Abstract
Background
Emerging evidence has suggested a contribution of bone marrow (BM) cells to lymphatic vessel formation; however, the exact phenotype of the cells with lymphatic endothelial progenitor cell (LEPC) function has yet to be identified. Here we investigate the identity of BM-derived LEPCs and their role in lymphatic neovascularization.
Methods and Results
Culture of BM-mononuclear cells (MNCs) in the presence of VEGFA, VEGFC and EGF resulted in expression of lymphatic endothelial cell (LEC) markers. Among these cells, podoplanin+ cells were isolated by magnetic-labeled cell separation system (MACS) and characterized by FACS and immunocytochemistry. These podoplanin+ cells highly express markers for LECs, hematopoietic lineages, and stem/progenitor cells, and upon further cultivation, generate LECs. We further confirmed that podoplanin+ cells exist in small numbers in BM and peripheral blood (PB) of normal mice, but are significantly (15 fold) augmented upon lymphangiogenic stimuli such as tumor implantation. Next, to evaluate the potential of podoplanin+ cells for the formation of new lymphatic vessels in vivo, we injected culture-isolated or freshly isolated BM-derived podoplanin+ cells into wound and tumor models. Immunohistochemistry demonstrated that the injected cells were incorporated into the lymphatic vasculature, displayed LEC phenotypes, and increased lymphatic vascular density in tissues, suggesting lymphvasculogenesis. Podoplanin+ cells also expressed high levels of lymphangiogenic cytokines and increased proliferation of LECs during co-culture, suggesting a lymphangiogenic or paracrine role.
Conclusions
Our results provide compelling evidence that BM-derived podoplanin+ cells, a previously unrecognized cell type, function as LEPCs and participate in postnatal lymphatic neovascularization through both lymphvasculogenesis and lymphangiogenesis.
Keywords: Bone Marrow, Podoplanin, Lymphangiogenesis, Lymphvasculogenesis
INTRODUCTION
Lymphatic vessels play an important role in the pathogenesis of diseases such as lymphedema and tumors1, 2, and an understanding of lymphatic biology is crucial in order to develop strategies to prevent or treat these diseases. Investigation of lymphatic vascular growth was made possible by the discovery of specific markers for lymphatic endothelial cells (LECs). The establishment of the lymphatic vasculature during mouse embryogenesis begins with expression of homeobox transcriptional factor PROX-1 in a subset of the venous endothelial cells of the cardinal vein3 which express VEGFR-34 and LYVE-15. Subsequently, the PROX-1 expressing (PROX-1+) cells migrate out to form the primary lymphatic plexus, which undergoes further remodeling to form a mature network of lymphatic endothelial cells (LECs) which express other lymphatic endothelial cell markers such as podoplanin6, 7. Podoplanin (pod) is a 38 kDa integral membrane mucoprotein which is predominantly expressed in the endothelium of lymphatic capillaries7. Mice deficient in pod die at birth due to respiratory failure accompanied by malfunctioning lymphatic, but not blood, vessels with impaired lymphatic transport and congenital lymphedema8.
The generation of lymphatic vessels in adults was previously believed to be achieved exclusively by a process called lymphangiogenesis, the formation of new lymphatic vessels from preexisting lymphatic vasculature9–13. However, emerging evidence has suggested that lymphvasculogenesis may also occur through putative progenitor cells for LECs. Studies have shown that BM contains cells with the potential to generate LECs14–17. An early study reported that human fetal liver-derived, non-adherent CD34+CD133+VEGFR-3+ cells, when cultured, became adherent and expressed LYVE-1 or pod, implying a role for these cells as common blood vascular and lymphatic endothelial progenitor cells (LEPCs)14. Subsequently, Religa and colleagues showed that BM-derived cells were incorporated into newly formed lymphatic vessels in corneas, using a chimeric mouse model in which BM of wild-type mice was reconstituted by BM transplantation from donor green fluorescent protein (GFP) mice16. Maruyama and his colleagues demonstrated incorporation of mouse GFP-BM cells into lymphatic vessels in inflamed corneas15. This study showed that tissue resident CD11b+ macrophages, presumably derived from BM, were incorporated into inflammation-induced lymphatics in the cornea. Kerjaschki et al demonstrated the presence of male recipient-derived LECs in the lymphatic vessels in kidneys transplanted from female donors17. Together, these studies support the idea that a certain population of BM cells, likely of monocyte-macrophage lineages, can give rise to LECs in the foci of new lymphatic vessel formation (lymphatic neovascularization) in various pathologic conditions. However, no studies have clearly addressed the exact identity of BM-derived LEPCs in adults and tested their potential for lymphatic neovascularization by external implantation of isolated LEPCs.
In this study, we for the first time show that pod+CD11b+ cells exist in adult BM and can function as LEPCs, and further demonstrate that these cells contribute to lymphatic neovascularization through dual lymphvasculogenic and lymphangiogenic roles.
METHODS
All protocols for animal experiments were approved by the Institutional Animal Care and Use Committees of Emory University.
Culture of BM-MNCs
Mouse BM-MNCs fractionated by density gradient centrifugation with Histopaque®-1083 (Sigma, St. Louis, MO) were seeded onto 100 mm culture dishes coated with rat vitronectin. To optimize culture conditions to generate LEPCs, four different combinations of media were employed (online-only Data Supplemental Table 1). To differentiate sorted putative LEPCs derived from cultured BM-MNCs into LECs, the cells were maintained in EBM-2 supplemented with cytokine cocktail (SingleQuots; Clonetics) for 7 days.
LEC proliferation assay
The sorted pod+ and pod− cells (2.5 × 103) from 4 day-cultured BM-MNCs were mixed with human dermal lymphatic endothelial cells (hDLECs, 1.5 × 104)(Cambrex) which were pre-labeled with CM-Dil (Invitrogen), seeded onto 96 well culture plates and cocultured in EGM media with 1% FBS. Twenty four hours later, cells were stained with Ki67 antibody and counterstained with DAPI. Cells positive for Dil, Ki67 and DAPI were counted.
Mouse Cornea Model
A micropocket was created followed by implantation of a micropellet containing VEGF-C and FGF-218. Subsequently, CM-Dil-labeled 4-day cultured pod+ cells were injected into the surrounding area in the cornea. Eyeballs were isolated 7 days later and subjected to immunohistochemistry.
Skin and Ear Wound Models
After creating full-thickness excisional skin wounds on backs or ears of the mice, pod+ cells labeled with DiI or derived from GFP mice were injected into the wound bed around the wound. Seven days later, the wound tissues including a perimeter of 1 to 2 mm of normal skin tissue were harvested for immunohistochemistry.
Mouse Tumor (Melanoma) Model
Tumor cells (B16-F1 melanoma cell line) were subcutaneously injected into the middle dorsum of C57BL/6 mice. Seven days later, Dil-labeled pod+ cells isolated from cultured BM-MNCs were injected into the tumor vicinity and the mice were sacrificed 7 days later for immunohistochemistry.
Measurement of lymphatic capillary density
After implantation of tumors (melanomas) and injection of pod+ and pod− cells or the same volume of PBS as described above, tumors and surrounding peritumoral tissues including skin were harvested and subjected to immunohistochemistry with a VEGFR-3 antibody19. Lymphatic capillary density was calculated from at least 10 randomly selected fields.
Statistical Analysis
All results were presented as mean ± SE. Statistical analyses were performed with the Mann-Whitney U test for comparisons between two groups and the Kruskal-Wallis ANOVA test for more than two groups. P values < 0.05 were considered to denote statistical significance.
Details on the above and other methods can be found in the online-only Data Supplement.
The authors have full access to and take full responsibility for the integrity of the data. All authors have read and agree to the manuscript as written.
RESULTS
BM-MNCs cultured under defined conditions express LEC markers
To explore whether BM-MNCs include potential LEPCs, we first examined LEC marker expression in freshly isolated BM-MNCs of FVB mice by qRT-PCR. Uncultured BM-MNCs expressed Cd31 and Lyve1 but not Prox1, Vegfr3 or pod (Figure 1A). Next, to investigate whether culture of BM-MNCs under defined conditions can induce LEC gene expression, we cultured BM-MNCs in EBM-2 supplemented with VEGF-A, VEGF-C, and EGF individually or in various combinations (online-only Data Supplemental Table 1). We selected VEGF-A and VEGF-C based on their known role as lymphatic growth factors11, 20 and EGF due to its role in cell proliferation21. Expression of Prox1 and Vegfr3 emerged at day 1, reached a peak at day 4, and decreased but continued until day 7. Pod expression went up from day 1 and was sustained until day 10, while Cd31 and Lyve1 expression decreased over the 4 days (Figure 1A). Another lymphatic marker, Foxc2, was also expressed in the VEGF-A, VEGF-C and EGF (ACE) condition with a peak at day 4 (data not shown). From these experiments, we found that cultivation of BM-MNCs with lymphangiogenic factors can generate LEC-like cells. We further found that 4 day-culture under the ACE condition was optimal for generating LEC-like cells, as all the LEC markers were significantly expressed while expression of a pan-vascular endothelial cell marker, Cd31, was significantly reduced at day 4. Immunocytochemistry and FACS analysis also confirmed the qRT-PCR results (Figure 1B and 1J). FACS analyses showed a significant increase in pod+ and VEGFR-3+ cells and similar levels of LYVE-1+ cells in cultured BM-MNCs compared to the freshly isolated BM-MNCs (Figure 1J). The expression levels of LEC markers in the pod+ cells were similar to those of mouse LECs isolated from a mouse EC line, SVEC4-1022 (online-only Data Supplemental Figure S1). Taken together, these results indicate that culture of BM-MNCs under the ACE condition can generate cells expressing LEC markers. We therefore used this culture condition for the following experiments.
