Abstract
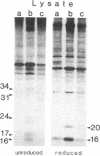
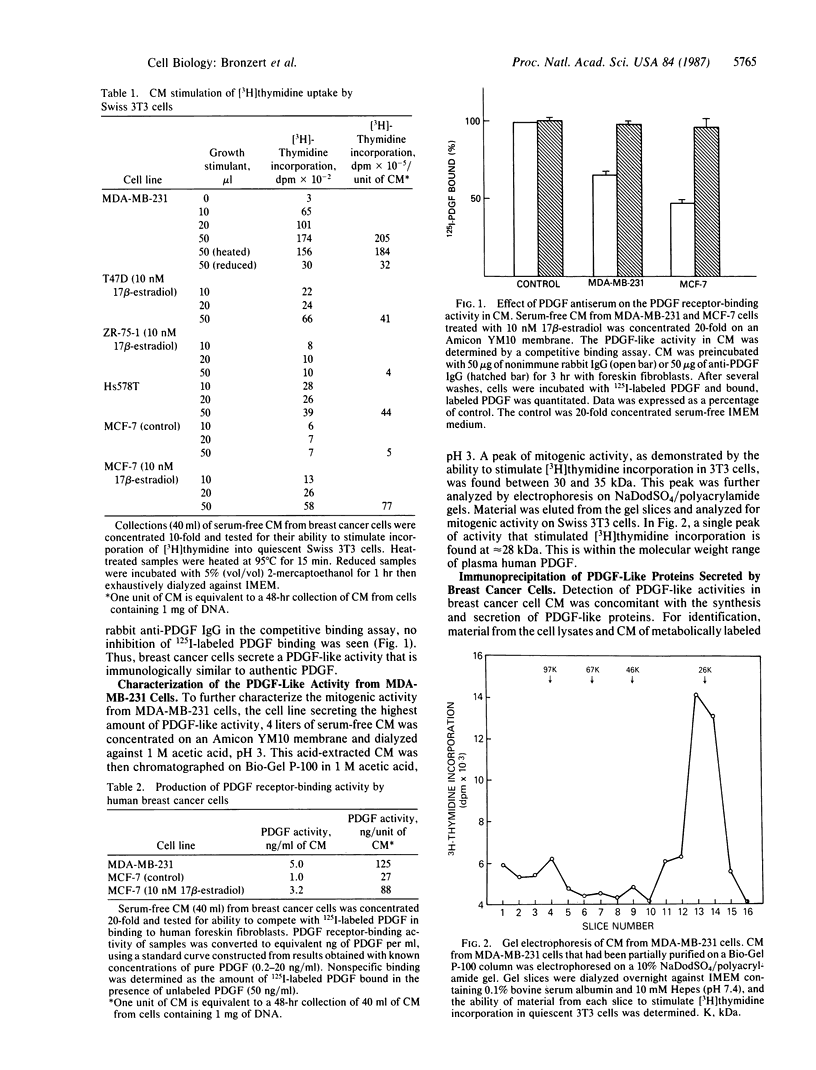
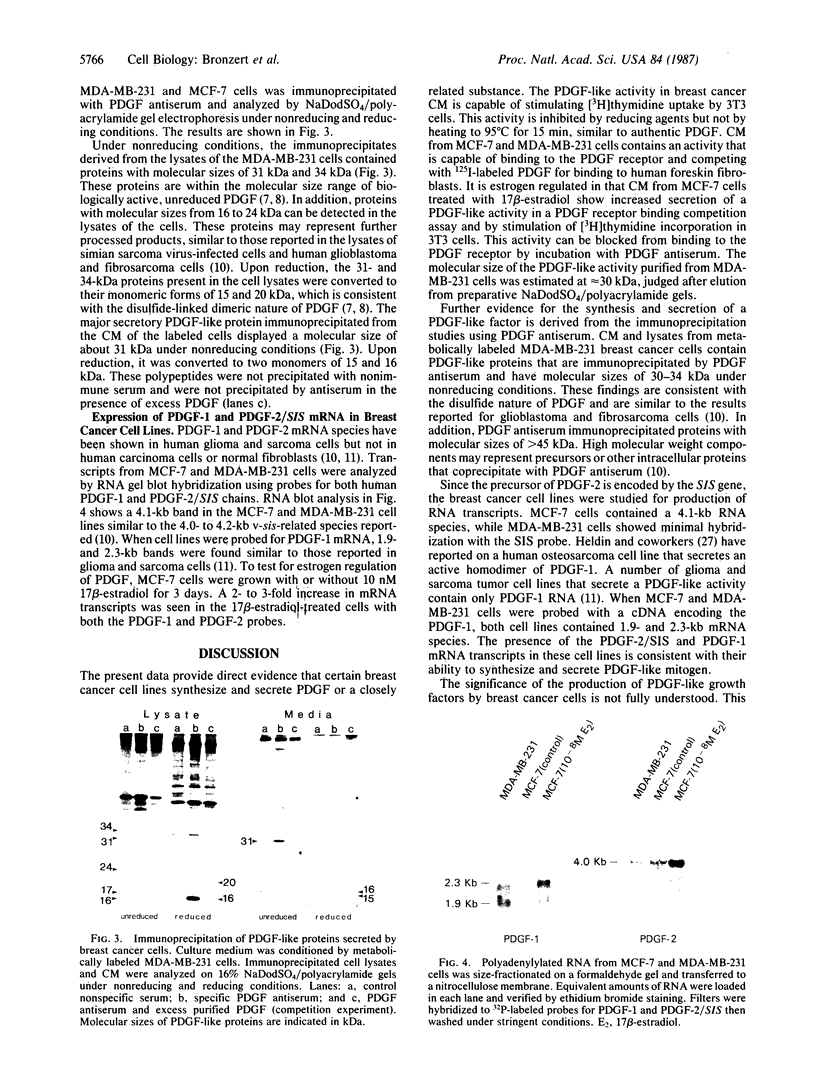
We report that human breast cancer cells secrete a growth factor that is biologically and immunologically similar to platelet-derived growth factor (PDGF). Serum-free medium conditioned by estrogen-independent MDA-MB-231 or estrogen-dependent MCF-7 cells contains a mitogenic or "competence" activity that is capable of inducing incorporation of [3H]thymidine into quiescent Swiss 3T3 cells in the presence of platelet-poor plasma. In addition, the conditioned medium contains an activity that competes with 125I-labeled PDGF for binding to PDGF receptors on normal human fibroblasts. The secretion of PDGF-like activity by the hormone-responsive cell line MCF-7 is stimulated by 17 beta-estradiol. Like authentic PDGF, the PDGF-like activity produced by breast cancer cells is stable after acid and heat treatment (95 degrees C) and inhibited by reducing agents. The mitogenic activity comigrates with a material of approximately equal to 30 kDa on NaDodSO4/polyacrylamide gels. Immunoprecipitation with PDGF antiserum of proteins from metabolically labeled cell lysates and conditioned medium followed by analysis on nonreducing NaDodSO4/polyacrylamide gels identified proteins of 30 and 34 kDa. Upon reduction, the 30- and 34-kDa bands were converted to 15- and 16-kDa bands suggesting that the immunoprecipitated proteins were made up of two disulfide-linked polypeptides similar to PDGF. Hybridization studies with cDNA probes for the A chain of PDGF and the B chain of PDGF/SIS identified transcripts for both PDGF chains in the MCF-7 and MDA-MB-231 cells. The data summarized above provide conclusive evidence for the synthesis and hormonally regulated secretion of a PDGF-like mitogen by breast carcinoma cells. Production of a PDGF-like growth factor by breast cancer cell lines may be important in mediating paracrine stimulation of tumor growth.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Antoniades H. N. Human platelet-derived growth factor (PDGF): purification of PDGF-I and PDGF-II and separation of their reduced subunits. Proc Natl Acad Sci U S A. 1981 Dec;78(12):7314–7317. doi: 10.1073/pnas.78.12.7314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoniades H. N., Hunkapiller M. W. Human platelet-derived growth factor (PDGF): amino-terminal amino acid sequence. Science. 1983 May 27;220(4600):963–965. doi: 10.1126/science.6844921. [DOI] [PubMed] [Google Scholar]
