Abstract
Objective
To investigate systematically the natural history of visual outcome in central retinal vein occlusion (CRVO).
Design
Cohort study.
Participants
667 consecutive CRVO patients (697 eyes) with CRVO, first seen in our clinic from 1973 to 2000.
Methods
At first visit, all patients had a detailed ophthalmic and medical history, and comprehensive ophthalmic evaluation. Visual evaluation was done by recording visual acuity, using the Snellen visual acuity chart, and visual fields with a Goldmann perimeter. The same ophthalmic evaluation was performed at each follow-up visit. CRVO was classified into non-ischemic (588 eyes) and ischemic (109 eyes) at initial visit, based on functional and morphological criteria.
Main Outcome Measures
Visual acuity and visual fields.
Results
Of the eyes first seen within 3 months of onset, visual acuity was 20/100 or better in 78% in non-ischemic CRVO and only 1% in ischemic CRVO (p<0.0001), and visual field defects were minimal or mild in 91% and 8% respectively (p<0.0001). Final visual acuity, on resolution of macular edema, was 20/100 or better in 83% in non-ischemic CRVO and only 12% in ischemic CRVO (p<0.0001), and visual field defects minimal or mild in 95% and 18% respectively (p<0.0001). On resolution of macular edema, in eyes with initial visual acuity 20/70 or worse, visual acuity improved in 59% of the non-ischemic CRVO, with no significant (p=0.55) improvement in ischemic CRVO. Similarly, on resolution of macular edema, in eyes with moderate to severe initial visual field defect, improvement was seen in 86% of non-ischemic CRVO eyes but no significant (p=0.83) improvement in ischemic CRVO. In non-ischemic CRVO, development of foveal pigmentary degeneration and/or epiretinal membrane was the main cause of poor final visual acuity. This shows that initial presentation and the final visual outcome in the two types of CRVO are totally different.
Conclusion
A clear differentiation of CRVO into non-ischemic and ischemic types, based primarily on functional criteria, is crucial and fundamental in determining visual outcome. Visual outcome is good in non-ischemic CRVO and poor in ischemic CRVO
Understanding the natural history of a disease is paramount to its management. If it is not understood, natural recovery may be attributed to a treatment which is actually ineffective. Although the clinical entity of central retinal vein occlusion (CRVO) has been known since 18781 and it is a common, visually disabling disorder, there is little firm information in the literature on the natural history of its visual outcome. Those few reports in the literature, which deal with the natural history of visual outcome in CRVO2–10, have some fundamental flaws. First, it is well-established now that CRVO is of two types: non-ischemic and ischemic CRVO11–17, with very different outcomes. In all the previous natural history studies, either the two clinical entities are lumped together into one category or the criteria used to differentiate CRVO into its two types are inadequate, so that the findings are based on a mixture of the two types, which makes the results unreliable. Second, retinopathy in CRVO involves the entire retina and not only the macular region. Unfortunately, all these CRVO studies deal only with visual acuity, without any information on the outcome of visual fields which provide information about the function of the entire retina. Although visual acuity is critical in fine work, the peripheral visual fields are equally, if not more, essential for normal navigation and day to day living. The objective of the present study was to evaluate prospectively, in a large cohort of CRVO patients, (i) the natural history of visual outcome (both visual acuity and visual fields), and (ii) the factors that influence the natural history of visual outcome, by differentiating CRVO into ischemic and non-ischemic types using a combination of functional and morphologic criteria15,16.
Patients and Methods
We have investigated various aspects of CRVO systematically in the Ocular Vascular Clinic at the Tertiary Care University of Iowa Hospitals and Clinics since 1973. The current study was part of a prospective study on ocular vascular occlusive disorders funded by the National Institute of Health (RO1 grant), approved by the Institutional Review Board. The data for this study were collected prospectively after the award of the NIH research grant. In the present study, we investigated the natural history of visual outcome in CRVO; it included patients who were first seen in our clinic from 1973 to 2000. The data on visual outcome were compiled from 667 consecutive CRVO patients (637 patients with data for one eye and 30 patients with data from both– a total of 697 eyes) who fulfilled our inclusion criteria for this study. There was no built-in bias for the severity, type or laterality of CRVO seen in the Ocular Vascular Clinic because of extremely good co-operation from our referring community of ophthalmologists, who sent us all cases with CRVO.
Inclusion Criteria
Only those patients for whom a definite diagnosis of CRVO could be made were included. CRVO consists of two distinct entities: non-ischemic CRVO and ischemic CRVO11–17. The distinction (between ischemic and non-ischemic CRVO) was based on the COMBINED data acquired from six tests which we evaluated in our study16, specifically designed to do so. Of the six tests, the study showed that combined information from the relative afferent pupillary defect and electroretinography differentiated 97% of the cases. Thus, relative afferent pupillary defect and electroretinography often played the most important deciding role about the type of CRVO. Fluorescein fundus angiography (when it was of reliable quality) also provided reliable information in differentiation. The other 3 tests: visual acuity, visual fields and ophthalmoscopy, although providing useful information, were not as sensitive as combined relative afferent pupillary defect and electroretinography and good quality fluorescein angiograms. No one test was decisive per se.
Exclusion criteria
We excluded all other retinopathies mimicking CRVO. All patients with inadequate information or doubtful diagnosis were excluded. Patients who had any retinal or optic nerve lesion or any other factor (e.g., cataract), including any treatment for CRVO, which could have influenced the visual status, were excluded. CRVO patients with only background diabetic retinopathy were included, but those who had active neovascularization, vitreous hemorrhages, traction detachment or other complications influencing the visual acuity or fields were excluded. Patients who had a diagnosis of glaucoma and visual field loss were excluded; however, those with elevated intraocular pressure with a documented normal field prior to the onset of CRVO were included. Eyes with unreliable visual fields were excluded.
STUDIES PERFORMED
The intention was to document the natural history of visual outcome by recording best corrected visual acuity and visual field defects on manual kinetic perimetry with a Goldmann perimeter. The data were collected prospectively and systematically. At the initial visit, all patients were seen by one of us (SSH) in the Ocular Vascular Clinic and had a detailed ocular and medical history as well as a comprehensive bilateral ophthalmic evaluation. This included, (i) careful testing of the best corrected visual acuity using the Snellen visual acuity chart, (ii) visual field plotting with a Goldmann perimeter (using I-2e, I-4e and V-4e targets regularly), (iii) intraocular pressure recording with a Goldmann applanation tonometer, (iv) relative afferent pupillary defect, (v) a thorough anterior segment examination, including slit lamp examination of the anterior segment, lens and vitreous, (vi) a meticulous fundus evaluation by direct and indirect ophthalmoscopy, and, if required, by contact lens, (vii) stereoscopic color fundus photography, (viii) stereoscopic fluorescein fundus angiography (only in the involved eye), and (ix) electroretinography.
In addition, the patients had a full systemic evaluation performed either by an internist at the University of Iowa Hospitals & Clinics, or by their local internist/physician.
At each follow-up visit, the same ophthalmic evaluation and stereoscopic color fundus photography were done, except that fluorescein fundus angiography was performed only when considered essential. Electroretinography was usually performed only during the initial visit; however, if a change in status of CRVO from non-ischemic to ischemic was suspected, it was repeated. Both eyes were examined at each visit.
Follow-up protocol for all patients
All patients were followed (by SSH) according to a protocol practiced in this Clinic for CRVO patients - at about 3 monthly intervals for 3 visits, then 6 monthly intervals for 4 visits, then annually. Both eyes were examined at each visit. Since this is a natural history study, no intervention of any kind whatsoever was undertaken in this cohort of patients with CRVO.
VISUAL STATUS EVALUATION
Visual acuity
Best corrected visual acuity was tested using the Snellen visual acuity chart and under identical testing conditions, almost invariably by the same person (SSH), encouraging the patient to look around and take his/her own time in responding, to ensure that the testing provided the most reliable information about the visual acuity. The following steps of visual acuity were checked: 20/15, 20/20, 20/25, 20/30, 20/40, 20/50, 20/60, 20/70, 20/80, 20/100, 20/200, 20/400, counting fingers (CF), hand motion (HM), perception of light (PL), and no perception of light (NPL).
Visual fields
Throughout this study we used kinetic perimetry. Automated perimetry did not exist when we started the study in 1973; moreover, the changing face of automated perimetry would make such long-term studies difficult - indeed, automated perimetry is still evolving. Both types of perimetry have their advantages and disadvantages, which are discussed elsewhere.18,19 Visual field plotting was attempted in all patients with a visual acuity of hand motion or better at all visits, with a Goldmann perimeter using I-2e, I-4e and V-4e targets regularly, although occasionally other targets were used if it was felt that that would provide additional information for evaluation of the visual status. In a few patients, at the initial visit, for various logistic reasons we could not do the visual fields. The method of testing visual fields used is described in detail elsewhere.18,19
Central visual field was also tested by using the Amsler grid chart which provided very useful information about the central defect, particularly metamorphopsia which was usually missed on perimetry.
Evaluation of changes in the visual acuity and visual field defects
Each was evaluated separately in a masked fashion, i.e., changes in visual acuity and visual fields were evaluated independently of each other, as well as independent of presence or absence of macular edema, so that the severity of one did not influence the evaluation of the other. Also, in eyes that developed recurrence of non-ischemic CRVO20, only the data on visual evaluation collected up to the last follow-up visit of the first episode were used, i.e., before the onset of recurrence.
Visual acuity evaluation
A change of at least 3 lines in the Snellen visual acuity chart was considered a significant change, which is equivalent to a logMAR (logarithm of the minimum angle of resolution) change of at least 0.30. We divided visual acuity into two categories for evaluation purposes. (i) Normal visual acuity was defined as 20/30 or better, because that category cannot show an improvement of 3 lines to achieve 20/20. (ii) As in our natural history study on visual outcome in non-arteritic anterior ischemic optic neuropathy21 and the Ischemic Optic Neuropathy Decompression Trial study22, poor visual acuity was defined as 20/70 or worse, because that is markedly disabling and persons with that visual acuity are not considered fit to drive a vehicle.
Visual field evaluation
We wanted to evaluate quantitative and qualitative changes in visual fields plotted with the Goldmann perimeter during the follow-up period. Having learnt from our previous experience of dealing with visual field evaluation for various studies over the years 21,23–26, we found that the most reliable method was overall subjective grading of the visual fields.
Visual fields were evaluated in the following 3 ways:
Peripheral visual field evaluation: These were evaluated as normal or abnormal, and if abnormal, with what isopter, and what was the defect at initial visit.
Central 30° visual field evaluation: In this part of the visual field, the presence of various types of scotomas was evaluated on initial and follow-up visits with different isopters.
Overall grading of the entire visual field: The subjective grading of the severity of visual loss took into consideration all the parameters one considers while clinically evaluating a change in visual fields plotted with manual kinetic perimetry (because of the complexity of the Goldmann visual field defects, it is unfortunately difficult to define the exact parameters). In general, deterioration was defined as development of a new scotoma, a deepening or expanding scotoma, a generalized constriction not accounted for by any other ocular parameter, or overall deterioration. Improvement was the reverse of the above. Subtle changes were confirmed on more than one examination. The entire visual field was graded into 4 levels - from 0 (normal) to 4 (severe loss) in steps of 0.5 (and occasionally 0.25 when the differences were subtle), and the dates when each change was noted during the entire follow-up. The grade was judged by qualitatively assessing clinical computation of the amount of visual field loss, factoring in the functional disability produced by that defect; for example, inferior and/or central visual field defect, producing far more functional disability, was assigned a much higher grade than a corresponding loss in the upper field or elsewhere. The grading was started from the first visual field. A change from one grade to another was noted. The findings were then condensed for descriptive purposes into minimal (grade 0.5), mild (grades >0.5 –1.0), moderate (1.5 to 2.0), marked (2.5 to 3.0) and severe (3.5 to 4.0) loss. These grades are best described by examples given elsewhere.23
Evaluation of macular edema
Visual acuity depends upon the macular function. Poor visual acuity in CRVO, particularly in non-ischemic CRVO, is due primarily to macular edema. Therefore, in each eye macular edema was evaluated critically at each visit. Critical evaluation of the macular edema was done by using: (i) direct ophthalmoscopy, (ii) Hruby contact lens, (iii) stereoscopic fundus photographs of the macular region, and (iv) frequent stereoscopic fluorescein fundus angiography of the macular region. OCT or other modern technologies to evaluate macular edema were not available during the period of this study.