Figure 1. Expression of LEC markers in BM-MNCs cultured with lymphangiogenic cytokines.
A, BM-MNCs were cultured under various culture conditions, harvested at day 1, 4, 7, or 10, and subjected to qRT-PCR. Each value is the average of three independent experiments (n = 4 per experiment). BM-MNC, bone marrow mononuclear cells; EBM-2, endothelial cell basal medium-2; A, VEGF-A; C, VEGF-C; E, EGF. B through J, Expression of LEC markers in 4-day cultured BM-MNCs which were cultured under the ACE condition. Immunocytochemistry (B–I): DAPI (blue) for nuclear staining, merged image (I) of images in F–H, scale bar = 100 µm. FACS analysis (J): the numbers in boxes are the percentage of cells positive for the indicated proteins. Each value is the average of three independent experiments (n = 4 per experiment).
Pod+ cells isolated from cultured BM-MNCs express LEC markers, hematopoietic and stem/progenitor cell markers, and lymphangiogenic cytokines
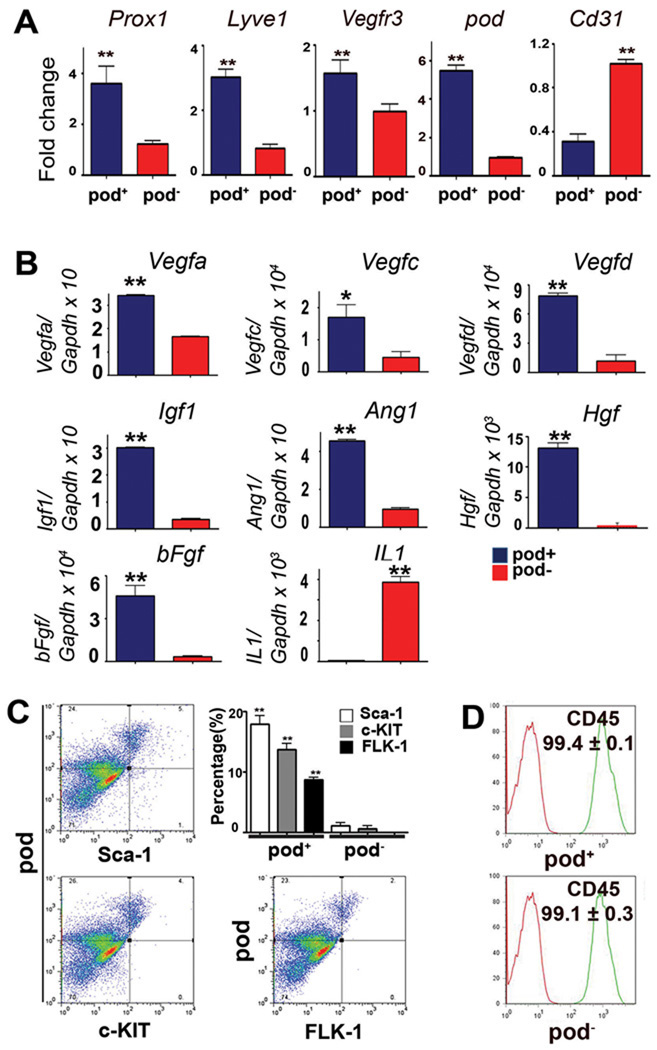
Since these cultured cells were still heterogeneous, we next sought to enrich cells with the LEC phenotype using a cell sorting strategy. Among the three representative LEC surface markers, LYVE-1, VEGFR-3 and pod, we selected pod based on the qRT-PCR data (Figure 1A), which showed pod expression to be the most stable from day 4 and on. Pod has been implicated in proper patterning of lymphatic vessels and lymphedema7, 8. Accordingly, we isolated pod+ or pod− cells from the cultured BM-MNCs at day 4 by MACS and examined the LEC markers. qRT-PCR analysis demonstrated augmented expression of Prox1 (3.7-fold), Lyve1 (3.0-fold) and pod (6.0-fold) in the pod+ fraction compared to the pod− fraction (Figure 2A). Vegfr3 expression was slightly higher in the pod+ than the pod− cells. However, pod+ cells showed lower expression of Cd31 (3.6-fold). qRT-PCR analysis further revealed that representative lymphangiogenic cytokines such as Vegfa (2.1-fold), Vegfc (3.8-fold), Vegfd (6.8-fold), Igf1 (8.9-fold), Ang1 (4.8-fold), Hgf (48.1-fold), and bFgf (14.4-fold), were more highly expressed in pod+ cells than pod− cells, implying more potent paracrine lymphangiogenic activity of pod+ cells (Figure 2B)2, 23, 24 Next, we examined the progenitor character of pod+ cells. FACS analysis showed that hematopoietic stem cell (HSC) markers Sca-1, c-KIT and FLK-1 were expressed in 17.8 ± 3.4%, 13.6 ± 2.4% and 8.6 ± 0.9% of the pod+ cells, respectively (Figure 2C). In contrast, pod− cells virtually did not express HSC markers. More than 99% of cultured pod+ cells expressed CD45 (Figure 2D) indicating that these pod+ cells are hematopoietic in origin and are not contaminating pre-existing LECs from BM. Importantly, when further cultured for 7 days in conventional LEC culture media, which is EBM-2 supplemented with SingleQuots® (complete EGM)25, the pod+ cells maintained expression of pod and LYVE-1, whereas pod− cells hardly expressed these markers (Figure 2E). We also found that the pod+ cells have multi-lineage differentiation potential, as demonstrated by expression of markers of blood endothelial cells, smooth muscle cells, and fibroblasts depending upon culture conditions (online-only Data Supplemental Figure S2). Taken together, these findings indicate that pod+ cells isolated from cultured BM-MNCs are enriched with LEC markers and lymphangiogenic cytokines, are hematopoietic in origin, possess stem/progenitor cell characteristics, and differentiate into LECs when further cultured, suggestive of putative LEPCs.
Figure 2. Enriched expression of lymphangiogenic cytokines and markers for LECs and HSCs in the culture-isolated pod+ cells.
A through D, The MACS-sorted pod+ and pod− cells from 4 day-cultured BM-MNCs were subjected to qRT-PCR (A, B) and FACS analysis (C, D). Each value is the average of three independent experiments (** P<0.01, * P<0.05, n=4 per experiment). E, The sorted cells were further cultured for 7 days and subjected to immunocytochemistry for LYVE-1 and pod.
Culture-isolated pod+ cells contribute to lymphatic vessel formation in animal models
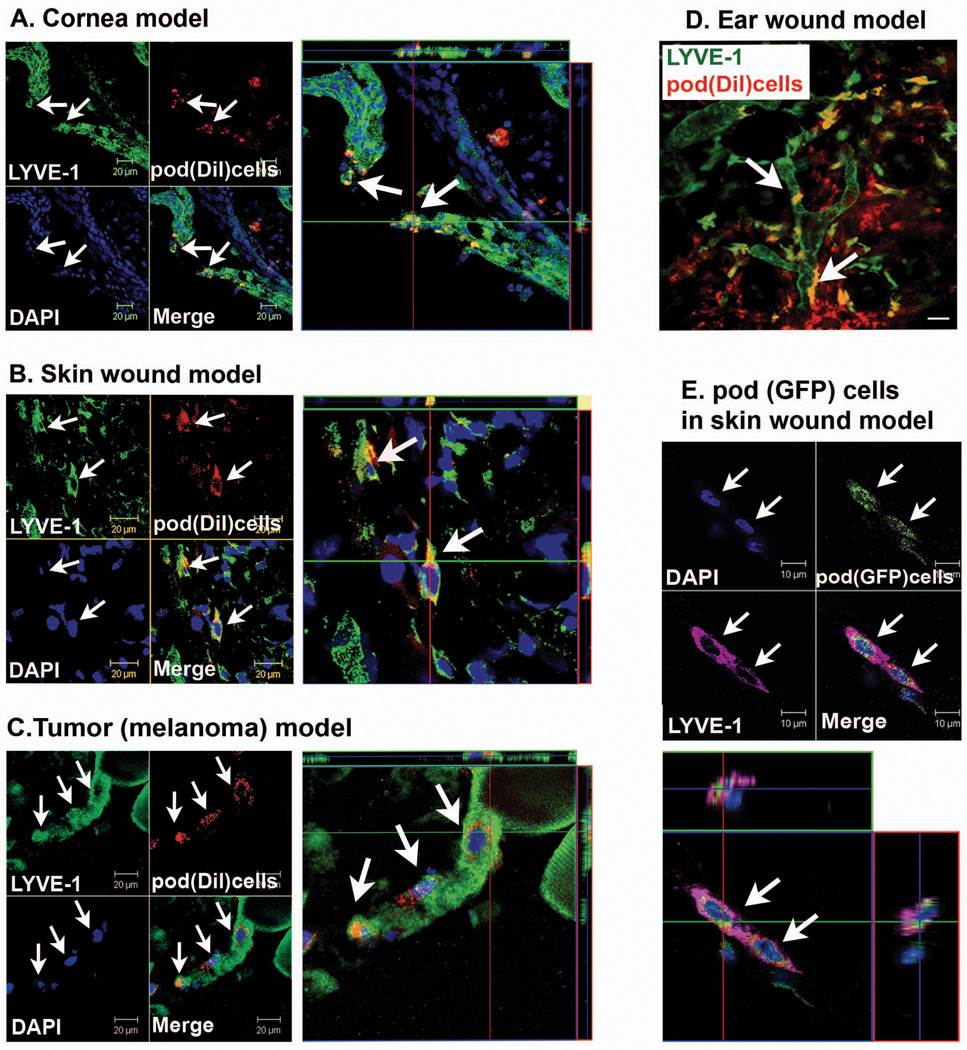
Next, we sought to determine the lymphatic neovascularization potential of these pod+ cells. We thus injected pod+ cells into four different animal models which are well-known to induce lymphatic vessel formation, a corneal micropocket model15 (Figure 3A), ear and skin wound models26 (Figure 3B, 3D and 3E) and a tumor (melanoma) model27 (Figure 3C). Culture-isolated pod+ cells pre-labeled with DiI, a red fluorescent dye used for cell tracking, were injected into the margin of the cornea (cornea model) or into the subcutaneous tissues (wound models or tumor model). The tissues were harvested 7 days later and subjected to immunohistochemistry. Three dimensional (3-D) reconstruction of the confocal microscopic images demonstrated that pod+ cells were clearly incorporated into lymphatic vessels and colocalized with cells expressing LEC markers in tissues harvested from all four models (Figure 3A through C and online-only Data Supplemental Video 1). The percentage of the LYVE-1+ lymphatic vessels containing Dil+ cells was around 5.2 ± 2.1% in the cornea, 5.5 ± 2.5% in wound models and 8.5 ± 3.7% in the tumor model, respectively. Of note, the Z-stacked, 3-D images of Figure 3C (tumor model) unequivocally showed that pod+ cells were incorporated into lymphatic vessels, exhibited a LEC marker, and had single nuclei, suggesting lymphvasculogenesis from the injected pod+ cells. In addition, in vivo Video-rate Laser Scanning microscopy demonstrated incorporation and colocalization of the injected pod+ cells into LYVE-1+ lymphatic vessels at multiple sites in the ear wound (Figure 3D) and the cornea (data not shown) models. Immunohistochemistry further confirmed the incorporation of the injected cells into pod-expressing vessels, which displayed typical lymphatic vessel morphology (online-only Data Supplemental Figure S3). To complement these results with Dil-labeled pod+ cells, we performed another experiment in which pod+ cells culture-isolated from GFP mice were injected into a skin wound model (Figure 3E and online-only Data Supplemental Video 2). Confocal microscopic imaging revealed that GFP+ cells were incorporated into lymphatic vessels and expressed LYVE-1.