- Antoniades H. N., Stathakos D., Scher C. D. Isolation of a cationic polypeptide from human serum that stimulates proliferation of 3T3 cells. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2635–2639. doi: 10.1073/pnas.72.7.2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assoian R. K., Grotendorst G. R., Miller D. M., Sporn M. B. Cellular transformation by coordinated action of three peptide growth factors from human platelets. 1984 Jun 28-Jul 4Nature. 309(5971):804–806. doi: 10.1038/309804a0. [DOI] [PubMed] [Google Scholar]
- Berthois Y., Katzenellenbogen J. A., Katzenellenbogen B. S. Phenol red in tissue culture media is a weak estrogen: implications concerning the study of estrogen-responsive cells in culture. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2496–2500. doi: 10.1073/pnas.83.8.2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Betsholtz C., Johnsson A., Heldin C. H., Westermark B., Lind P., Urdea M. S., Eddy R., Shows T. B., Philpott K., Mellor A. L. cDNA sequence and chromosomal localization of human platelet-derived growth factor A-chain and its expression in tumour cell lines. Nature. 1986 Apr 24;320(6064):695–699. doi: 10.1038/320695a0. [DOI] [PubMed] [Google Scholar]
- Bowen-Pope D. F., Vogel A., Ross R. Production of platelet-derived growth factor-like molecules and reduced expression of platelet-derived growth factor receptors accompany transformation by a wide spectrum of agents. Proc Natl Acad Sci U S A. 1984 Apr;81(8):2396–2400. doi: 10.1073/pnas.81.8.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunk C. F., Jones K. C., James T. W. Assay for nanogram quantities of DNA in cellular homogenates. Anal Biochem. 1979 Jan 15;92(2):497–500. doi: 10.1016/0003-2697(79)90690-0. [DOI] [PubMed] [Google Scholar]
- Clemmons D. R., Underwood L. E., Van Wyk J. J. Hormonal control of immunoreactive somatomedin production by cultured human fibroblasts. J Clin Invest. 1981 Jan;67(1):10–19. doi: 10.1172/JCI110001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalla-Favera R., Gallo R. C., Giallongo A., Croce C. M. Chromosomal localization of the human homolog (c-sis) of the simian sarcoma virus onc gene. Science. 1982 Nov 12;218(4573):686–688. doi: 10.1126/science.6291150. [DOI] [PubMed] [Google Scholar]
- Dickson R. B., Bates S. E., McManaway M. E., Lippman M. E. Characterization of estrogen responsive transforming activity in human breast cancer cell lines. Cancer Res. 1986 Apr;46(4 Pt 1):1707–1713. [PubMed] [Google Scholar]
- Dickson R. B., McManaway M. E., Lippman M. E. Estrogen-induced factors of breast cancer cells partially replace estrogen to promote tumor growth. Science. 1986 Jun 20;232(4757):1540–1543. doi: 10.1126/science.3715461. [DOI] [PubMed] [Google Scholar]
- Haslam S. Z. Mammary fibroblast influence on normal mouse mammary epithelial cell responses to estrogen in vitro. Cancer Res. 1986 Jan;46(1):310–316. [PubMed] [Google Scholar]