Statistical Analysis
Demographic and clinical characteristics of patients with non-ischemic and ischemic CRVO were compared using either two-sample t-test for continuous variables or Pearson Chi-square test for categorical variables. Wilcoxon rank-sum test was used to compare Initial and final visual acuity and visual defect grade. The outcomes of visual acuity and visual field defect of improvement (for those that initially presented with 20/70 or worse visual acuity, and moderate to severe visual field defect grade) and deterioration (for those that initially presented with 20/60 or better visual acuity, and minimal to mild visual field defect grade) over time were compared between non-ischemic and ischemic CRVO using logistic regression that was fitted by the method of generalized estimating equations to account for correlation of outcomes over time from the same eye. The same analysis was used to assess the effect of presence/absence of macular edema on visual outcomes over time. The effect of age, systemic conditions, foveal pigmentation, and epiretinal membrane on the final visual outcome of improvement or deterioration after macular edema has resolved was tested using logistic regression analysis.
Results
Demographic characteristics
This study consisted of 559 patients (588 eyes) with non-ischemic CRVO, of whom 47 (48 eyes) later converted to ischemic CRVO, and 108 (109 eyes) that presented initially with ischemic CRVO. Table 1 gives the demographic characteristics of these patients. The ischemic CRVO patients were significantly older as initial diagnosis, with a mean age of 70 years, compared to 61 years for non-ischemic CRVO patients (p<0.0001). The ischemic CRVO also had a higher prevalence of arterial hypertension (p=0.052) and diabetes mellitus (p=0.018) compared to those with non-ischemic. There was a significantly higher percentage that smoked (current or past) in the non-ischemic CRVO compared to the ischemic group (p=0.012). Comparison of age, gender, smoking history, and systemic conditions between those that converted from non-ischemic to ischemic CRVO and those that initially presented with ischemic CRVO showed a higher prevalence of history of cerebrovascular accidents in those that converted from non-ischemic to ischemic (p=0.066). No significant difference was seen for the other variables.
Table 1.
Demographic and Clinical characteristics
| Demographic/Clinical Variable | Non-ischemic CRVO n=559 patients 588 eyes | Ischemic CRVO |
|
|---|---|---|---|
| As first diagnosis n=108 patients 109 eyes | Converted from non-ischemic CRVO n=47 patients* 48 eyes | ||
| Gender (Male) | 294 (53%) | 51 (47%) | 28 (60%) |
| Age at initial visit | |||
| Mean±SD | 61±16 | 70±13 | 70±13 |
| Range | 18–90 | 30–90 | 26–100 |
| Distribution by age (count, %) | |||
| <45 | 87 (16%) | 8 (7%) | 2 (4%) |
| 45–<65 | 212 (38%) | 19 (18%) | 11 (24%) |
| 65 and older | 260 (47%) | 81 (75%) | 33 (72%) |
| Eye involvement | |||
| OD | 266 (48%) | 45 (42%) | 18 (39%) |
| OS | 265 (47%) | 62 (57%) | 27 (59%) |
| OU | 29 (5%) | 1 (1%) | 1 (2%) |
| Follow-up@ | (n=422 eyes) | (n=86 eyes) | (n=41 eyes) |
| Median (25th–75th percentile) | 2.6 (0.7–5.3) years | 2.6 (0.8–6.0) years | 4.2 (1.1–5.9) years |
| Minimum-Maximum | (2.1 months-28 years) | (3 months-15.3 years) | (4.0 months-19 years) |
| Systemic Conditions | (n=557) | (n=107) | (n=46) |
| Arterial hypertension | 240 (43%) | 57 (53%) | 19 (42%) |
| Ischemic heart disease | 65 (12%) | 17 (16%) | 7 (15%) |
| Diabetes mellitus | 55 (10%) | 19 (18%) | 6 (13%) |
| TIA/CVA | 27 (5%) | 4 (4%) | 6 (13%) |
| (n=536) | (n=97) | (n=46) | |
| Smoked current/past | 243 (45%) | 38 (39%) | 21 (47%) |
CRVO=Central retinal Vein Occlusion; CVA = Cerebrovascular accident; OD = Right eye; OS = Left eye; OU = Both eyes; SD=Standard deviation; TIA = Transient ischemic attack
48 eyes (47 patients) with ischemic CRVO that converted from non-ischemic CRVO are also included among the eyes with non-ischemic CRVO
Follow-up of at least 3 months.
Initial visual acuity and visual fields
Comparing visual status at initial visit showed a significantly worst initial visual acuity and visual field defect in eyes with ischemic CRVO compared to those with non-ischemic CRVO (both p<0.0001; Table 2). Of the eyes first seen within 3 months of onset, initial visual acuity of 20/200 or worse was observed in 99% of eyes with ischemic CRVO as first diagnosis and 22% in those with non-ischemic CRVO. Forty four percent of eyes with ischemic CRVO had an initial visual field defect grade of marked to severe, compared to 0.4% of eyes with non-ischemic CRVO.
Table 2.
Visual acuity and visual field at initial visit by time from onset of visual loss
| Visual assessment | Time from onset of visual loss to initial visit |
||
|---|---|---|---|
| ≤3 months | >3–6 months | >6–12 months | |
|
Non-ischemic CRVO | |||
| Visual acuity | (n=492 eyes) | (n=70 eyes) | (n=26 eyes) |
| 20/15–20/20 | 115 (23%) | 9 (13%) | 6 (23%) |
| 20/25–20/30 | 86 (18%) | 7 (10%) | 1 (4%) |
| 20/40–20/60 | 122 (25%) | 18 (26%) | 3 (12%) |
| 20/70–20/100 | 61 (12%) | 17 (24%) | 7 (27%) |
| 20/200–400 | 96 (20%) | 16 (23%) | 6 (23%) |
| CF or worse | 12 (2%) | 3 (4%) | 3 (12%) |
| Visual field defect | (n=478 eyes, 14 missing) | (n=69 eyes, 1 missing) | (n=25 eyes, 1 missing) |
| Minimal | 321 (67%) | 40 (58%) | 18 (72%) |
| Mild | 117 (24%) | 19 (28%) | 5 (20%) |
| Moderate | 38 (8%) | 7 (10%) | 2 (8%) |
| Marked | 2 (0.4%) | 2 (3%) | 0 (0%) |
| Severe | 0 (0%) | 1 (1%) | 0 (0%) |
|
Ischemic CRVO as first diagnosis | |||
| Visual acuity | (n=85 eyes, 3 missing) | (n=13 eyes, 2 missing) | (n=5 eyes, 1 missing) |
| 20/15–20/20 | 0 | 0 | 0 |
| 20/25–20/30 | 0 | 0 | 0 |
| 20/40–20/60 | 0 | 0 | 0 |
| 20/70–20/100 | 1 (1%) | 1 (8%) | 0 |
| 20/200–400 | 18 (21%) | 2 (15%) | 1 (20%) |
| CF or worse | 66 (78%) | 10 (77%) | 4 (80%) |
| Visual field defect | (n=80 eyes, 8 missing) | (n=13, 2 missing) | (n=5, 1 missing) |
| Minimal | 0 | 0 | 1 (20%) |
| Mild | 6 (8%) | 0 | 0 |
| Moderate | 39 (49%) | 3 (23%) | 0 |
| Marked | 32 (40%) | 8 (62%) | 4 (80%) |
| Severe | 3 (4%) | 2 (15%) | 0 |
|
Ischemic CRVO (converted from non-ischemic CRVO)* |
|||
| Visual acuity | (n=48 eyes) | ||
| 20/15–20/20 | 0 | ||
| 20/25–20/30 | 0 | ||
| 20/40–20/60 | 0 | ||
| 20/70–20/100 | 0 | ||
| 20/200–400 | 16 (33%) | ||
| CF or worse | 32 (67%) | ||
| Visual field defect | (n=44, 4 missing) | ||
| Minimal | 0 | ||
| Mild | 10 (23%) | ||
| Moderate | 20 (45%) | ||
| Marked | 13 (30%) | ||
| Severe | 1 (2%) | ||
CF = counting fingers; CRVO=Central retinal Vein Occlusion
Visual acuity and field defect at the visit where conversion was recorded
Missing visual field information at the initial visit was due to inability to do visual fields for various logistic reasons.
With V4e isopter, 55% of the eyes seen within 3 months of onset with ischemic CRVO presented with scotoma, compared to only 5% of the eyes with non-ischemic CRVO (p<0.0001). Most of the scotomas were central scotoma, which was found in 41% of eyes with ischemic CRVO and 4% of eyes with non-ischemic CRVO. For the peripheral visual field, I-2e target was seen by 89% of the eyes with non-ischemic CRVO compared to only 7% of the eyes with ischemic CRVO as first diagnosis, and I-4e target by 99% and 74% respectively. Peripheral visual field defect was present in 3% of eyes with non-ischemic CRVO and in 17% of eyes with ischemic CRVO as the first diagnosis. For ischemic CRVO, the type of peripheral visual defect most frequently observed was peripheral inferior nasal defect, which was present in 10% of eyes.
Change in visual acuity on follow-up
Visual acuity changes in the eyes with non-ischemic and ischemic CRVO, first seen within 3 months of onset, are presented in Table 3 (available at http://aaojournal.org). These were as follows:
Table 3.