Figure 3. Lymphvasculogenesis from pod+ cells in animal models.
A through C, Mice which had received surgery for cornea micropocket, wound or implantation of tumor cells (B16-F1 Melanoma) were injected with Dil-labeled pod+ cells (red) and the tissues were harvested 7 days later for immunohistochemistry. A through C, Representative confocal images from cornea (A), wounded skin (B) and peritumoral subcutaneous tissues (C) demonstrated that DiI-labeled pod+ cells were incorporated into lymphatic vessels and exhibited a LEC marker, LYVE-1. D, In vivo live confocal microscopic image from an ear wound model showed that multiple pod+ cells (DiI) were clearly incorporated into lymphatic vessels and colocalized with LYVE-1. Arrows indicate cells positive for Dil and LYVE-1. Scale bar = 20 µm. E, Skin wound tissues injected with pod+ cells from GFP mice were stained for LYVE-1 and examined by confocal microscopy. Injected pod+ GFP cells (arrows) were incorporated into the lymphatic vessels and expressed LYVE-1. Representative images from at least two independent experiments for each animal model are shown (n = 3 per experiment). Scale bar = 10 µm. Blue fluorescence: DAPI
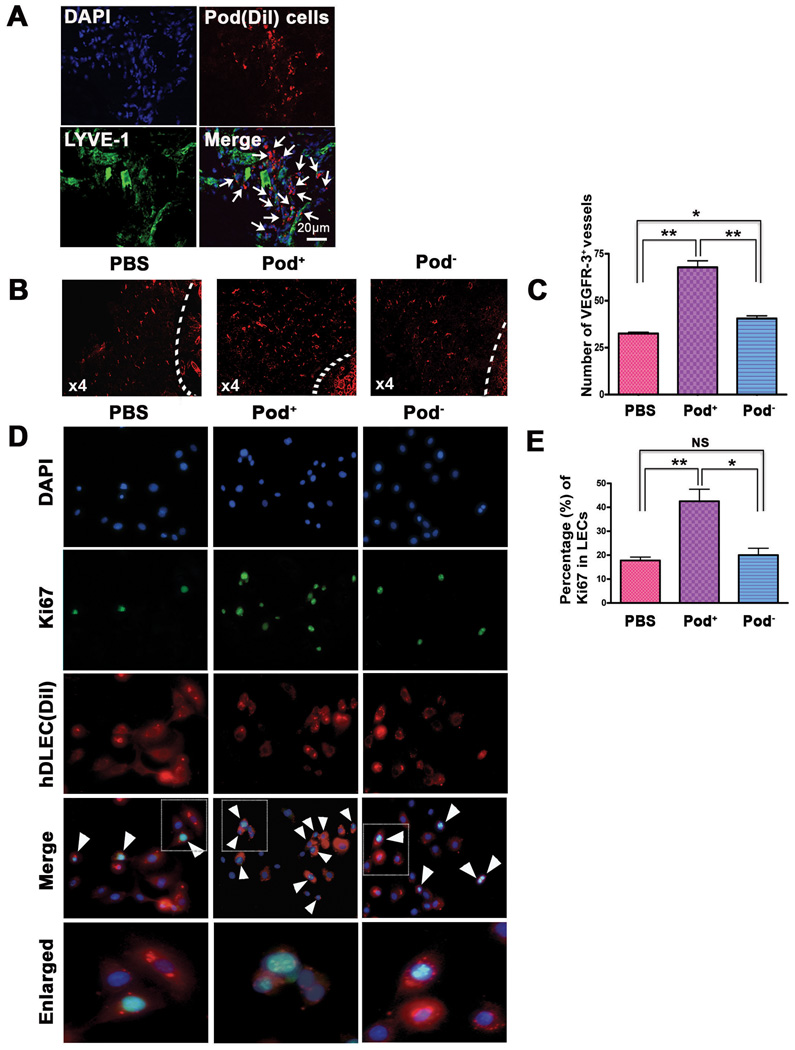
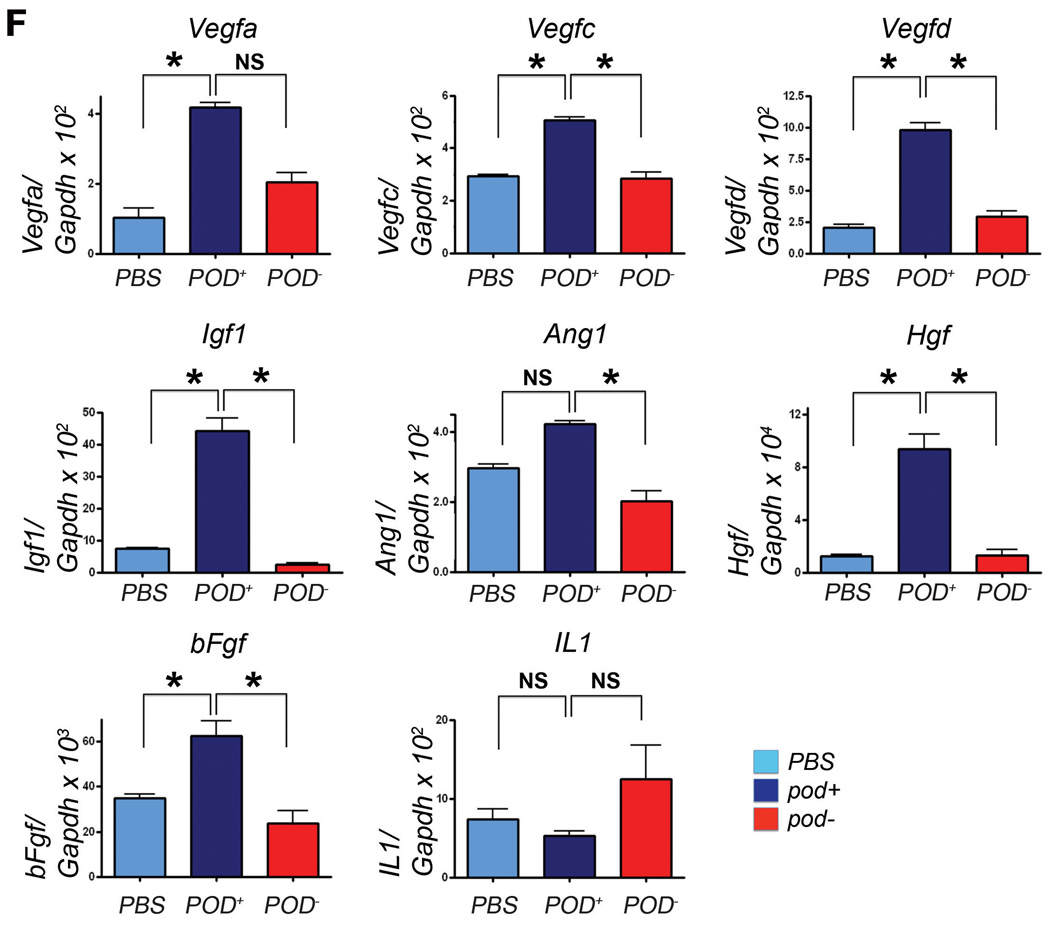
We also found that a larger portion of the injected cells were localized in close proximity to the lymphatic vasculature in the ear wound, tumor and cornea models (Figure 3D, 4A, online-only Data Supplemental Figure S4), suggesting their lymphangiogenic role. To determine the overall lymphatic neovascularization potential of pod+ cells, we measured lymphatic vascular density in peritumoral tissues and found that pod+ cell-injected tissues showed significantly higher lymphatic vascular density than PBS- or pod− cell-injected tissues (Figure 4B and 4C). To further explore their lymphangiogenic role, we cocultured pod+ cells with DiI-labeled human dermal LECs and determined the proliferation of LECs by Ki67 staining. The number of Ki67-positive LECs were more than 2 fold higher in the pod+ cell-cocultured LECs than PBS-added or pod− cell-cocultured LECs (Figure 4D and 4E). In agreement with these data, lymphangiogenic cytokines in tissues measured at day 7 after injection of 2 × 106 pod+ cells in the skin wound model were significantly increased in the pod+ cell-injected mice, compared to PBS- or pod−-cell injected tissues (Figure 4F). From these in vitro and in vivo studies, we concluded that culture-isolated pod+ cells have dual lymphvasculogenic and lymphangiogenic roles and augment lymphatic neovascularization.