- Heldin C. H., Johnsson A., Wennergren S., Wernstedt C., Betsholtz C., Westermark B. A human osteosarcoma cell line secretes a growth factor structurally related to a homodimer of PDGF A-chains. Nature. 1986 Feb 6;319(6053):511–514. doi: 10.1038/319511a0. [DOI] [PubMed] [Google Scholar]
- Heldin C. H., Westermark B., Wasteson A. Platelet-derived growth factor. Isolation by a large-scale procedure and analysis of subunit composition. Biochem J. 1981 Mar 1;193(3):907–913. doi: 10.1042/bj1930907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff K. K., Kaufman D., Gabbay K. H., Spencer E. M., Lippman M. E., Dickson R. B. Secretion of an insulin-like growth factor-I-related protein by human breast cancer cells. Cancer Res. 1986 Sep;46(9):4613–4619. [PubMed] [Google Scholar]
- Johnsson A., Heldin C. H., Wasteson A., Westermark B., Deuel T. F., Huang J. S., Seeburg P. H., Gray A., Ullrich A., Scrace G. The c-sis gene encodes a precursor of the B chain of platelet-derived growth factor. EMBO J. 1984 May;3(5):921–928. doi: 10.1002/j.1460-2075.1984.tb01908.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kao R. T., Hall J., Engel L., Stern R. The matrix of human breast tumor cells is mitogenic for fibroblasts. Am J Pathol. 1984 Apr;115(1):109–116. [PMC free article] [PubMed] [Google Scholar]
- Kaplan P. L., Anderson M., Ozanne B. Transforming growth factor(s) production enables cells to grow in the absence of serum: an autocrine system. Proc Natl Acad Sci U S A. 1982 Jan;79(2):485–489. doi: 10.1073/pnas.79.2.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe E. J., Pledger W. J. A model of cell cycle control: sequential events regulated by growth factors. Mol Cell Endocrinol. 1983 Aug;31(2-3):167–186. doi: 10.1016/0303-7207(83)90147-8. [DOI] [PubMed] [Google Scholar]
- Pledger W. J., Stiles C. D., Antoniades H. N., Scher C. D. An ordered sequence of events is required before BALB/c-3T3 cells become committed to DNA synthesis. Proc Natl Acad Sci U S A. 1978 Jun;75(6):2839–2843. doi: 10.1073/pnas.75.6.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J., Taylor-Papadimitriou J. Production of PDGF-like growth factor by breast cancer cell lines. Int J Cancer. 1985 Aug 15;36(2):247–252. doi: 10.1002/ijc.2910360218. [DOI] [PubMed] [Google Scholar]
- Stiles C. D., Capone G. T., Scher C. D., Antoniades H. N., Van Wyk J. J., Pledger W. J. Dual control of cell growth by somatomedins and platelet-derived growth factor. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1279–1283. doi: 10.1073/pnas.76.3.1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakimoto H., Oka T. Involvement of collagen formation in the hormonally induced functional differentiation of mouse mammary gland in organ culture. J Biol Chem. 1983 Mar 25;258(6):3775–3779. [PubMed] [Google Scholar]