;68(14):5972–5978. doi: 10.1158/0008-5472.CAN-07-6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miller AM, Pisa P. Tumor escape mechanisms in prostate cancer. Cancer Immunol Immunother. 2007;56(1):81–87. doi: 10.1007/s00262-005-0110-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koh YT, Gray A, Higgins SA, Hubby B, Kast WM. Androgen ablation augments prostate cancer vaccine immunogenicity only when applied after immunization. Prostate. 2009;69(6):571–584. doi: 10.1002/pros.20906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