Figure 4. Lymphangiogenic characteristics of pod+ cells.
A, Pod+ cells sorted from 4-day cultured BM-MNCs labeled with Dil were injected into the periphery of tumors in mice that had been injected with melanoma cells 7 days before. Seven days after cell implantation, tumor and peritumoral tissues including skin were harvested and underwent immunostaining for LYVE-1 (green). The arrows indicate engraftment of pod+ cells in close proximity to lymphatic vessels. Representative images from more than two independent experiments are shown (n = 3 per experiment). DAPI (blue). Scale bar = 20 µm. B, The peritumoral tissues which were injected with pod+ cells, pod− cells or the same volume of PBS similarly to the procedure described above were harvested 7 days later and subjected to immunohistochemistry using a VEGFR-3 antibody. Representative images from at least two independent experiments are shown (n = 2 per group). Magnification, × 4. C, The number of VEGFR-3+ vessels in peritumoral tissues was higher in mice implanted with pod+ cells than pod− cells or PBS. The indicated values were calculated from two independent experiments (B) using more than 10 randomly selected fields (** P<0.01, * P<0.05). D, The pod+- and pod− cells from 4 day-cultured BM-MNCs or PBS were mixed with Dil-labeled hDLECs (red), cultured for 24 hrs and stained for Ki67 (green). DAPI (blue). The cells positive for Dil, Ki67 and DAPI (white arrowheads) were counted. Representative images from three independent experiments are shown. Scale bar = 20 µm. E, hDLECs cocultured with pod+ cells exhibited a higher rate of Ki67+ cells compared to controls, suggesting a lymphangiogenic role of pod+ cells on surrounding LECs. The indicated values are the averages calculated from 14 randomly selected fields of each group of three independent experiments. (** P<0.01, * P<0.05). F, Tissues from mice injected with pod+ or pod− cells were subjected to qRT-PCR analyses. Graphs from three independent experiments are shown (* P<0.05, n=3 per group).
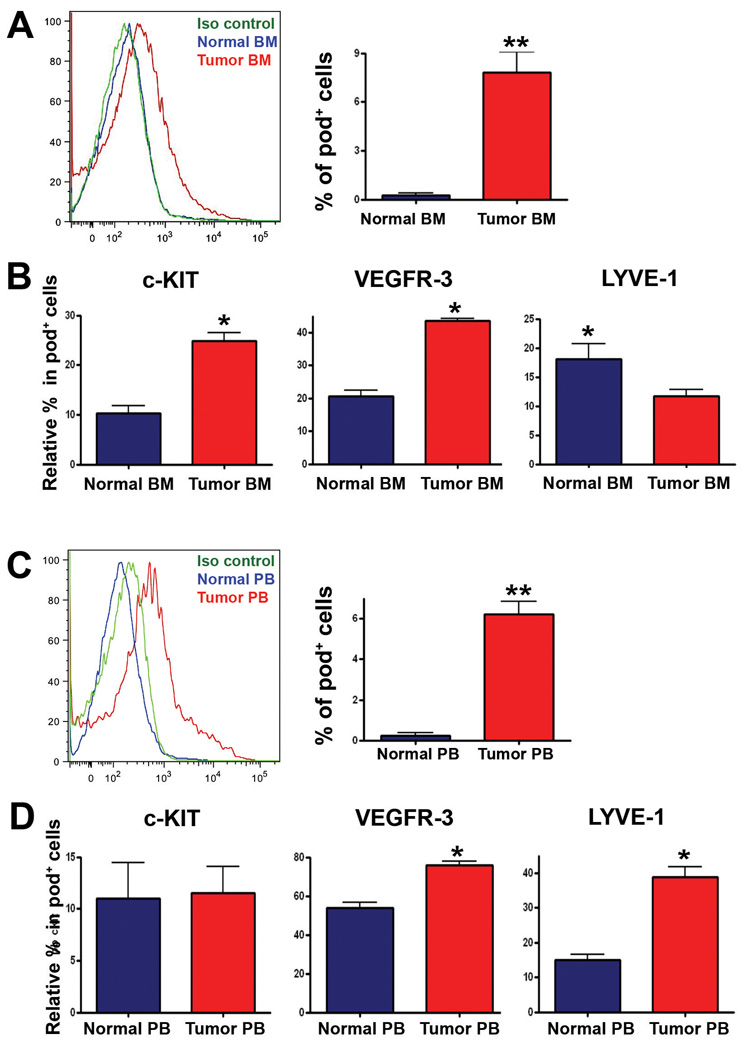
Pod+ cells are increased in BM and PB under lymphangiogenic conditions, and pod+ cells contribute to lymphatic neovascularization
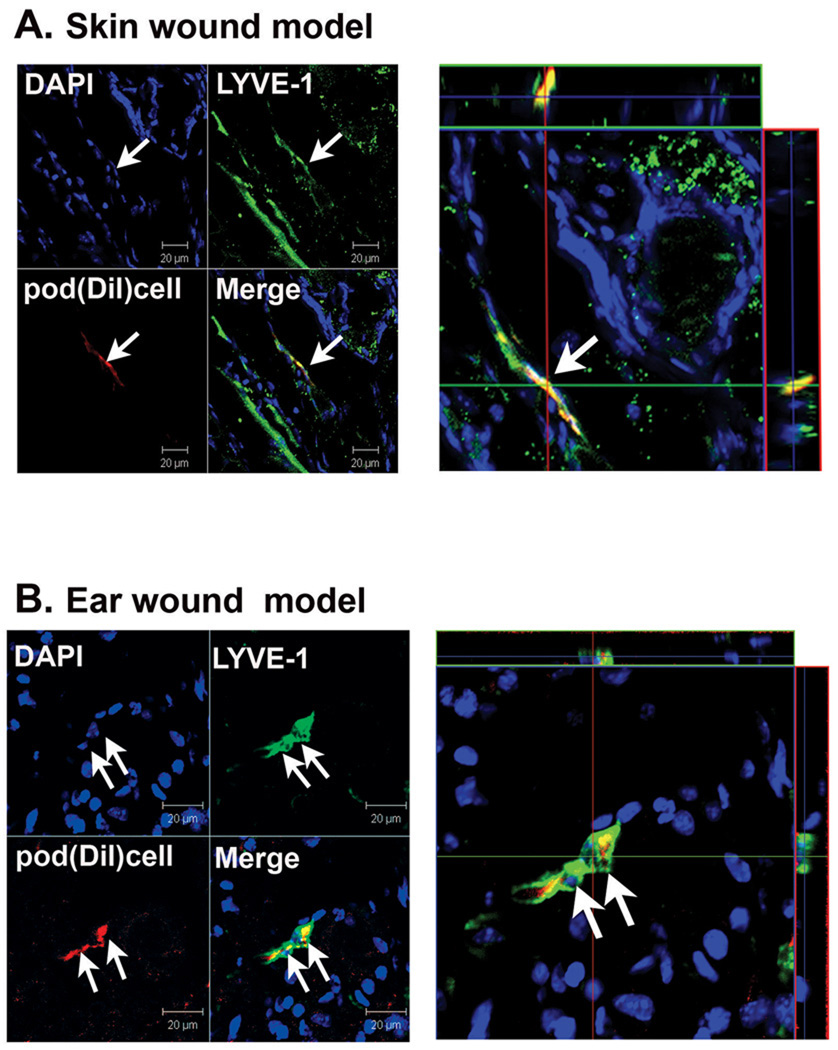
Next, we investigated whether pod+ cells directly isolated from animals could function similarly to culture-driven pod+ cells. As only a small number of BM-MNCs are pod+ in the steady-state normal animal (Figure 1J), we hypothesized that if these cells function as LEPCs, the number would be increased in the BM and/or PB under conditions promoting lymphatic vascular growth. Hence, we injected B16-F1 melanoma cells27 subcutaneously into the backs of mice, and the number of pod+ cells in BM and PB was examined 7 days later by FACS. FACS analysis showed that pod+ cells in BM and PB were less than 0.5% in healthy C57/BL6 mice but increased about 15-fold both in BM and PB upon the growth of melanoma (BM: 0.2 ± 0.5% vs. 3.2 ± 1.0%, PB: 0.4 ± 0.3% vs. 6.7 ± 2.0%, P < 0.01) (Figure 5A and 5C). Similar results were found in nude mice implanted with MDA-MB-231 human breast cancer cells (data not shown). Next, we examined the expression of VEGFR-3, LYVE-1 and c-KIT in pod+ cells isolated from BM and PB of normal and tumor-bearing mice to determine the progenitor and lymphatic character of the pod+ cells. FACS analysis of BM cells showed that among the pod+ population, the c-KIT+ or VEGFR-3+, but not LYVE-1+ cells, were more highly enriched in the tumor (melanoma)-bearing mice than in the normal mice (Figure 5B). The frequency of c-KIT+ cells in the pod+ population of tumor bearing mice was 24.8 ± 3.3%, whereas approximately 16.3 ± 2.0% of c-KIT+ cells were positive for podoplanin (online-only Data Supplemental Figure S5). In PB cells, among the pod+ population, VEGFR-3+ or LYVE-1+, but not c-KIT+ cells were more enriched in the tumor-bearing mice than in the normal mice. These findings suggest that during the process of lymphatic vascular growth, pod+ cells not only increase in number but also undergo qualitative changes into more lymphatic and progenitor-like cells, as evidenced by an increase in VEGFR-3, LYVE-1, and c-KIT, respectively. We also observed differences between BM and