Visual acuity change at 3 months, 6 months, 9 months, 15 months, and 2 to 5 years follow-up from initial visit, in those seen within 3 months of onset.
| Follow-up/Initial Visual Acuity | Non-ischemic CRVO (n=374**) |
Ischemic CRVO (as first diagnosis) (n=64**) |
Ischemic CRVO (converted from non-ischemic) (n=43**) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n | Number (%) of eyes |
n** | Number (%) of eyes |
n** | Number (%) of eyes |
||||
| Improved | Worsened | Improved | Worsened | Improved | Worsened | ||||
| 3 months* | (n=317) | (n=59) | (n=40) | ||||||
| 20/15–20/30 | 135 | 2 (1%) | 18 (13%) | 0 | -- | -- | 0 | -- | -- |
| 20/40–20/60 | 70 | 14 (20%) | 17 (24%) | 0 | -- | -- | 0 | -- | -- |
| 20/70–20/100 | 39 | 6 (15%) | 15 (38%) | 1 | 0 (0%) | 1 (100%) | 0 | -- | -- |
| 20/200–20/400 | 65 | 25 (38%) | 14 (22%) | 13 | 2 (15%) | 7 (54%) | 13 | 0 (0%) | 8 (62%) |
| CF or worse | 8 | 5 (62%) | 0 (0%) | 45 | 4 (9%) | 1 (2%) | 27 | 1 (4%) | 1 (4%) |
| 20/60 or better | 205 | 16 (8%) | 35 (17%) | 0 | -- | -- | 0 | -- | -- |
| 20/70 or worse | 112 | 36 (32%) | 29 (26%) | 59 | 6 (10%) | 9 (15%) | 40 | 1 (2%) | 9 (22%) |
| 6 months* | (n=258) | (n=52) | (n=33) | ||||||
| 20/15–20/30 | 89 | 2 (2%) | 18 (20%) | 0 | -- | -- | 0 | -- | -- |
| 20/40–20/60 | 70 | 17 (24%) | 18 (26%) | 0 | -- | -- | 0 | -- | -- |
| 20/70–20/100 | 37 | 9 (24%) | 15 (41%) | 0 | -- | -- | 0 | -- | -- |
| 20/200–20/400 | 58 | 23 (41%) | 13 (22%) | 11 | 2 (18%) | 6 (55%) | 8 | 0 (0%) | 6 (75%) |
| CF or worse | 4 | 3 (75%) | 1 (25%) | 41 | 6 (15%) | 5 (12%) | 25 | 3 (12%) | 2 (8%) |
| 20/60 or better | 159 | 19 (12%) | 36 (23%) | 0 | -- | -- | 0 | -- | -- |
| 20/70 or worse | 99 | 35 (35%) | 29 (29%) | 52 | 8 (15%) | 11 (21%) | 33 | 3 (9%) | 8 (24%) |
| 9 months* | (n=226) | (n=52) | (n=35) | ||||||
| 20/15–20/30 | 89 | 1 (1%) | 17 (19%) | 0 | -- | -- | 0 | -- | -- |
| 20/40–20/60 | 61 | 12 (20%) | 18 (30%) | 0 | -- | -- | 0 | -- | -- |
| 20/70–20/100 | 28 | 6 (21%) | 14 (50%) | 0 | -- | -- | 0 | -- | -- |
| 20/200–20/400 | 43 | 18 (42%) | 8 (19%) | 11 | 1 (9%) | 7 (64%) | 11 | 1 (9%) | 7 (64%) |
| CF or worse | 5 | 4 (80%) | 0 (0%) | 41 | 7 (17%) | 4 (10%) | 24 | 6 (27%) | 1 (4%) |
| 20/60 or better | 150 | 13 (9%) | 25 (17%) | 0 | -- | -- | 0 | -- | -- |
| 20/70 or worse | 76 | 28 (37%) | 22 (29%) | 52 | 8 (15%) | 11 (21%) | 35 | 7 (20%) | 8 (23%) |
| 15 months* | (n=190) | (n=38) | (n=25) | ||||||
| 20/15–20/30 | 69 | 0 (0%) | 14 (20%) | 0 | -- | -- | 0 | -- | -- |
| 20/40–20/60 | 51 | 13 (25%) | 11 (22%) | 0 | -- | -- | 0 | -- | -- |
| 20/70–20/100 | 29 | 3 (10%) | 15 (52%) | 0 | -- | -- | 0 | -- | -- |
| 20/200–20/400 | 37 | 17 (46%) | 8 (22%) | 6 | 1 (17%) | 4 (67%) | 8 | 2 (25%) | 4 (50%) |
| CF or worse | 4 | 4 (100%) | 0 (0%) | 32 | 9 (28%) | 6 (19%) | 17 | 2 (12%) | 1 (6%) |
| 20/60 or better | 120 | 13 (11%) | 25 (21%) | 0 | -- | -- | 0 | -- | -- |
| 20/70 or worse | 70 | 24 (34%) | 23 (33%) | 38 | 10 (26%) | 10 (26%) | 25 | 4 (16%) | 5 (20%) |
| 2 to 5 years | (n=205) | (n=26) | (n=20) | ||||||
| 20/15–20/30 | 84 | 0 (0%) | 16 (19%) | 0 | -- | -- | 0 | -- | -- |
| 20/40–20/60 | 53 | 16 (30%) | 12 (23%) | 0 | -- | -- | 0 | -- | -- |
| 20/70–20/100 | 25 | 6 (24%) | 10 (40%) | 0 | -- | -- | 0 | -- | -- |
| 20/200–20/400 | 40 | 24 (60%) | 6 (15%) | 6 | 0 (0%) | 4 (67%) | 6 | 2 (33%) | 4 (67%) |
| CF or worse | 3 | 2 (67%) | 0 (0%) | 20 | 6 (30%) | 4 (20%) | 14 | 3 (21%) | 2 (14%) |
| 20/60 or better | 137 | 16 (12%) | 28 (20%) | 0 | -- | -- | 0 | -- | -- |
| 20/70 or worse | 68 | 32 (47%) | 16 (24%) | 26 | 6 (23%) | 8 (31%) | 20 | 5 (25%) | 6 (30%) |
CF = counting fingers; CRVO=Central retinal Vein Occlusion
± 6 weeks for 3, 6, and 9 months; ±12 weeks for 15 months
Total number of eyes that had at least one follow-up period in which visual acuity change was assessed
In the eyes with initial visual acuity of 20/60 or better: Only the non-ischemic CRVO eyes presented with initial visual acuity of 20/60 or better. For these eyes, the proportion of visual acuity that worsened did not significantly change over the follow-up periods (p=0.64). There were 17% (95% confidence interval (CI): 13%, 23%) that showed visual acuity deterioration at 3 months of follow-up, and 20% (95% CI: 15%, 28%) during the 2 to 5 years follow-up.
In the eyes with initial visual acuity of 20/70 or worse: In the eyes with non-ischemic CRVO, there was a significant increase in the proportion of eyes with improvement in visual acuity over the follow-up periods (p=0.034). There were 32% (95% CI: 27%, 45%) that showed visual acuity improvement at 3 months of follow-up, and 47% (95% CI: 40%, 63%) during the 2 to 5 years follow-up. In contrast, a smaller proportion of the eyes with ischemic CRVO as first diagnosis (10% and 23%, respectively) showed improvement. Overall, the rate of improvement in non-ischemic CRVO was significantly higher (p=0.0004), with odds ratio of 2.96 (95% CI: 1.55, 5.66) for visual acuity improvement for non-ischemic CRVO relative to those with ischemic CRVO.
Change in visual fields on follow-up
The changes in visual field defect grade were similar to those seen for visual acuity (Table 4 - available at http://aaojournal.org)).
Table 4.
Visual field grade change at 3 months, 6 months, 9 months, 15 months, and 2 to 5 years from initial visit, in those seen within 3 months of onset.
| Follow-up/Initial Visual Field | Non-ischemic CRVO (n=365**) |
Ischemic CRVO (as first diagnosis) (n=66**) |
Ischemic CRVO (converted from non-ischemic) (n=41**) |
||||||
|---|---|---|---|---|---|---|---|---|---|
| n** | Number (%) of eyes |
n** | Number (%) of eyes |
n** | Number (%) of eyes |
||||
| Improved | Worsened | Improved | Worsened | Improved | Worsened | ||||
| 3 months* | (n=306) | (n=60) | (n=36) | ||||||
| Minimal | 199 | 17 (9%) | 36 (18%) | 0 | -- | -- | 0 | -- | -- |
| Mild | 80 | 38 (48%) | 12 (15%) | 5 | 0 (0%) | 3 (60%) | 10 | 0 (0%) | 6 (60%) |
| Moderate | 25 | 10 (40%) | 6 (24%) | 31 | 4 (13%) | 14 (45%) | 17 | 3 (18%) | 8 (47%) |
| Marked | 2 | 1 (50%) | 0 | 22 | 4 (18%) | 7 (32%) | 8 | 1 (12%) | 2 (25%) |
| Severe | 0 | -- | -- | 2 | 0 | 0 | 1 | 0 | 0 |
| Minimal to mild | 279 | 55 (20%) | 48 (17%) | 5 | 0 (0%) | 3 (60%) | 10 | 0 (0%) | 6 (60%) |
| Moderate to severe | 27 | 11 (41%) | 6 (22%) | 55 | 8 (15%) | 21 (38%) | 26 | 4 (15%) | 10 (38%) |
| 6 months* | (n=249) | (n=54) | (n=33) | ||||||
| Minimal | 162 | 23 (14%) | 35 (22%) | 0 | -- | -- | 0 | -- | -- |
| Mild | 66 | 35 (53%) | 12 (18%) | 4 | 0 (0%) | 3 (75%) | 9 | 0 (0%) | 7 (78%) |
| Moderate | 20 | 8 (40%) | 4 (20%) | 30 | 6 (20%) | 15 (50%) | 13 | 2 (15%) | 10 (77%) |
| Marked | 1 | 1 (100%) | 0 | 19 | 4 (21%) | 8 (42%) | 10 | 2 (20%) | 2 (20%) |
| Severe | 0 | -- | -- | 1 | 0 | 0 | 1 | 1 (100%) | 0 |
| Minimal to mild | 228 | 58 (25%) | 47 (21%) | 4 | 0 (0%) | 3 (75%) | 9 | 0 (0%) | 7 (78%) |
| Moderate to severe | 21 | 9 (43%) | 4 (19%) | 50 | 10 (20%) | 23 (46%) | 24 | 5 (21%) | 12 (50%) |
| 9 months* | (n=220) | (n=50) | (n=34) | ||||||
| Minimal | 144 | 15 (10%) | 29 (20%) | 0 | -- | -- | 0 | -- | -- |
| Mild | 53 | 34 (64%) | 10 (19%) | 6 | 0 (0%) | 4 (67%) | 8 | 0 (0%) | 6 (75%) |
| Moderate | 22 | 13 (59%) | 4 (18%) | 28 | 5 (18%) | 16 (57%) | 17 | 3 (18%) | 13 (76%) |
| Marked | 1 | 0 | 0 | 14 | 2 (14%) | 6 (43%) | 8 | 2 (25%) | 1 (12%) |
| Severe | 0 | -- | -- | 2 | 0 | 0 | 1 | 1 (100%) | 0 |
| Minimal to mild | 197 | 49 (25%) | 39 (20%) | 6 | 0 (0%) | 4 (67%) | 8 | 0 (0%) | 6 (75%) |
| Moderate to severe | 23 | 13 (57%) | 4 (17%) | 44 | 7 (16%) | 22 (50%) | 26 | 6 (23%) | 14 (54%) |
| 15 months* | (n=185) | (n=42) | (n=30) | ||||||
| Minimal | 116 | 15 (13%) | 22 (19%) | 0 | -- | -- | 0 | -- | -- |
| Mild | 51 | 34 (67%) | 8 (16%) | 5 | 0 (0%) | 4 (80%) | 7 | 0 (0%) | 7 (100%) |
| Moderate | 17 | 11 (65%) | 4 (24%) | 23 | 6 (26%) | 13 (57%) | 15 | 2 (13%) | 12 (80%) |
| Marked | 1 | 1 (100%) | 0 | 13 | 3 (23%) | 7 (54%) | 8 | 2 (25%) | 3 (38%) |
| Severe | 0 | -- | -- | 1 | 0 | 0 | 1 | 1 (100%) | 0 |
| Minimal to mild | 167 | 49 (29%) | 30 (18%) | 5 | 0 (0%) | 4 (80%) | 7 | 0 (0%) | 7 (100%) |
| Moderate to severe | 18 | 12 (67%) | 4 (22%) | 37 | 9 (24%) | 20 (54%) | 24 | 5 (21%) | 15 (62%) |
| 2 to 5 years* | (n=199) | (n=37) | (n=30) | ||||||
| Minimal | 134 | 26 (19%) | 22 (16%) | 0 | -- | -- | 0 | -- | -- |
| Mild | 46 | 37 (80%) | 5 (11%) | 4 | 0 (0%) | 3 (75%) | 7 | 0 (0%) | 7 (100%) |
| Moderate | 19 | 15 (79%) | 3 (16%) | 21 | 5 (24%) | 12 (57%) | 14 | 3 (21%) | 10 (71%) |
| Marked | 0 | -- | -- | 12 | 4 (33%) | 7 (58%) | 9 | 1 (11%) | 5 (56%) |
| Severe | 0 | -- | -- | 0 | -- | -- | 0 | -- | -- |
| Minimal to mild | 180 | 63 (35%) | 27 (15%) | 4 | 0 (0%) | 3 (75%) | 7 | 0 (0%) | 7 (100%) |
| Moderate to severe | 19 | 15 (79%) | 3 (16%) | 33 | 9 (27%) | 19 (58%) | 23 | 4 (17%) | 15 (65%) |
± 6 weeks for 3, 6, and 9 months; ±12 weeks for 15 months
Total number of eyes that had at least one follow-up period in which visual field change was assessed
CRVO = Central retinal vein occlusion
In eyes with minimal to mild initial visual field defect: In non-ischemic CRVO eyes with initial visual field defect of minimal to mild (primarily the central field defect), the proportion that worsened stayed about the same over the follow-up periods (p=0.63), with visual field deterioration of 17% (95% CI: 14%, 23%) at 3 months and 15% (95% CI: 12%, 22%) during the 2 to 5 years follow-up.
In eyes with moderate to severe initial visual field defect: In the non-ischemic CRVO eyes with visual field defect grade of moderate to severe (primarily the central field defect), there was a suggestion of increase in the proportion with visual field improvement over the follow-up periods (p=0.090). There were 41% (95% CI: 23%, 57%) that showed visual field improvement at 3 months of follow-up, and 79% (95% CI: 54%, 88%) during the 2 to 5 years follow-up. In contrast, a smaller proportion of the eyes with ischemic CRVO as first diagnosis (15% and 27%, respectively) had visual field improvement. Overall, the odds ratio of improvement was 5.16 (95% CI: 2.13, 12.42) for non-ischemic CRVO relative to ischemic CRVO (p=0.0006).
The visual changes after conversion of the non-ischemic to ischemic CRVO are also shown in Tables 3 and 4 (both tables available at http://aaojournal.org). They were similar to those seen in the eyes with ischemic CRVO as the first diagnosis.
Examining the relation of change in visual acuity to change in visual field, among the eyes with non-ischemic CRVO, of the 27 eyes with minimal to mild initial visual field defect that had visual field deterioration at the 2–5 year follow-up, 22 (81%) had a corresponding deterioration in visual acuity. Of the 15 eyes that presented with moderate to severe visual field defect and showed improvement in visual field at the 2–5 year follow-up, 9 (60%) also had improvement in visual acuity.
Macular edema and visual acuity outcome
The median time to macular edema resolution was 23 months in those with non-ischemic CRVO and 29 months in those with ischemic CRVO as first diagnosis. Visual acuity changes in relation to the presence/absence of macular edema at the follow-up periods, and the overall cumulative change are shown on Table 5.
Table 5.
Visual acuity change from initial visit to 3 months, 6 months, 9 months, 15 months, and 2 to 5 years follow-up, and overall cumulative change@ in those first seen within 3 months from onset, by macular edema status at follow-up
| Follow-up/Initial Visual Acuity | With Macular Edema* |
No Macular Edema* |
||||
|---|---|---|---|---|---|---|
| n | Number (%) of eyes |
n | Number (%) of eyes |
|||
| Improved | Worsened | Improved | Worsened | |||
|
Non-ischemic CRVO | ||||||
| 3 months** | ||||||
| 20/60 or better | 104 | 6 (6%) | 35 (37%) | 98 | 10 (10%) | 0 (0%) |
| 20/70 or worse | 88 | 23 (26%) | 27 (31%) | 15 | 13 (87%) | 1 (7%) |
| 6 months** | ||||||
| 20/60 or better | 86 | 6 (7%) | 33 (39%) | 67 | 13 (19%) | 1 (1%) |
| 20/70 or worse | 75 | 17 (23%) | 25 (33%) | 18 | 16 (89%) | 1 (6%) |
| 9 months** | ||||||
| 20/60 or better | 72 | 4 (5%) | 29 (40%) | 72 | 9 (12%) | 2 (3%) |
| 20/70 or worse | 60 | 15 (25%) | 21 (35%) | 13 | 11 (85%) | 1 (8%) |
| 15 months** | ||||||
| 20//60 or better | 44 | 1 (2%) | 18 (41%) | 76 | 12 (15%) | 7 (9%) |
| 20/70 or worse | 48 | 8 (17%) | 19 (34%) | 17 | 12 (73%) | 3 (18%) |
| 2 to 5 years** | ||||||
| 20//60 or better | 31 | 1 (3%) | 12 (39%) | 101 | 14 (14%) | 15 (15%) |
| 20/70 or worse | 20 | 6 (30%) | 8 (40%) | 41 | 23 (56%) | 5 (12%) |
| Cumulative@ | ||||||
| 20//60 or better | 39 | 2 (5%) | 15 (42%) | 204 | 28 (14%) | 24 (12%) |
| 20/70 or worse | 35 | 9 (26%) | 15 (42%) | 71 | 42 (59%) | 10 (14%) |
|
Ischemic CRVO as first diagnosis | ||||||
| 3 months** | ||||||
| 20/70 or worse | 41 | 4 (10%) | 3 (7%) | 3 | 2 (67%) | 0 (0%) |
| 6 months** | ||||||
| 20/70 or worse | 35 | 6 (17%) | 5 (14%) | 6 | 2 (33%) | 1 (17%) |
| 9 months** | ||||||
| 20/70 or worse | 30 | 5 (17%) | 5 (17%) | 10 | 3 (30%) | 0 (0%) |
| 15 months** | ||||||
| 20/70 or worse | 21 | 7 (33%) | 3 (14%) | 9 | 2 (22%) | 4 (44%) |
| 2 to 5 years | ||||||
| 20/70 or worse | 6 | 1 (17%) | 0 (0%) | 13 | 4 (31%) | 4 (31%) |
| Cumulative@ | ||||||
| 20/70 or worse | 21 | 5 (24%) | 3 (14%) | 22 | 9 (41%) | 4 (18%) |
Status of macular edema at time of follow-up
± 6 weeks for 3, 6, and 9 months; ±12 weeks for 15 months
Change from initial visit to last follow-up (after at least 6 months) for eyes where macular edema did not resolve, and to visit following resolution for eyes where macular edema resolved,
CRVO = Central retinal vein occlusion
In eyes with initial visual acuity of 20/60 or better: In the non-ischemic CRVO eyes that presented with 20/60 or better visual acuity within 3 months of onset, there was a significant association of presence of macular edema with deterioration of visual acuity (p<0.0001). The odds ratio of visual acuity deterioration was 3.22 (95% CI: 1.86, 5.57) for eyes where macular edema was still present relative to those where it had resolved. At the 2 to 5 years follow-up, there was visual acuity deterioration in 39% (95% CI: 22%, 48%) where macular edema was still present compared to 15% (95% CI: 10%, 22%) where macular edema had resolved.
In eyes with initial visual acuity of 20/70 or worse: In non-ischemic CRVO eyes that presented with 20/70 or worse visual acuity, resolution of macular edema was associated with improvement in visual acuity (p<0.0001). The odds ratio of improvement is 4.00 (95% CI: 2.29, 6.98) for those with macular edema resolved relative to those where macular edema was still present. Overall, for non-ischemic CRVO with initial visual acuity 20/70 or worse, improvement was seen in 59% where macular edema had resolved compared to 26% where macular edema was still present (Table 5). In contrast, among the eyes with ischemic CRVO as first diagnosis and initial visual acuity of 20/70 or worse, there was no significant association of presence/absence of macular edema with improvement in visual acuity (p=0.55).
Macular edema and visual field outcome
Changes in overall visual field grades (based on combined information of central and peripheral visual fields) in relation to the presence/absence of macular edema at the follow-up periods, and the overall cumulative change are shown in Table 6. Visual acuity and visual field achieved at 15 months and 2–5 years follow-up and at final follow-up after resolution of macular edema in non-ischemic and ischemic CRVO are given in Table 7.
Table 6.
Visual field change from initial visit to 3 months, 6 months, 9 months, 15 months, and 2 to 5 years follow-up, and overall cumulative change@ in those first seen within 3 months from onset, by macular edema status at follow-up
| Follow-up/Initial Visual Acuity | With Macular Edema* |
No Macular Edema* |
||||
|---|---|---|---|---|---|---|
| n | Number (%) of eyes |
n | Number (%) of eyes |
|||
| Improved | Worsened | Improved | Worsened | |||
|
Non-ischemic CRVO | ||||||
| 3 months** | ||||||
| Minimal to mild | 161 | 23 (14%) | 47 (29%) | 108 | 29 (27%) | 2 (2%) |
| Moderate to severe | 22 | 8 (36%) | 5 (23%) | 3 | 2 (67%) | 1 (33%) |
| 6 months** | ||||||
| Minimal to mild | 140 | 26 (19%) | 42 (30%) | 80 | 28 (35%) | 1 (1%) |
| Moderate to severe | 16 | 5 (31%) | 4 (25%) | 3 | 3 (100%) | 0 (0%) |
| 9 months** | ||||||
| Minimal to mild | 111 | 22 (20%) | 35 (32%) | 81 | 26 (32%) | 2 (2%) |
| Moderate to severe | 19 | 11 (58%) | 4 (21%) | 2 | 3 (100%) | 0 (0%) |
| 15 months** | ||||||
| Minimal to mild | 79 | 18 (23%) | 22 (28%) | 85 | 29 (34%) | 7 (8%) |
| Moderate to severe | 11 | 7 (64%) | 2 (22%) | 5 | 4 (80%) | 1 (20%) |
| 2 to 5 years | ||||||
| Minimal to mild | 47 | 13 (28%) | 15 (32%) | 129 | 49 (38%) | 12 (9%) |
| Moderate to severe | 5 | 2 (40%) | 2 (40%) | 13 | 12 (92%) | 1 (8%) |
| Cumulative@ | ||||||
| Minimal to mild | 60 | 11 (18%) | 20 (33%) | 222 | 79 (36%) | 18 (8%) |
| Moderate to severe | 14 | 7 (50%) | 4 (28%) | 35 | 30 (86%) | 4 (11%) |
|
Ischemic CRVO as first diagnosis | ||||||
| 3 months** | ||||||
| Moderate to severe | 37 | 5 (14%) | 12 (32%) | 3 | 2 (67%) | 1 (33%) |
| 6 months** | ||||||
| Moderate to severe | 33 | 7 (21%) | 14 (42%) | 5 | 2 (40%) | 1 (20%) |
| 9 months** | ||||||
| Moderate to severe | 27 | 5 (19%) | 14 (52%) | 9 | 2 (22%) | 2 (22%) |
| 15 months** | ||||||
| Moderate to severe | 20 | 7 (35%) | 9 (45%) | 8 | 2 (25%) | 3 (38%) |
| 2 to 5 years | ||||||
| Moderate to severe | 8 | 3 (38%) | 2 (25%) | 16 | 6 (38%) | 8 (50%) |
| Cumulative@ | ||||||
| Moderate to severe | 17 | 5 (29%) | 7 (41%) | 23 | 7 (30%) | 9 (39%) |
Status of macular edema at the time of follow-up
± 6 weeks for 3, 6, and 9 months; ±12 weeks for 15 months
Change from initial visit to last follow-up (after at least 6 months) for eyes where macular edema did not resolve, and to visit following resolution for eyes where macular edema resolved,
CRVO = Central retinal vein occlusion
Table 7.