PB in the composition of these markers. In BM, c-KIT expression was higher in tumor mice than normal mice; however in PB, there was no difference in c-KIT expression between the two (left panels in Figure 5B and 5D). On the other hand, there was no difference in LYVE-1 expression in BM between tumor mice and normal mice, but in PB, the expression was higher in tumor mice than normal mice (right panels in Figure 5B and 5D). In summary, among pod+ cells, c-KIT+ cells were more enriched in BM and LYVE-1+ cells were more enriched in PB in tumor mice compared to normal mice. Since stem/progenitor cells are more predominant in BM and undergo differentiation during mobilization into PB, these results imply that among pod+ cells, progenitor forms are more prevalent in BM, and during and after mobilization into PB, the composition of pod+ cells shifts to more lymphatic and less progenitor-like phenotypes. The increase in absolute percentage of VEGFR-3+ cells among the pod+ population in PB in comparison to BM further supports this interpretation (middle panels in Figure 5B and 5D). Finally, to determine the lymphvasculogenic potential of freshly isolated BM pod+ cells in vivo, we injected the pod+ cells (Dil-labeled) derived from BM of tumor-bearing mice into skin and ear wound models. Immunohistochemistry showed that the injected cells were incorporated into lymphatic vessels and exhibited a LEC marker (Figure 6A and 6B). Together, these results demonstrated that pod+ cells are present in BM and PB, are increased in number under lymphatic vascular growth conditions, and contribute to lymphvasculogenesis, suggesting a pathophysiologic role of pod+ cells as LEPCs in vivo.
Figure 5. Augmentation of pod+ cells in BM and PB upon tumor induction.
Cells prepared from BM (A, B) or PB (C, D) of normal or tumor (B16-F1 melanoma cells)-bearing mice were subjected to FACS analysis. Representative images from three independent experiments are shown (** P<0.01, * P<0.05, n=3 per group).
Figure 6. Incorporation of freshly isolated pod+ cells into lymphatic vessels.
Pod+ cells isolated from BM of tumor-bearing mice were labeled with Dil and injected into mice in the skin wound (A) or the ear wound model (B). Confocal imaging showed colocalization of the injected pod+ cells (red) with LYVE-1+ (green) lymphatic vessels in mice. Representative images from at least two independent experiments for each animal model are shown (n = 3 per experiment). Scale bar = 20 µm.
Podoplanin is a determining marker to confer lymphatic and progenitor cell properties
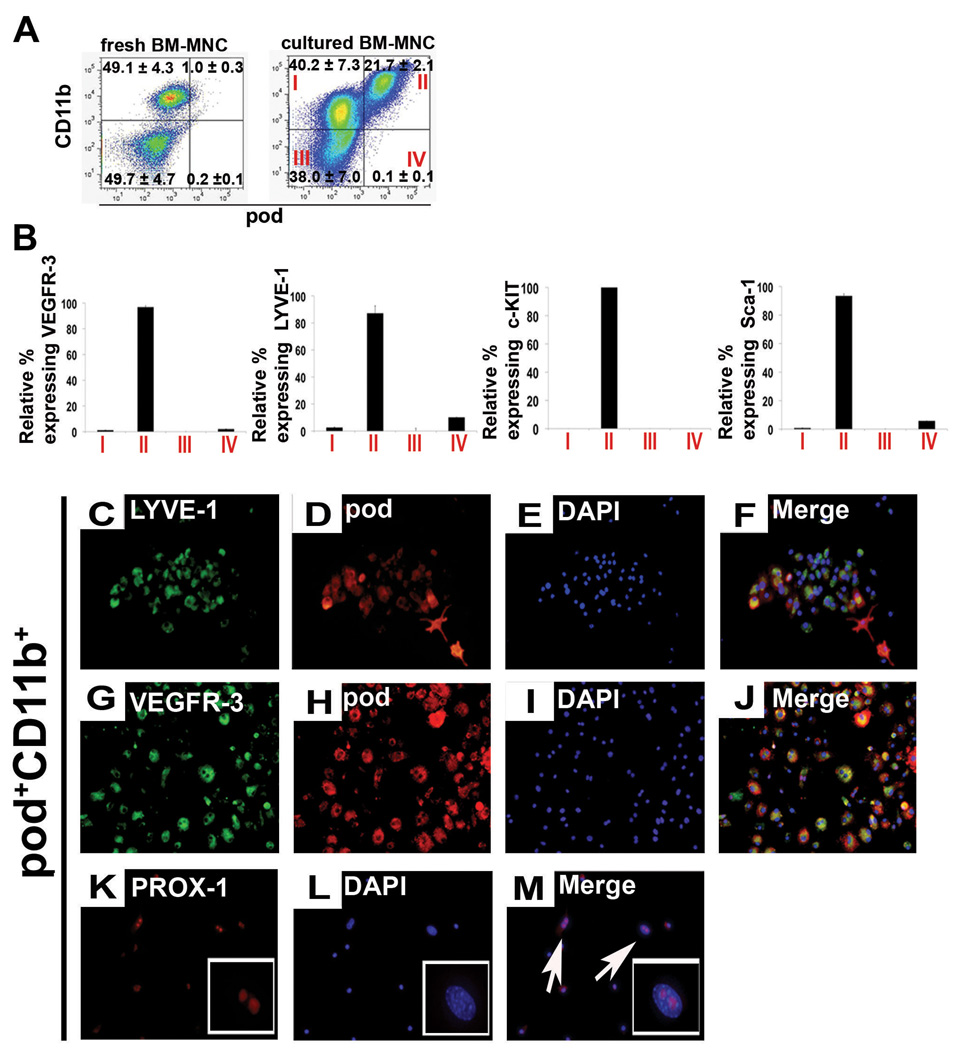
Since previous studies documented that tissue resident CD11b+ macrophages express LEC markers and could contribute to lymphatic vessel formation15, we further explored the relationship between pod and CD11b in BM-MNCs using FACS analysis. Approximately 50% of the freshly isolated BM-MNCs expressed CD11b, but less than 1% expressed pod (Figure 7A, left panel). In freshly isolated BM-MNCs, 85% of the pod+ cells expressed CD11b; however, only 2% of the CD11b+ cells expressed pod, showing that the majority of pod+ cells express CD11b but most CD11b+ cells are negative for pod. When cultured in the ACE condition for 4 days, 22% of total cells expressed pod and 62% expressed CD11b, (Figure 7A, right panel) indicating an increase in both pod+ cells and CD11b+ cells, but greater enrichment of pod+, cells during lymphatic activation. More than 99% of these pod+ cells expressed CD11b but only 33% of CD11b+ cells expressed pod, indicating that when activated by lymphangiogenic cytokines, pod+ cells can virtually represent pod+CD11b+ cells; however, two-thirds of CD11b+ cells are negative for pod (Figure 7A, right panel). This argument is supported by the in vivo finding that the frequency of pod+CD11b+ cells was increased in BM of tumor bearing mice undergoing active lymphatic neovascularization (online-only Data Supplemental Figure S6). Together, these results suggest that during lymphatic activation, the frequency of BM cells expressing either pod or CD11b is increased, but especially so for cells expressing both pod and CD11b.
Figure 7. The relationship between podoplanin, CD11b, stem cell markers and LEC markers.