Visual acuity and visual field achieved at 15 months and 2–5 years of follow-up, and overall final follow-up in eyes without macular edema
| Visual assessment | Non-ischemic CRVO | Ischemic CRVO as first diagnosis | Ischemic CRVO converted from non-ischemic |
|---|---|---|---|
|
Visual Acuity | |||
| 15 months* | (n=93) | (n=9) | (n=4) |
| 20/15–20/20 | 43 (46%) | 1 (11%) | -- |
| 20/25–20/30 | 25 (27%) | 0 (0%) | -- |
| 20/40–20/60 | 9 (10%) | 1 (11%) | -- |
| 20/70–20/100 | 5 (5%) | 0 (0%) | 0 (0%) |
| 20/200–400 | 10 (11%) | 0 (0%) | 1 (25%) |
| CF or worse | 1 (1%) | 7 (78%) | 3 (75%) |
| 2–5 years | (n=146) | (n=13) | (n=10) |
| 20/15–20/20 | 61 (42%) | 0 (0%) | -- |
| 20/25–20/30 | 29 (20%) | 0 (0%) | -- |
| 20/40–20/60 | 20 (14%) | 0 (0%) | -- |
| 20/70–20/100 | 9 (6%) | 0 (0%) | 1 (10%) |
| 20/200–400 | 25 (17%) | 5 (38%) | 3 (30%) |
| CF or worse | 2 (1%) | 8 (62%) | 6 (60%) |
| Final@ | (n=316) | (n=26) | (n=10) |
| 20/15–20/20 | 138 (44%) | 0 (0%) | -- |
| 20/25–20/30 | 54 (17%) | 1 (3%) | -- |
| 20/40–20/60 | 45 (14%) | 2 (6%) | -- |
| 20/70–20/100 | 26 (8%) | 1 (3%) | 1 (10%) |
| 20/200–400 | 46 (15%) | 9 (35%) | 4 (40%) |
| CF or worse | 7 (2%) | 13 (50%) | 5 (50%) |
|
Visual Field Defect | |||
| 15 months* | (n=90 eyes) | (n=9) | (n=3) |
| Minimal | 81 (90%) | 0 (0%) | 0 (0%) |
| Mild | 8 (9%) | 2 (22%) | 1 (33%) |
| Moderate | 0 (0%) | 3 (33%) | 0 (0%) |
| Marked | 0 (0%) | 2 (22%) | 1 (33%) |
| Severe | 1 (1%) | 2 (22%) | 1 (33%) |
| 2–5 years | (n=142 eyes) | (n=17) | (n=12) |
| Minimal | 122 (86%) | 0 (0%) | 1 (8%) |
| Mild | 18 (13%) | 3 (18%) | 4 (33%) |
| Moderate | 1 (1%) | 7 (41%) | 1 (8%) |
| Marked | 1 (1%) | 4 (23%) | 4 (33%) |
| Severe | 0 (0%) | 3 (18%) | 2 (17%) |
| Final@ | (n=312 eyes) | (n=28) | (n=12) |
| Minimal | 271 (87%) | 1 (4%) | 1 (8%) |
| Mild | 24 (8%) | 4 (14%) | 5 (42%) |
| Moderate | 13 (4%) | 9 (32%) | 0 (0%) |
| Marked | 3 (1%) | 6 (21%) | 3 (25%) |
| Severe | 0 (0%) | 8 (29%) | 3 (25%) |
±12 weeks
of those with at least 3 months follow-up; includes all eyes regardless of time from onset of the first visit
CF = counting fingers; CRVO=Central retinal Vein Occlusion
In eyes with minimal to mild initial visual field defect: There was a significant association between the presence of macular edema and deterioration of visual field (primarily of the central visual field) (p<0.0001) among the non-ischemic CRVO eyes. The odds ratio of visual field deterioration was 2.50 (95% CI: 1.68, 3.70) for eyes where macular edema was still present relative to those where it had resolved. At the 2 to 5 year follow-up, there was visual field deterioration in 32% (95% CI: 19%, 40%) where macular edema was still present compared to 9% (95% CI: 7%, 16%) where macular edema had resolved.
In eyes with moderate to severe initial visual field defect: In non-ischemic CRVO eyes, improvement in visual field was associated with the absence of macular edema (p=0.037). The odds ratio of improvement is 2.60 (95% CI: 1.23, 5.47) for those in whom macular edema had resolved relative to those where macular edema was still present. Overall, for non-ischemic CRVO, improvement was seen in 86% where macular edema had resolved compared to 50% where macular edema was still present (Table 7). Among the eyes with ischemic CRVO as first diagnosis, there was no significant association of the presence/absence of macular edema with improvement in visual field (p=0.83).
Final visual acuity and final visual field defect at the final visit after macular edema has resolved showed worst visual acuity and visual field defect in eyes with ischemic CRVO as first diagnosis compared to those with non-ischemic CRVO (both p<0.0001; Table 7). There were 85% of the ischemic CRVO eyes with visual acuity of 20/200 or worse compared to 17% of those with non-ischemic CRVO. There were 50% of eyes with ischemic CRVO had an final visual field defect grade of marked to severe compared to 1% of eyes with non-ischemic CRVO. With V4e isopter, scotoma was found in 78% of the eyes with ischemic CRVO compared to only 6% of the eyes with non-ischemic CRVO (p<0.0001).
Effect of retinociliary collaterals on visual outcome
Retinociliary collaterals developed in 46% of the eyes with non-ischemic CRVO with median time of 15 months. Grouping and comparing the non-ischemic CRVO eyes based on those that developed retinociliary collaterals (n=174) and those that did not after at least 15 months of follow-up (n=109), showed that those that developed the collaterals were the eyes with poorer initial vision with 52% having 20/70 or worse visual acuity compared to 35% in those that did not develop collaterals. In the eyes that did not develop collaterals, median time to resolution of macular edema was 21 months. In those that developed collaterals, resolution of macular edema occurred at a median time 40 months from onset of CRVO, or 29 months after the development of the collaterals. Visual acuity change after macular edema had resolved in those with initial visual acuity of 20/70 or worse showed 43% with improved vision, 35% with no change, and 22% with deterioration for the eyes that developed collaterals. In contrast those that did not develop collaterals had better visual outcome, having 76% with improved vision, 18% with no change, and 6% with deterioration (p=0.015).
In eyes with ischemic CRVO as first diagnosis, 41% developed retinociliary collaterals with median time of 13 months. The development of collaterals had no significant effect on time of resolution of macular edema (p=0.54). In the eyes that did not develop retinociliary collaterals after at least 15 months of follow-up, median time to resolution of macular edema was 30 months. In those that developed collaterals, resolution of macular edema occurred at a median time 26 months from onset of CRVO, or 21 months after the development of the collaterals. In eyes with initial visual acuity of 20/70 or worse, there was no significant effect of presence of collaterals in change in visual acuity (p=0.81). In eyes with collaterals, 33% had improved visual acuity, 53% with no change, and 13% with deterioration, compared to 31% improved, 46% no change, and 23% deterioration in those without collaterals. As discussed below, the primary cause of poor visual acuity and visual fields in ischemia CRVO is ischemic damage of the macular ganglion cell and not so much the macular edema, while in non-ischemic CRVO the primary factor for poor visual acuity and visual fields is macular edema – this distinction is important.
Effect of foveal pigmentation and epiretinal membrane on visual outcome
The association between the presence of foveal pigmentation and epiretinal membrane and visual acuity deterioration after macular edema resolution was also examined. In eyes with non-ischemic CRVO with initial visual acuity of 20/60 or better, there were 42% that had deterioration in those with pigmentation compared to 3% without pigmentation. For epiretinal membrane, deterioration was present in 45% with epiretinal membrane compared to 8% without epiretinal membrane. From the logistic regression model with foveal pigmentation and epiretinal membrane as independent variables, both the presence of foveal pigmentation (odds ratio 20.84; 95% CI: 6.65, 65.36; p<0.0001) and epiretinal membrane (odds ratio 9.54; 95% CI: 2.62, 34.73; p=0.0006) showed a significant association with visual acuity deterioration. With respect to visual acuity improvement in non-ischemic CRVO that had initial visual acuity of 20/70 or worse, improvement in visual acuity was associated with absence of pigmentation (p=0.006; 78% in those without pigmentation vs. 44% in those with pigmentation), whereas no significant association was seen for epiretinal membrane (p=0.99). For the eyes with ischemic CRVO as first diagnosis, after macular edema had resolved, there was no significant association of change in visual acuity with foveal pigmentation and epiretinal membrane (both p>0.86).
Effect of neovascular glaucoma on visual outcome in ischemic CRVO
Of the eyes with ischemic CRVO that were first seen within 3 months of onset and had follow-up of at least 6 months, neovascular glaucoma developed in 36% (23 of 64) of those with ischemic CRVO as first diagnosis and 33% (14 of 43) of those that converted from non-ischemic CRVO. In those with ischemic CRVO as first diagnosis, there was further deterioration in visual acuity from initial visual acuity of 20/70 or worse in 44%, with no eyes showing improvement, of those with neovascular glaucoma, compared to 12% deterioration and 32% with improvement in those without neovascular glaucoma (p=0.002). Similar findings were observed for visual field changes. In those with moderate to severe initial visual field defect, those with neovascular glaucoma had 76% with worsening and 5% improvement of visual field compared to 38% worsening and 35% improvement in those without neovascular glaucoma (p=0.008).
Effect of age on visual acuity
In non-ischemic CRVO where macular edema had resolved, logistic regression adjusting for the effect of foveal pigmentation and epiretinal membrane showed a significant association of age with visual acuity change, with increasing age positively associated with visual acuity deterioration (p=0.001) in those that presented with 20/60 or better visual acuity, and negatively associated with visual acuity improvement (p=0.012) in eyes with initial visual acuity of 20/70 or worse. Among younger (<45 years) patients, of those with initial visual acuity of 20/60 or better, none (0 of 48) had visual acuity deterioration, and, among those with initial visual acuity of 20/70 or worse, 80% (8 of 10) had visual acuity improvement. In contrast, in persons 45 or older visual acuity deterioration was seen in 15% (24 of 156) of those with initial visual acuity of 20/60 or better, while among those with initial visual acuity of 20/70 or worse, 56% (34 of 61) improved.
Effect of systemic conditions on visual acuity
The effect of systemic conditions and smoking on visual acuity change in non-ischemic CRVO after macular edema had resolved was also examined by logistic regression analysis. In eyes with initial visual acuity of 20/60 or better, after adjusting for the effect of foveal pigmentation and epiretinal membrane, stroke (p=0.036) and diabetes mellitus (p=0.049) showed a significant association with visual acuity deterioration. The odds ratio of visual acuity deterioration for those with history of stroke relative to those without was 8.94 (95% CI: 1.15, 68.96). For those with diabetes mellitus relative to those without, the odds ratio of visual acuity deterioration was 6.04 (95% CI: 1.01, 36.04). There was no significant association between visual acuity deterioration and arterial hypertension (p=0.14), ischemic heart disease (p=0.58), or smoking (p=0.31). In those that presented with initial visual acuity of 20/70 or worse, no significant association, with respect to reducing the percentage with visual acuity improvement, was found for arterial hypertension (p=0.84), stroke (p=0.82), ischemic heart disease (p=0.47), diabetes mellitus (p=0.58), and smoking (p=0.24).
Discussion
From the results of our present study, we have shown that there are significant differences in initial visual acuity and visual field defects between non-ischemic and ischemic CRVO (Table 2). The changes in visual outcome, as well as the effect of macular edema and retinopathy on the visual outcomes were found to differ between these two types of CRVO. Thus, a distinction between the two types of CRVO is the most crucial and fundamental in determining visual outcome in CRVO. It is most unfortunate that this basic fact has been ignored in the vast majority of CRVO studies dealing with the visual outcome in CRVO. This is also true of other complications of CRVO - most importantly ocular neovascularization, which is a complication of only the ischemic CRVO and is not seen in non-ischemic CRVO, unless the latter is associated with diabetic retinopathy or ocular ischemia.17 Therefore, in the management of CRVO, the first, crucial step is to determine which type of CRVO one is dealing with. Combining the two types of CRVO into one group is like lumping benign and malignant tumors in one category to determine their outcome.
This distinct difference in visual outcomes between the two types of CRVO is not at all surprising if one considers basic facts about the pathogenesis of visual loss in the two types of CRVO. The primary cause of poor visual acuity in non-ischemic CRVO is macular edema, while in ischemia CRVO retinal ischemia is usually the major factor with macular edema a minor factor. Macular retinal ganglion cells are most vulnerable to ischemic damage27. It is well known that the macular region has more than one layer of retinal ganglion cells, unlike the rest of the retina, and it is the thickest part of the retina - maximum thickness being close to the foveola. Experimental27,28 and clinical12,29 studies on central retinal artery occlusion have shown that ischemic damage is most marked in the central part of the macular retina, which results in development of central scotoma, most commonly the only visual field defect seen in patients following transient central retinal artery occlusion.29 Once the retinal ganglion cells in the macular region have suffered irreversible ischemic damage in ischemic CRVO, there is little chance of any visual acuity improvement, even if the macular edema resolves, since it is not the primary cause of poor visual acuity in theses cases. This is evident from the fact that, in the present study, resolution of macular edema had a significant effect on visual improvement in non-ischemic CRVO (visual acuity: p<0.0001, visual fields: p=0.037) but not in ischemic CRVO (visual acuity: p=0.55, visual fields: p=0.83).
Criteria to differentiate ischemic from non-ischemic CRVO
The distinct difference in the visual outcome between ischemic and non-ischemic CRVO makes the importance of differentiating ischemic CRVO from non-ischemic CRVO crucial in any study dealing with visual outcome. Studies claiming to differentiate the two types have used very variable criteria (see below). The most common, sole criterion used for making that differentiation has been “10 disc area of retinal capillary obliteration” on fluorescein fundus angiography - this somehow seems to have become a “gold standard”.30 Unfortunately, this is not a reliable criterion in most cases. The large multicenter CRVO study31 showed that eyes with less than 30 disc diameters of retinal capillary nonperfusion are at low risk for developing iris/angle neovascularization, which means that their chance of being ischemic CRVO are very low; most of them are therefore non-ischemic CRVO. That study showed that “eyes with 75 disc diameters or more are at highest risk”, i.e., they are definitely ischemic CRVO. Retinal capillary non-perfusion on fluorescein in CRVO was also investigated by our prospective study of 128 eyes with CRVO in a study specifically designed to investigate the validity, sensitively and specificity of various tests to differentiate the two types of CRVO16. It showed that fluorescein fundus angiography during the early acute phase of CRVO, provided reliable information about retinal capillary nonperfusion in only about 60% because of many limitations (discussed at length in that paper16); it also showed the presence of multiple isolated focal capillary nonperfusion areas in eyes which otherwise fulfilled the criteria for non-ischemic CRVO on all other tests. That study showed that during the early acute phase of CRVO, because of problem with evaluation of retinal capillary non-perfusion fluorescein fundus angiography in about a third of the eyes, functional tests, i.e., relative afferent pupillary defect, electroretinography, visual acuity and peripheral visual fields, provided reliable information in all eyes. Sensitivity and specificity of the various functional tests is described in detail elsewhere. In fluorescein angiograms, where capillary non-perfusion could be seen satisfactory, provided very useful information in differentiation of the two types of CRVO; however, that was possible in about 60% of the eyes in that study.
It could very well be argued that the use of criterion of visual acuity and visual field to differentiate ischemic from non-ischemic CRVO might have biased the initial visual acuity and visual fields at baseline and visual outcome in the two types of CRVO in our study. However, in fact, as discussed in the inclusion criteria above, that was not true at all. Briefly, the distinction (between ischemic and non-ischemic CRVO) was based on the COMBINED data acquired from six tests which we evaluated in our study.16 Combined information from relative afferent pupillary defect and electroretinography differentiated 97% of the cases. Thus, relative afferent pupillary defect and electroretinography often played the most important deciding role about the type of CRVO. Fluorescein fundus angiography (when it was of reliable quality) also provided useful information in differentiation. In some cases the information from other tests in fact over-ruled the information from the visual acuity or visual fields. This is evident from fact that: (i) the initial visual acuity was 20/200 or worse in 22% of the non-ischemic CRVO eyes and it was 20/70 – 20/100 in 8% of the ischemic CRVO, and (ii) initial visual field defects were moderate to marked in 8.4% of the non-ischemic CRVO and mild in 8% of the ischemic CRVO.
Mixing or combing the two types of CRVO or use of unreliable criteria, as is almost invariably the case in most of the studies dealing with natural history of visual outcome, as well as those dealing with different advocated invasive and non-invasive mode of treatments, results in misleading information. That is responsible for much of the controversy and confusion on management of CRVO.
Importance of evaluation of visual fields in evaluation of visual outcome in CRVO
The visual outcome recorded in various studies on CRVO is invariably based entirely on the visual acuity, but this provides information only about the function of the macula. CRVO, however, involves the entire retina and NOT only the macular retina. By contrast, visual fields provide information on the function of the entire retina. Therefore, it is crucial to evaluate visual outcome by a combination of both the tests in all eyes with CRVO. Moreover, a central scotoma may improve markedly in size and severity, but so long as it involves central fixation, the visual acuity may not improve, thus providing misleading information about the change in visual outcome.
Currently, visual fields are usually plotted using automated perimetry. Visual field information provided by manual kinetic perimetry performed with a Goldmann perimeter is very different from that by automated static threshold perimetry (Humphrey 30-2 or 24-2 SITA). Both types of perimetry have their advantages and disadvantages, and one should be aware of those when interpreting the findings. It is well established that the constant tracking provided by the peripheral visual fields is essential for sensory input for day to day activity and for “navigating”. In view of that, to assess the visual function disability, it is important to have complete information about the peripheral visual fields and any impairment in them. Automated perimetry provides information about peripheral visual fields only up to 24° – 30°. Manual kinetic perimetry, by contrast, provides information all the way to about 80° – 90° temporally, 70° inferiorly, 60° – 70° nasally and 50° – 60° superiorly. Thus, a visual field plotted with manual kinetic perimetry provides far more comprehensive information about the peripheral visual field defects, for evaluating visual functional disability; it is most unfortunate that is rapidly being replaced by automated perimetry.
In the present study the central visual fields showed a variety of scotomas - the most common being the central scotoma. Peripheral visual fields plotted with a Goldmann perimeter remained perfectly normal in all eyes with non-ischemic CRVO throughout the course of follow-up, unless there was some other associated problem, e.g., cilioretinal artery occlusion32. By contrast, in ischemic CRVO, there was always a relative peripheral visual defect with I-2e and/or with I-4e isopters, but V-4e isopter was still normal in the vast majority - even in eyes with visual acuity of counting fingers and in some of those with hand motion. This information about the peripheral visual fields is important, as discussed above, to: (i) differentiate ischemic from non-ischemic CRVO, as shown by a previous study16, and (ii) to evaluate the disability caused by the CRVO. For example, a study of panretinal photocoagulation in ischemic CRVO showed marked loss of peripheral visual fields24, which, combined with a permanent large central scotoma, would result in marked visual disability.
Natural history of visual outcome in CRVO
Since Moore2 in 1924 first reported the natural history of visual outcome in CRVO in 18 eyes, there seem to be only a few such studies3–10 of an appreciable number of cases. It is essential to put all those studies in proper perspective; they are not at all comparable - each is based on an entirely different study design, variable number of patients, some combining all the CRVO patients under one category without differentiating ischemic from non-ischemic CRVO, and others differentiating by means of totally different criteria (see below). Thus, no two studies are really alike, and therefore, it is not feasible to compare their results. Moreover, in all studies visual outcome was based only on the visual acuity outcome except for that of Moore2 who is the only one who evaluated visual field defects also - this is a serious flaw in almost all the reported studies.
Moore,2 in his study of 18 eyes with CRVO mostly followed for several years, found almost invariably marked deterioration in visual acuity. It seems most of his cases had ischemic CRVO. He recorded the visual fields in his cases and commented: “Perhaps the most striking fact with regards to the field of vision in central vein thrombosis is the relative frequency with which the peripheral fields are but little constricted, and this is true not only when they were taken within a short time of the thrombosis but they may remain nearly full for many years”. This is supported by our study and is an important finding.
Zegarra et al.3 in a study of 25 eyes, followed for 1–8 years, found that in 10 eyes with “non-ischemic CRVO”, the final visual acuity was 20/30 or better in 50% and 20/60 in 30%, while among the “ischemic CRVO” eyes 82% had a final VA of 20/400 or less.
Quinlan et al.4 in a retrospective study of 107 non-ischemic and 61 ischemic CRVO eyes (using a criterion of ≥5 disc diameter area of capillary non-perfusion) and a follow-up of 6 months to 6 years (mean 22 months) reported the following: in “non-ischemic CRVO” eyes, initial visual acuity varied from 20/15 to count fingers; among them, in eyes with initial visual acuity of 20/40 or better, the final visual acuity was 20/200 or less in 21%, and in those with initial visual acuity of 20/200 or less it was 20/200 or less in 88%. In this group, 15% improved by 3 or more lines from the baseline, and 31% lost 3 or more lines. In all eyes with “ischemic CRVO”, initial visual acuity was 20/100 or less; the final visual acuity was 20/200 or less in 93%, counting fingers or less in 54%, and hand motion or less in 36%. They concluded that good initial visual acuity provided no guarantee of a satisfactory visual outcome.
Chen et al.5 in a case series of 59 eyes with “non-ischemic CRVO”, followed for at least 1 year (average 2.5 years), found that visual acuity improved by two or more lines in 15%, remained stable in 56% and decreased in 29%. They concluded that “non-ischemic CRVO” frequently results in significant, permanent visual loss. They found that initial visual acuity had no predictive value of progression.
The multicenter Central Vein Occlusion Study Group9 reported their results on visual acuity in 714 eyes with CRVO. The only criterion used by them to classify their cases into ischemic CRVO was the presence of at least a 10 disc area of retinal nonperfusion30. However, to evaluate the natural history of visual outcome, they combined their ischemic and non-ischemic CRVOs into one group. They divided their cases into 3 groups based on initial visual acuity: group 1 with visual acuity of 20/40 or better (29%), group 2 with acuity between 20/50 and 20/200 (43%), and group 3 with acuity worse than 20/200 (28%). The final visual acuity in group 1 was stable in 65% and deteriorated in 35% - in 10% to worse than 20/200; in group 2 it improved to better than 20/40 in 19%, stayed the same in 44% and deteriorated to worse than 20/200 in 37%; and in group 3 in 79% it was less than 20/200, in 19% improved to 20/50 to 20/200 and in 1% to 20/40. They concluded that “Visual acuity at baseline is a strong predictor of visual acuity at 3 years” for eyes in group 1 and 3 but a poor predictor for group 2.