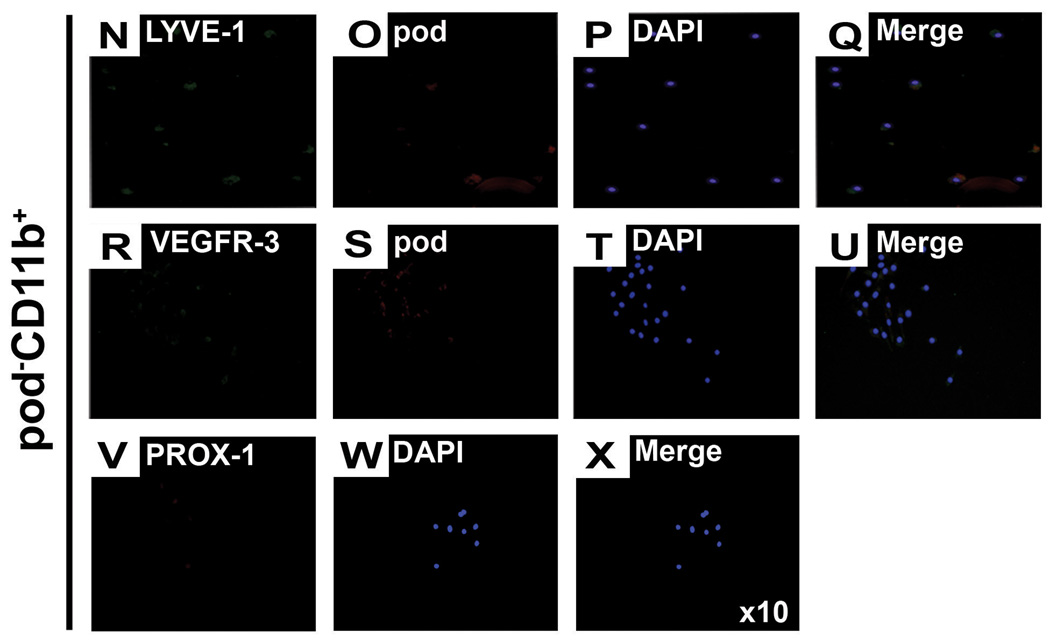
A, The uncultured or 4-day cultured BM-MNCs were subjected to FACS analysis for pod and CD11b. The numbers in each box represent percentage of positive cells. B, The Y axis represents the relative percentage cells expressing VEGFR-3, LYVE-1, c-KIT or Sca-1 in the indicated cell fraction of (A). The indicated values are the averages of three independent experiments (n=4 per experiment). C through X, The culture-isolated pod+CD11b+ or pod−CD11b+ cells from BM-MNCs were cultured for another 7 days under complete EGM media and stained for lymphatic markers. Representative images from at least three independent experiments are shown (n=4 per experiment). Magnification, × 10.
Next, to further define the phenotype of podoplanin- or CD11b-expressing cells, we investigated expression of other LEC markers (VEGFR-3, LYVE1) and stem/progenitor markers (c-KIT and Sca-1) in each cell fraction (Figure 7B). FACS analysis showed that VEGFR-3+ cells and LYVE-1+ cells were present almost exclusively in the pod+ population, of which the majority was pod+CD11b+ (fraction II in Figure 7A). These results suggest that the pod+ cell population includes almost all the cells having LEC phenotypes. Approximately 99% of c-KIT+ cells and 90% of Sca-1+ cells were also restricted to the pod+CD11b+ cell fraction (Figure 7B), i.e., pod+ cell fraction, and only a negligible number of those cells were present in the pod−CD11b+ cell fraction (Figure 7A, fraction II vs. I). Morphology of the two cell populations was distinctively different. The pod+CD11b+ cells were attached and grew as a monolayer, whereas the pod−CD11b+ cells were floating and maintained a round cell morphology (online-only Data Supplemental Figure S7). Importantly, when the isolated pod+CD11b+ or pod−CD11b+ cells were further cultured for 7 days in complete EGM, the pod+CD11b+ cells robustly expressed pod, LYVE-1, VEGFR-3 and PROX-1, while the pod−CD11b+ cells minimally expressed pod and did not express LYVE-1, VEGFR-3 or PROX-1 (Figure 7C through 7X). These data indicate that almost all the stem/progenitor cells were exclusively restricted to the pod+CD11b+, i.e., pod+ cell population. As a whole, these findings suggest that pod, more than CD11b, is a determining marker to confer lymphatic as well as progenitor cell properties upon BM cells, robustly supporting the role of pod+ cells as LEPCs.
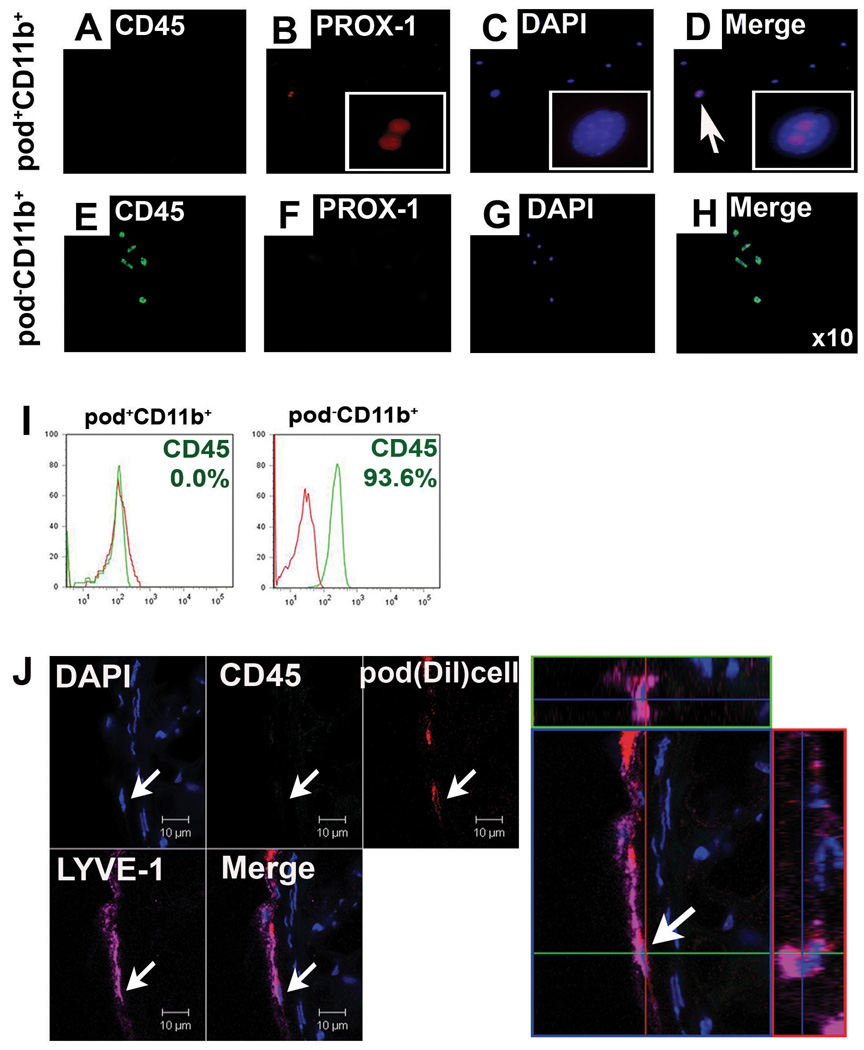
Loss of hematopoietic properties of pod+CD11b+ cells during differentiation into LECs
We next investigated whether the hematopoietic character of the putative LEPCs or pod+CD11b+ cells is lost during differentiation into LECs. Pod+CD11b+ or pod−CD11b+ cells isolated from cultured BM-MNCs were further cultured for 7 days in complete EGM. Immunocytochemistry showed that 7-day cultured pod+CD11b+ cells do not express CD45 while expressing LEC markers including PROX-1 (Figure 7 and Figure 8A–D). In contrast, 7-day cultured pod−CD11b+ cells still maintained CD45 expression with concomitant loss of LEC markers (Figure 7 and Figure 8E–H). FACS analysis additionally confirmed loss of CD45 in the further cultured pod+CD11b− cells. Approximately 94% of the pod−CD11b+ cells were CD45 positive (Figure 8I). To further verify these findings in vivo, we injected DiI-labeled pod+CD11b+ cells sorted from the cultured BM-MNCs into skin wounded mice. Figure 8J clearly shows that expression of CD45 was not detected in the pod+CD11b+ cells incorporated into lymphatic vessels (Figure 8J). These data indicate that pod+CD11b+ cells lost hematopoietic properties during differentiation into LECs.
Figure 8. The pod+CD11b+ cells, but not pod−CD11b+ cells, lose expression of CD45.
The culture-isolated pod+CD11b+ or pod−CD11b+ cells from BM-MNCs were further cultured for 7 days under complete EGM medium and subjected to immuocytochemistry (A–H) and FACS analysis for CD45 (I). For in vivo injection experiments (J), DiI-labeled pod+CD11b+ cells from the cultured BM-MNCs were injected into skin wounded mice and subjected to immunohistochemistry (n = 3 per experiment). The injected DiI-labeled cells did not express CD45 in tissues.
DISCCUSION
This is the first study to demonstrate the presence of pod+ cells in the circulatory system and to provide evidence that these cells can function as LEPCs. Pod+ cells exist in BM and PB in very low amounts at steady state and increase in number under conditions which promote lymphatic vascular growth such as melanoma27. Pod+ cells are expandable by culture with lymphangiogenic growth factors. As lymphangiogenic tumors, such as melanoma or breast cancer28, or inflammation15, 29 induce an increase in the local concentration of lymphatic growth factors, the cell culture conditions closely mimic the local environment of these conditions. We also successfully generated LECs in vitro by further cultivation of these culture-isolated pod+ cells. Thus this in vitro and in vivo evidence strongly suggests a crucial role for pod+ cells in lymphatic vessel formation. High expression of other LEC genes and lymphangiogenic cytokines in pod+ cells further supports their role in lymphatic neovascularization. The almost exclusive restriction of c-KIT, Sca-1 and FLK-1 expression to pod+ cells suggests their stem/progenitor cell character.