In a recent SCORE study10, where all CRVO eyes were combined into one group, without any differentiation into ischemic and non-ischemic, in the 73 eyes without any treatment, at 12 months of follow-up, visual acuity improved in 26%, remained the same in 19% and deteriorated in 55%.
A detailed account of the initial visual acuity and visual fields in the present study and the changes in them during follow-up are given above. In our study, we categorized patients into ischemic and non-ischemic CRVO based on combined information from functional and morphologic tests16. We found that among the non-ischemic eyes with initial visual acuity of 20/70 or worse, 59 % improved, 27% showed no change and 14% deteriorated on resolution of macular edema, while in ischemic CRVO 41% improved, 41% did not change and 18% deteriorated (Table 5). This showed that there was a difference in improvement in visual outcome between the two types. Since the other studies used different criteria, no comparison is possible.
Conversion of non-ischemic to ischemic CRVO
In the present study, of the 588 eyes with non-ischemic CRVO, 48 converted to the ischemic type; this may be an under estimate for overall conversion, because of the inclusion criteria used in this study. The subjects of conversion of non-ischemic CRVO to ischemic CRVO20 and mechanism of conversion33 are discussed at length elsewhere.
In the present study, we compared various aspects of ischemic CRVO eyes that presented initially with ischemic CRVO versus those that converted from non-ischemic to ischemic. There was a higher prevalence of history of cerebrovascular disease in those that converted from non-ischemic to ischemic (p=0.066). Visual changes following conversion to ischemic CRVO were similar to those seen in the eyes with ischemic CRVO as the first diagnosis. Based on the experience of dealing with more than a thousand CRVO eyes by one of us (SSH), it seems that most CRVO eyes start as non-ischemic and convert later to ischemic CRVO; they are not usually seen by most ophthalmologists during the non-ischemic phase, because there are no, or only slight, symptoms.
Effect of foveal pigmentation and epiretinal membrane on visual outcome
Development of foveal pigmentation and epiretinal membrane following chronic macular edema, in non-ischemic CRVO adversely influences the visual outcome after resolution of macular edema (foveal pigmentation: odds ratio 20.84; p<0.0001, and epiretinal membrane: odds ratio 9.54; p=0.0006); this is an important piece of information. However, in ischemic CRVO there was no significant association of change in visual acuity with foveal pigmentation and epiretinal membrane (both p>0.86).
Effect of neovascular glaucoma on visual outcome in ischemic CRVO
In the present study, neovascular glaucoma developed in 36% of the ischemic CRVO eyes. As expected, that resulted in further deterioration of visual outcome (visual acuity p=0.002; visual fields p=0.008) compared to those without neovascular glaucoma.
Effect of age on natural history of visual outcome
Chen et al.,5 in a study of 59 eyes, found that the factors significantly related to visual outcome were initial visual acuity (p = 0.0001) and age - older patients having a worse visual outcome (p = 0.0029). Glacet-Bernard et al.34 in 120 CRVO eyes found older age a prognostic factor for poor visual outcome. Similarly, in our study, no patients aged <45 years with initial visual acuity of 20/60 or better had visual acuity deterioration, and among those with initial visual acuity of 20/70 or worse visual acuity improved in 80%. In contrast, in those ≥45 years, 15% of those with initial visual acuity of 20/60 or better experienced deterioration, while only 56% of those with initial visual acuity of 20/70 or worse showed any improvement. Thus, increasing age was associated with visual acuity deterioration.
Effect of systemic diseases on natural history of visual outcome
Among the systemic diseases, only cerebrovascular disease (odds ratio 8.94; p=0.036) and diabetes mellitus (odds ratio 6.04; p=0.049) showed a significant association with visual acuity deterioration in eyes with initial visual acuity of 20/60 or better. There was no significant association with arterial hypertension (p=0.14), ischemic heart disease (p=0.58) or smoking (p=0.31). In eyes that presented with initial visual acuity of 20/70 or worse, there as no significant association between visual acuity improvement and arterial hypertension (p=0.84), cerebrovascular disease (p=0.82), ischemic heart disease (p=0.47), diabetes mellitus (p=0.58), or smoking (p=0.24).
Effect of development of retinociliary collaterals on natural history of visual outcome
There is a prevalent impression among ophthalmologists that development of retinociliary collaterals has a beneficial effect on the course and visual outcome of CRVO, by improving the circulation in the central retinal vein by by-passing the occlusion - some have claimed visual improvement after radial optic neurotomy is due to development of these collaterals. Priluck et al. 6 in a retrospective study of 42 patents with CRVO, aged 40 or younger, stated that the presence of collaterals closely correlated with a favorable prognosis. Giuffré et al.,8, 35 in 94 patients with CRVO, found that the visual acuity of the eyes with CRVO that developed collaterals was not significantly different from the visual acuity of the eyes without collaterals. Quinlan et al.,4 in a retrospective study of 168 eyes with CRVO, also found that the collaterals were not related to any improvement in visual acuity. Garcia-Arumi et al.,36 in their radial optic neurotomy study, found no significant difference in visual acuity between those with and without these collaterals. Thus, literature gives firm evidence that development of retinociliary collaterals does not make any difference in the visual outcome in CRVO.
We studied the role of retinociliary collaterals in detail because there is little information on its different aspects, based on a comprehensive study in a large cohort. In our study, 46% of non-ischemic CRVO eyes developed the collaterals with a median time of 15 months. Our study revealed an interesting phenomenon, not previously described. Non-ischemic CRVO (in eyes with initial visual acuity of 20/70 or worse) showed the following:
The eyes that developed collaterals had poorer initial visual acuity than the eyes that did (52% versus 35%).
Resolution of macular edema took much longer in those with collaterals than in those without (median time 40 months versus 21 months).
After resolution of macular edema, 35% of eyes that developed collaterals showed no change in visual acuity, 43% improved, and 22% deteriorated. Compared to that, in eyes without collaterals, this was 6%, 76% and 6% respectively.
Eyes with ischemic CRVO, as first diagnosis, had similar findings.
These findings pose an intriguing question about the retinociliary veins in CRVO: why presence of cilioretinal collaterals is related to poor initial and final visual acuity? Based on our anatomic, basic, experimental and clinical studies, the following seems the most plausible explanation. In all eyes with CRVO, fluorescein fundus angiography shows the presence of blood flow. Therefore, in CRVO, after the vein is occluded by a thrombus, the eye must develop collaterals to maintain its blood flow. The collaterals develop from the pre-existing venous tributaries anterior to the site of occlusion in the central retinal vein. Within the optic nerve the central retinal vein has multiple prominent tributaries and only a few inconstant ones in the prelaminar region of the optic nerve head (Fig. 1). The severity of retinopathy in CRVO varies widely, indicating that severity depends upon the site of occlusion in the CRV and the number of tributaries available anterior to the site of occlusion to develop collaterals, i.e., farther back the site of occlusion in the optic nerve, the greater the number of tributaries within the optic nerve available to establish collateral circulation, and milder the retinopathy; conversely, the closer the site of occlusion to the lamina cribrosa, the fewer tributaries are available in the optic nerve to establish collateral circulation, and the worse the retinopathy. In the latter case, there is a far greater stress on the few available tributaries in the prelaminar region to develop into collaterals than when the site of occlusion is far back in the optic nerve. Therefore, eyes with retinociliary collaterals on the optic disc have a much greater chance of having a severer type of retinopathy, with much worse visual acuity and more marked macular edema than do those without those collaterals, as shown by our study. The severity of retinopathy in turn determines the visual acuity outcome. Therefore, not surprisingly the higher the prevalence of retinociliary collaterals on the optic disc, the smaller is the chance of visual improvement and the greater the chance of visual deterioration – where there are no retinociliary collaterals, there are plenty of venous tributaries available within the optic nerve and no need to develop collaterals on the disc. On clinical evaluation, there is no way to determine the number and locations of the collaterals that develop within the optic nerve in CRVO. The other factor one has to keep in mind is that in our study 54% of the non-ischemic CRVO eyes with CRVO did not develop venous tributaries in the prelaminar region. Thus, the prevalence of collaterals on the optic disc does not represent a true incidence of all the collaterals that develop with CRVO, and to judge the prevalence of collaterals in CRVO simply from the ones seen on the optic disc is highly misleading. Incidentally, the prevalent misconception that the site of occlusion in CRVO is always at the lamina cribrosa is based on histopathology of rare eyes enucleated for painful, uncontrollable neovascular glaucoma due to ischemic CRVO – the worst type of CRVO37; that does not apply to the vast majority of the CRVO eyes where the site of occlusion is within the optic nerve posterior to the lamina cribrosa.
Figure 1.
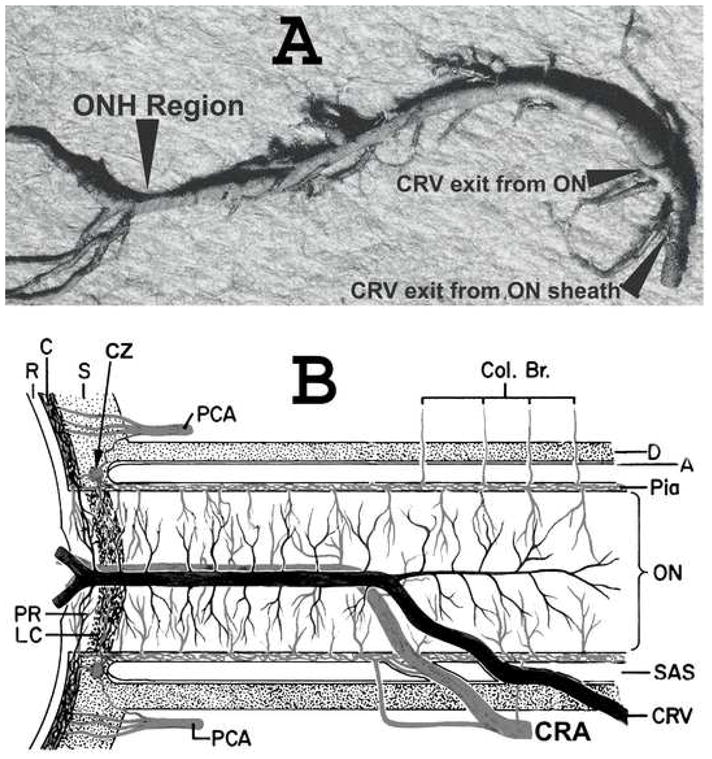
(A) Cast of the central retinal vein showing its entire course from the optic disc to its exit from the optic nerve sheath. Note the presence of a large number of prominent collaterals within the optic nerve and none in the optic nerve head region in this specimen.
(B). Schematic representation of the blood vessels in the optic nerve. (B modified from Hayreh SS: Trans Am Acad Ophthalmol Otolaryngol 1974; 78:OP240-OP254.)
Abbreviations: A = arachnoid; C = choroid; CAR and CRA = central retinal artery; Col. Br. = Collateral branches; CRV = central retinal vein; CZ = circle of Zinn and Haller; D = dura; LC = lamina cribrosa; ON = optic nerve; ONH = optic nerve head; PCA = posterior ciliary artery; PR = prelaminar region; R = retina; Rec. Br. CZ = recurrent pial branches from peripapillary choroid/CZ; S = sclera; SAS = subarachnoid space.
Visual outcome in CRVO in young adults
Because of a widespread misconception that CRVO does not occur in young persons, they often undergo extensive investigations to find the cause of retinopathy. In our study, 16% of the non-ischemic CRVO patients were under the age of 45 years, and 7% of those with ischemic CRVO as initial diagnosis were in that age group (Table 1). There are other reports of CRVO in young persons6–8.
The reports of visual outcome in young individuals vary widely from study to study. Priluck et al.6 in a retrospective study of 42 patients with CRVO, who were 40 years or younger, found that their final visual prognosis could not be predicted by the severity of the venous occlusion at the time of diagnosis. Fong et al. 7 in a retrospective study of 103 cases of CRVO in young, nondiabetic adults, followed for at least six months, found that 32% had a final visual acuity of 20/200 or worse and 6% no light perception. Giuffré et al.,8 in a study of 20 patients with CRVO aged 40 years or less, found that the visual prognosis of young people with CRVO is often poor. In contrast to all the above studies, in our study, none of those aged under 45, with initial visual acuity of 20/60 or better, had visual acuity deterioration, and, in those with initial visual acuity of 20/70 or worse, it improved in 80%. In those 45 or older, with initial visual acuity of 20/60 or better, it deteriorated in 15%, and among those with initial visual acuity of 20/70 or worse, it improved in 56%. This shows that the visual prognosis for young people is much better than that for those 45 or older.
In conclusion, visual outcome is totally different in the two types of CRVO, i.e., non-ischemic and ischemic - good in the former and poor in the latter. Therefore, a differentiation of CRVO into non-ischemic and ischemic types is most crucial and fundamental in determining visual outcome. For differentiation of CRVO into its two types during the initial acute phase, the most reliable are functional tests (visual acuity, visual fields, relative afferent pupillary defect and electroretinography) and much less reliable are morphological tests (fluorescein fundus angiography and ophthalmoscopy)16. Visual outcome is also adversely influenced to some extent by several other factors, including increasing age, cerebrovascular disease and diabetes mellitus, and in non-ischemic CRVO by development of foveal pigmentation and epiretinal membrane, and in ischemic CRVO by neovascular glaucoma.
Acknowledgments
Supported by grant EY-1151 from the National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Michel J. Ueber die anatomischen Ursachen von Veraenderungen des Augenhintergrundes bei einigen Allgemeinerkrankungen. Dtsch Arch Klin Med. 1878;22:339–45. [Google Scholar]
- 2.Moore RF. Br J Ophthalmol Monograph. suppl 2. London: Pulman; 1924. Retinal venous thrombosis: A clinical study of sixty-two cases followed over many years; pp. 1–90. [Google Scholar]
- 3.Zegarra H, Gutman FA, Conforto J. The natural course of central retinal vein occlusion. Ophthalmology. 1979;86:1931–42. doi: 10.1016/s0161-6420(79)35327-1. [DOI] [PubMed] [Google Scholar]
- 4.Quinlan PM, Elman MJ, Bhatt AK, et al. The natural course of central retinal vein occlusion. Am J Ophthalmol. 1990;110:118–23. doi: 10.1016/s0002-9394(14)76979-x. [DOI] [PubMed] [Google Scholar]
- 5.Chen JC, Klein ML, Watzke RC, et al. Natural course of perfused central retinal vein occlusion. Can J Ophthalmol. 1995;30:21–4. [PubMed] [Google Scholar]
- 6.Priluck IA, Robertson DM, Hollenhorst RW. Long-term follow-up of occlusion of the central retinal vein in young adults. Am J Ophthalmol. 1980;90:190–202. doi: 10.1016/s0002-9394(14)74853-6. [DOI] [PubMed] [Google Scholar]
- 7.Fong AC, Schatz H, McDonald HR, et al. Central retinal vein occlusion in young adults (papillophlebitis) Retina. 1992;12:3–11. doi: 10.1097/00006982-199212010-00002. [DOI] [PubMed] [Google Scholar]
- 8.Giuffré G, Randazzo-Papa G, Palumbo C. Central retinal vein occlusion in young people. Doc Ophthalmol. 1992;80:127–32. doi: 10.1007/BF00161238. [DOI] [PubMed] [Google Scholar]
- 9.Central Vein Occlusion Study Group. Natural history and clinical management of central retinal vein occlusion. Arch Ophthalmol. 1997;115:486–91. doi: 10.1001/archopht.1997.01100150488006. [DOI] [PubMed] [Google Scholar]
- 10.SCORE Study Research Group. A randomized trial comparing the efficacy and safety of intravitreal triamcinolone with observation to treat vision loss associated with macular edema secondary to central retinal vein occlusion: The Standard Care vs Corticosteroid for Retinal Vein Occlusion (SCORE) Study report 5. Arch Ophthalmol. 2009;127:1101–14. doi: 10.1001/archophthalmol.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hayreh SS. Occlusion of the central retinal vessels. Br J Ophthalmol. 1965;49:626–45. doi: 10.1136/bjo.49.12.626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hayreh SS. So-called “central retinal vein occlusion”: I. Pathogenesis, terminology, clinical features. Ophthalmologica. 1976;172:1–13. doi: 10.1159/000307579. [DOI] [PubMed] [Google Scholar]
- 13.Hayreh SS. Pathogenesis of occlusion of the central retinal vessels. Am J Ophthalmol. 1971;72:998–1011. doi: 10.1016/0002-9394(71)91706-5. [DOI] [PubMed] [Google Scholar]
- 14.Hayreh SS, van Heuven WA, Hayreh MS. Experimental retinal vascular occlusion I. Pathogenesis of central retinal vein occlusion. Arch Ophthalmol. 1978;96:311–23. doi: 10.1001/archopht.1978.03910050179015. [DOI] [PubMed] [Google Scholar]
- 15.Hayreh SS. Classification of central retinal vein occlusion. Ophthalmology. 1983;90:458–74. doi: 10.1016/s0161-6420(83)34530-9. [DOI] [PubMed] [Google Scholar]
- 16.Hayreh SS, Klugman MR, Beri M, et al. Differentiation of ischemic from non-ischemic central retinal vein occlusion during the early acute phase. Graefes Arch Clin Exp Ophthalmol. 1990;228:201–17. doi: 10.1007/BF00920022. [DOI] [PubMed] [Google Scholar]
- 17.Hayreh SS, Rojas P, Podhajsky P, et al. Ocular neovascularization with retinal vascular occlusion-III. Incidence of ocular neovascularization with retinal vein occlusion. Ophthalmology. 1983;90:488–506. doi: 10.1016/s0161-6420(83)34542-5. [DOI] [PubMed] [Google Scholar]
- 18.Hayreh SS, Zimmerman B. Visual field abnormalities in nonarteritic anterior ischemic optic neuropathy: their pattern and prevalence at initial presentation. Arch Ophthalmol. 2005;123:1554–62. doi: 10.1001/archopht.123.11.1554. [DOI] [PubMed] [Google Scholar]
- 19.Hayreh SS. Prevalent misconceptions about acute retinal vascular occlusive disorders. Prog Retin Eye Res. 2005;24:493–519. doi: 10.1016/j.preteyeres.2004.12.001. [DOI] [PubMed] [Google Scholar]
- 20.Hayreh SS, Zimmerman MB, Podhajsky P. Incidence of various types of retinal vein occlusion and their recurrence and demographic characteristics. Am J Ophthalmol. 1994;117:429–41. doi: 10.1016/s0002-9394(14)70001-7. [DOI] [PubMed] [Google Scholar]
- 21.Hayreh SS, Zimmerman MB. Nonarteritic anterior ischemic optic neuropathy: natural history of visual outcome. Ophthalmology. 2008;115:298–305. doi: 10.1016/j.ophtha.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ischemic Optic Neuropathy Decompression Trial Research Group. Optic nerve decompression surgery for nonarteritic anterior ischemic optic neuropathy (NAION) is not effective and may be harmful. JAMA. 1995;273:625–32. [PubMed] [Google Scholar]
- 23.Hayreh SS. Posterior ischaemic optic neuropathy: clinical features, pathogenesis, and management. Eye (Lond) 2004;18:1188–206. doi: 10.1038/sj.eye.6701562. [DOI] [PubMed] [Google Scholar]
- 24.Hayreh SS, Klugman MR, Podhajsky P, et al. Argon laser panretinal photocoagulation in ischemic central retinal vein occlusion: a 10-year prospective study. Graefes Arch Clin Exp Ophthalmol. 1990;228:281–96. doi: 10.1007/BF00920049. [DOI] [PubMed] [Google Scholar]
- 25.Hayreh SS, Zimmerman MB, Podhajsky P, Alward WL. Nocturnal arterial hypotension and its role in optic nerve head and ocular ischemic disorders. Am J Ophthalmol. 1994;117:603–24. doi: 10.1016/s0002-9394(14)70067-4. [DOI] [PubMed] [Google Scholar]
- 26.Hayreh SS, Podhajsky PA, Zimmerman B. Role of nocturnal arterial hypotension in optic nerve head ischemic disorders. Ophthalmologica. 1999;213:76–96. doi: 10.1159/000027399. [DOI] [PubMed] [Google Scholar]
- 27.Hayreh SS, Zimmerman MB, Kimura A, Sanon A. Central retinal artery occlusion: retinal survival time. Exp Eye Res. 2004;78:723–36. doi: 10.1016/s0014-4835(03)00214-8. [DOI] [PubMed] [Google Scholar]
- 28.Hayreh SS, Weingeist TA. Experimental occlusion of the central artery of the retina. I. Ophthalmoscopic and fluorescein fundus angiographic studies. Br J Ophthalmol. 1980;64:896–912. doi: 10.1136/bjo.64.12.896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hayreh SS, Zimmerman MB. Central retinal artery occlusion: visual outcome. Am J Ophthalmol. 2005;140:376–91. doi: 10.1016/j.ajo.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 30.Central Vein Occlusion Study Group. Baseline and early natural history report: the Central Vein Occlusion Study. Arch Ophthalmol. 1993;111:1087–95. doi: 10.1001/archopht.1993.01090080083022. [DOI] [PubMed] [Google Scholar]
- 31.A randomized clinical trial of early panretinal photocoagulation for ischemic central vein occlusion: the Central Vein Occlusion Study Group N report. Ophthalmology. 1995;102:1434–44. [PubMed] [Google Scholar]
- 32.Hayreh SS, Fraterrigo L, Jonas J. Central retinal vein occlusion associated with cilioretinal artery occlusion. Retina. 2008;28:581–94. doi: 10.1097/IAE.0b013e31815ec29b. [DOI] [PubMed] [Google Scholar]
- 33.Hayreh SS. Central retinal vein occlusion. Ophthalmol Clin North Am. 1998;11:559–90. [Google Scholar]
- 34.Glacet-Bernard A, Coscas G, Chabanel A, et al. Prognostic factors for retinal vein occlusion: prospective study of 175 cases. Ophthalmology. 1996;103:551–60. doi: 10.1016/s0161-6420(96)30653-2. [DOI] [PubMed] [Google Scholar]
- 35.Giuffrè G, Palumbo C, Randazzo-Papa G. Optociliary veins and central retinal vein occlusion. Br J Ophthalmol. 1993;77:774–7. doi: 10.1136/bjo.77.12.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Garcia- Arumíi J, Boixadera A, Martinez-Castillo V, et al. Chorioretinal anastomosis after radial optic neurotomy for central retinal vein occlusion. Arch Ophthalmol. 2003;121:1385–91. doi: 10.1001/archopht.121.10.1385. [DOI] [PubMed] [Google Scholar]
- 37.Green WR, Chan CC, Hutchins GM, Terry JM. Central vein occlusion: a prospective histological study of 29 eyes in 28 cases. Trans Am Ophthalmol Soc. 1981;79:371–422. [PMC free article] [PubMed] [Google Scholar]