More direct evidence of lymphatic neovascularization by pod+ cells was provided by the cell injection studies. Both freshly isolated and cultured pod+ cells were not only incorporated as LECs into lymphatic vasculature (lymphvasculogenesis) but also highly localized near the lymphatic vasculature, suggestive of a paracrine or lymphangiogenic role. Although there is controversy regarding the transdifferentiation potential of BM-derived hematopoietic cells30, 31, our data support transdifferentiation as a viable mechanism for lymphvasculogenesis from BM cells. In fact, the prevalent view that terminally differentiated cells do not change their phenotype has been challenged15–17, 32, 33. B lymphocytes can become macrophage-like cells upon overexpression of C/EBP transcription factor34. Fully differentiated somatic cells can be reprogrammed into pluripotent stem cells with ectopic expression of pluripotency-related transcription factors35. A battery of three transcription factors was able to convert differentiated exocrine cells into functional β-cells in adult mice36. Conditional inactivation of Prox1 in adult mice induced cell fate change from lymphatic endothelial cells into blood endothelial cells25, suggesting plasticity of differentiated cells. Our data also showed that BM-derived pod+ cells clearly express PROX-1 and could become LECs in pathological conditions. To determine the contribution of injected pod+ cells to LECs, we used comprehensive and multimodal approaches. Both freshly isolated- and culture-isolated pod+ cells were used as cell sources. For cell tracking in vivo, both DiI-labeled-and GFP-mice derived cells were used. As in vivo models, we used various animal models such as tumor, inflammation and wound models, which represent the most prevalent clinical conditions associated with lymphatic vascular growth. The fact that pod+ cells gave rise to LECs in all these models supports the identity of pod+ cells as LEPCs. To prove this transdifferentiation, we used both static and in vivo confocal microscopy technologies and adopted rigorous criteria as follows. As criteria for transdifferentiation, we required not only the incorporation of injected cells into lymphatic vessels but also exact colocalization of injected cells showing single nuclei with LEC marker staining at two different orthogonal images and at multiple z-stacked files. To further confirm this colocalization, three dimensional reconstructions were used. In addition, for identification of lymphatic vessels which harbor the injected cells, both positive staining for multiple lymphatic markers and clear morphology of the vessels were required. These multiple criteria and high standards insured that we were seeing true transdifferentiation of injected cells rather than artifacts. Common expression of LYVE-1 in LECs and a subpopulation of monocyte-macrophage lineage increases this likelihood of transdifferentiation as well15, 37, 38.
Our data indicate that pod+ cells also contribute to lymphatic neovascularization through paracrine lymphangiogenic effects on existing lymphatic vessels. Similar evidence of the role for BM-derived cells in postnatal lymphangiogenesis in pathologic conditions has been reported. He et al showed that BM-derived GFP+ cells were heavily recruited to the periphery of new lymphatic vessels following tumor implantation, although few were incorporated into lymphatic vessels9. In other reports, depletion of macrophages inhibited inflammation-induced new lymphatic vessel formation29, 39. Macrophages isolated from mice undergoing extensive lymphangiogenesis displayed a marked increase in lymphangiogenic cytokines39, 40. These studies support a specific population of BM-derived cells playing an important role in lymphangiogenesis.
In this study, we demonstrated that BM cells which express pod as well as CD11b can function as LEPCs. A recent report showed that CD11b+ macrophages may play an important role in inflammation-induced lymphvasculogenesis in the cornea15. However, this study demonstrated only that tissue resident CD11b+ macrophages, which are presumably derived from BM, show a LEC phenotype. In contrast, our study clearly demonstrated that BM hematopoietic cells are the source of lymphvasculogenic CD11b+ cells and further identified, using in vitro culture methods and in vivo physiologic and pathologic conditions, a specific cell population among the heterogeneous CD11b+, i.e. pod+CD11b+ cells, which can function as LEPCs.
Our study identifies pod+ cells as LEPCs and proposes their crucial role in lymphvasculogenesis and lymphangiogenesis. The identification of this phenotype has important clinical significance. Since a culture isolation protocol has been established, these cells can be utilized for treating diseases which can benefit from a lymphatic vascular supply, such as lymphedema or wound healing. Furthermore, the quantification of these cells in BM may be used to measure or predict tumor burden, progression or metastasis, allowing their use as a biomarker.
Clinical Summary.
In this study, we identified podoplanin-expressing lymphatic endothelial progenitor cells (LEPC) derived from bone marrow. Injection of these podoplanin-expressing LEPCs into various animal models showed contribution of the LEPCs to the formation of new lymphatic vessels through direct transdifferentiation as well as paracrine effects. This lymphatic vessel forming capability can be utilized for the treatment of lymphedema or chronic unhealed wounds, which are characterized by lymphatic vascular insufficiency. Moreover, this study demonstrated an increase in the number of circulating LEPCs in tumor-bearing mice, suggesting that LEPCs are correlated with tumor-associated lymphatic vascular growth. This property can be harnessed for development of a biomarker for monitoring tumor burden, tumor growth and/or metastasis.
Supplementary Material
Acknowledgements
We would like to thank Debby Martinson for confocal imaging; Mackenzie Houge and Min-Young Lee for technical assistance; Andrea Wecker for critical reading of the manuscript; Dr. Mejeong Lee for support for the initial steps of this project.
Funding
This work was supported in part by an Idea Grant Award from Department of Defense (W81XWH-09-1-0278), NIH grants (RO1HL084471, R21HL097353), PHS Grant (UL1 RR025008) from the Clinical and Translational Science Award program, NIH, NCRR, and a grant (SC4300) from the Stem Cell Research Center of the 21st Century Frontier Research Program funded by the Ministry of Science and Technology, Republic of Korea.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Subject codes: 129, 97
Disclosure
None
REFERENCES
- 1.Achen MG, McColl BK, Stacker SA. Focus on lymphangiogenesis in tumor metastasis. Cancer Cell. 2005;7:121–127. doi: 10.1016/j.ccr.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 2.Alitalo K, Tammela T, Petrova TV. Lymphangiogenesis in development and human disease. Nature. 2005;438:946–953. doi: 10.1038/nature04480. [DOI] [PubMed] [Google Scholar]
- 3.Wigle JT, Oliver G. Prox1 function is required for the development of the murine lymphatic system. Cell. 1999;98:769–778. doi: 10.1016/s0092-8674(00)81511-1. [DOI] [PubMed] [Google Scholar]
- 4.Kaipainen A, Korhonen J, Mustonen T, van Hinsbergh VW, Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci U S A. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Banerji S, Ni J, Wang SX, Clasper S, Su J, Tammi R, Jones M, Jackson DG. LYVE-1, a new homologue of the CD44 glycoprotein, is a lymph-specific receptor for hyaluronan. J Cell Biol. 1999;144:789–801. doi: 10.1083/jcb.144.4.789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wetterwald A, Hoffstetter W, Cecchini MG, Lanske B, Wagner C, Fleisch H, Atkinson M. Characterization and cloning of the E11 antigen, a marker expressed by rat osteoblasts and osteocytes. Bone. 1996;18:125–132. doi: 10.1016/8756-3282(95)00457-2. [DOI] [PubMed] [Google Scholar]
- 7.Breiteneder-Geleff S, Soleiman A, Kowalski H, Horvat R, Amann G, Kriehuber E, Diem K, Weninger W, Tschachler E, Alitalo K, Kerjaschki D. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: podoplanin as a specific marker for lymphatic endothelium. Am J Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Schacht V, Ramirez MI, Hong YK, Hirakawa S, Feng D, Harvey N, Williams M, Dvorak AM, Dvorak HF, Oliver G, Detmar M. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He Y, Rajantie I, Ilmonen M, Makinen T, Karkkainen MJ, Haiko P, Salven P, Alitalo K. Preexisting lymphatic endothelium but not endothelial progenitor cells are essential for tumor lymphangiogenesis and lymphatic metastasis. Cancer Res. 2004;64:3737–3740. doi: 10.1158/0008-5472.CAN-04-0088. [DOI] [PubMed] [Google Scholar]
- 10.Karpanen T, Egeblad M, Karkkainen MJ, Kubo H, Yla-Herttuala S, Jaattela M, Alitalo K. Vascular endothelial growth factor C promotes tumor lymphangiogenesis and intralymphatic tumor growth. Cancer Res. 2001;61:1786–1790. [PubMed] [Google Scholar]
- 11.Karkkainen MJ, Haiko P, Sainio K, Partanen J, Taipale J, Petrova TV, Jeltsch M, Jackson DG, Talikka M, Rauvala H, Betsholtz C, Alitalo K. Vascular endothelial growth factor C is required for sprouting of the first lymphatic vessels from embryonic veins. Nat Immunol. 2004;5:74–80. doi: 10.1038/ni1013. [DOI] [PubMed] [Google Scholar]
- 12.Nagy JA, Vasile E, Feng D, Sundberg C, Brown LF, Detmar MJ, Lawitts JA, Benjamin L, Tan X, Manseau EJ, Dvorak AM, Dvorak HF. Vascular permeability factor/vascular endothelial growth factor induces lymphangiogenesis as well as angiogenesis. J Exp Med. 2002;196:1497–1506. doi: 10.1084/jem.20021244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cao R, Bjorndahl MA, Gallego MI, Chen S, Religa P, Hansen AJ, Cao Y. Hepatocyte growth factor is a lymphangiogenic factor with an indirect mechanism of action. Blood. 2006;107:3531–3536. doi: 10.1182/blood-2005-06-2538. [DOI] [PubMed] [Google Scholar]
- 14.Salven P, Mustjoki S, Alitalo R, Alitalo K, Rafii S. VEGFR-3 and CD133 identify a population of CD34+ lymphatic/vascular endothelial precursor cells. Blood. 2003;101:168–172. doi: 10.1182/blood-2002-03-0755. [DOI] [PubMed] [Google Scholar]
- 15.Maruyama K, Ii M, Cursiefen C, Jackson DG, Keino H, Tomita M, Van Rooijen N, Takenaka H, D'Amore PA, Stein-Streilein J, Losordo DW, Streilein JW. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J Clin Invest. 2005;115:2363–2372. doi: 10.1172/JCI23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Religa P, Cao R, Bjorndahl M, Zhou Z, Zhu Z, Cao Y. Presence of bone marrow-derived circulating progenitor endothelial cells in the newly formed lymphatic vessels. Blood. 2005;106:4184–4190. doi: 10.1182/blood-2005-01-0226. [DOI] [PubMed] [Google Scholar]
- 17.Kerjaschki D, Huttary N, Raab I, Regele H, Bojarski-Nagy K, Bartel G, Krober SM, Greinix H, Rosenmaier A, Karlhofer F, Wick N, Mazal PR. Lymphatic endothelial progenitor cells contribute to de novo lymphangiogenesis in human renal transplants. Nat Med. 2006;12:230–234. doi: 10.1038/nm1340. [DOI] [PubMed] [Google Scholar]
- 18.Kenyon BM, Voest EE, Chen CC, Flynn E, Folkman J, D'Amato RJ. A model of angiogenesis in the mouse cornea. Invest Ophthalmol Vis Sci. 1996;37:1625–1632. [PubMed] [Google Scholar]
- 19.Yoon YS, Murayama T, Gravereaux E, Tkebuchava T, Silver M, Curry C, Wecker A, Kirchmair R, Hu CS, Kearney M, Ashare A, Jackson DG, Kubo H, Isner JM, Losordo DW. VEGF-C gene therapy augments postnatal lymphangiogenesis and ameliorates secondary lymphedema. J Clin Invest. 2003;111:717–725. doi: 10.1172/JCI15830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong YK, Lange-Asschenfeldt B, Velasco P, Hirakawa S, Kunstfeld R, Brown LF, Bohlen P, Senger DR, Detmar M. VEGF-A promotes tissue repair-associated lymphatic vessel formation via VEGFR-2 and the alpha1beta1 and alpha2beta1 integrins. FASEB J. 2004;18:1111–1113. doi: 10.1096/fj.03-1179fje. [DOI] [PubMed] [Google Scholar]
- 21.Deasy BM, Qu-Peterson Z, Greenberger JS, Huard J. Mechanisms of muscle stem cell expansion with cytokines. Stem Cells. 2002;20:50–60. doi: 10.1634/stemcells.20-1-50. [DOI] [PubMed] [Google Scholar]
- 22.Ledgerwood LG, Lal G, Zhang N, Garin A, Esses SJ, Ginhoux F, Merad M, Peche H, Lira SA, Ding Y, Yang Y, He X, Schuchman EH, Allende ML, Ochando JC, Bromberg JS. The sphingosine 1-phosphate receptor 1 causes tissue retention by inhibiting the entry of peripheral tissue T lymphocytes into afferent lymphatics. Nat Immunol. 2008;9:42–53. doi: 10.1038/ni1534. [DOI] [PubMed] [Google Scholar]
- 23.Skobe M, Hawighorst T, Jackson DG, Prevo R, Janes L, Velasco P, Riccardi L, Alitalo K, Claffey K, Detmar M. Induction of tumor lymphangiogenesis by VEGF-C promotes breast cancer metastasis. Nat Med. 2001;7:192–198. doi: 10.1038/84643. [DOI] [PubMed] [Google Scholar]
- 24.Stacker SA, Caesar C, Baldwin ME, Thornton GE, Williams RA, Prevo R, Jackson DG, Nishikawa S, Kubo H, Achen MG. VEGF-D promotes the metastatic spread of tumor cells via the lymphatics. Nat Med. 2001;7:186–191. doi: 10.1038/84635. [DOI] [PubMed] [Google Scholar]
- 25.Johnson NC, Dillard ME, Baluk P, McDonald DM, Harvey NL, Frase SL, Oliver G. Lymphatic endothelial cell identity is reversible and its maintenance requires Prox1 activity. Genes Dev. 2008;22:3282–3291. doi: 10.1101/gad.1727208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maruyama K, Asai J, Ii M, Thorne T, Losordo DW, D'Amore PA. Decreased macrophage number and activation lead to reduced lymphatic vessel formation and contribute to impaired diabetic wound healing. Am J Pathol. 2007;170:1178–1191. doi: 10.2353/ajpath.2007.060018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Padera TP, Kadambi A, di Tomaso E, Carreira CM, Brown EB, Boucher Y, Choi NC, Mathisen D, Wain J, Mark EJ, Munn LL, Jain RK. Lymphatic metastasis in the absence of functional intratumor lymphatics. Science. 2002;296:1883–1886. doi: 10.1126/science.1071420. [DOI] [PubMed] [Google Scholar]
- 28.Olmeda D, Moreno-Bueno G, Flores JM, Fabra A, Portillo F, Cano A. SNAI1 is required for tumor growth and lymph node metastasis of human breast carcinoma MDA-MB-231 cells. Cancer Res. 2007;67:11721–11731. doi: 10.1158/0008-5472.CAN-07-2318. [DOI] [PubMed] [Google Scholar]
- 29.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, D'Amore PA, Dana MR, Wiegand SJ, Streilein JW. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–1050. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wagers AJ, Sherwood RI, Christensen JL, Weissman IL. Little evidence for developmental plasticity of adult hematopoietic stem cells. Science. 2002;297:2256–2259. doi: 10.1126/science.1074807. [DOI] [PubMed] [Google Scholar]
- 31.Murry CE, Soonpaa MH, Reinecke H, Nakajima H, Nakajima HO, Rubart M, Pasumarthi KB, Virag JI, Bartelmez SH, Poppa V, Bradford G, Dowell JD, Williams DA, Field LJ. Haematopoietic stem cells do not transdifferentiate into cardiac myocytes in myocardial infarcts. Nature. 2004;428:664–668. doi: 10.1038/nature02446. [DOI] [PubMed] [Google Scholar]
- 32.Gao D, Nolan DJ, Mellick AS, Bambino K, McDonnell K, Mittal V. Endothelial progenitor cells control the angiogenic switch in mouse lung metastasis. Science. 2008;319:195–198. doi: 10.1126/science.1150224. [DOI] [PubMed] [Google Scholar]
- 33.Purhonen S, Palm J, Rossi D, Kaskenpaa N, Rajantie I, Yla-Herttuala S, Alitalo K, Weissman IL, Salven P. Bone marrow-derived circulating endothelial precursors do not contribute to vascular endothelium and are not needed for tumor growth. Proc Natl Acad Sci U S A. 2008;105:6620–6625. doi: 10.1073/pnas.0710516105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xie H, Ye M, Feng R, Graf T. Stepwise reprogramming of B cells into macrophages. Cell. 2004;117:663–676. doi: 10.1016/s0092-8674(04)00419-2. [DOI] [PubMed] [Google Scholar]
- 35.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 36.Zhou Q, Brown J, Kanarek A, Rajagopal J, Melton DA. In vivo reprogramming of adult pancreatic exocrine cells to beta-cells. Nature. 2008;455:627–632. doi: 10.1038/nature07314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schledzewski K, Falkowski M, Moldenhauer G, Metharom P, Kzhyshkowska J, Ganss R, Demory A, Falkowska-Hansen B, Kurzen H, Ugurel S, Geginat G, Arnold B, Goerdt S. Lymphatic endothelium-specific hyaluronan receptor LYVE-1 is expressed by stabilin-1+, F4/80+, CD11b+ macrophages in malignant tumours and wound healing tissue in vivo and in bone marrow cultures in vitro: implications for the assessment of lymphangiogenesis. J Pathol. 2006;209:67–77. doi: 10.1002/path.1942. [DOI] [PubMed] [Google Scholar]
- 38.Cho HJ, Lee N, Lee JY, Choi YJ, Ii M, Wecker A, Jeong JO, Curry C, Qin G, Yoon YS. Role of host tissues for sustained humoral effects after endothelial progenitor cell transplantation into the ischemic heart. J Exp Med. 2007;204:3257–3269. doi: 10.1084/jem.20070166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kataru RP, Jung K, Jang C, Yang H, Schwendener RA, Baik JE, Han SH, Alitalo K, Koh GY. Critical role of CD11b+ macrophages and VEGF in inflammatory lymphangiogenesis, antigen clearance, and inflammation resolution. Blood. 2009;113:5650–5659. doi: 10.1182/blood-2008-09-176776. [DOI] [PubMed] [Google Scholar]
- 40.Jeon BH, Jang C, Han J, Kataru RP, Piao L, Jung K, Cha HJ, Schwendener RA, Jang KY, Kim KS, Alitalo K, Koh GY. Profound but dysfunctional lymphangiogenesis via vascular endothelial growth factor ligands from CD11b+ macrophages in advanced ovarian cancer. Cancer Res. 2008;68:1100–1109. doi: 10.1158/0008-5472.CAN-07-2572. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.