Abstract
BACKGROUND AND PURPOSE
Levetiracetam, a novel antiepileptic drug, has recently been shown to have antinociceptive effects in various animal models of pain. The purpose of this study was to investigate the antihyperalgesic effect of levetiracetam and its mechanism of action, by examining the involvement of GABAergic, opioidergic, 5-hydroxytryptaminergic (5-HTergic) and adrenergic systems in its effect, in a rat model of inflammatory pain.
EXPERIMENTAL APPROACH
Rats were intraplantarly injected with the pro-inflammatory compound carrageenan. A paw pressure test was used to determine: (i) the effect of levetiracetam on carrageenan-induced hyperalgesia; and (ii) the effects of bicuculline (selective GABAA receptor antagonist), naloxone (non-selective opioid receptor antagonist), methysergide (non-selective 5-HT receptor antagonist) and yohimbine (selective α2-adrenoceptor antagonist) on the antihyperalgesic action of levetiracetam.
RESULTS
Levetiracetam (10–200 mg·kg−1; p.o.) significantly reduced, in a dose-dependent manner, the inflammatory hyperalgesia induced by carrageenan. The antihyperalgesic effect of levetiracetam was significantly decreased after administration of bicuculline (0.5–2 mg·kg−1; i.p.), naloxone (1–3 mg·kg−1; i.p.), methysergide (0.25–1 mg·kg−1; i.p.) and yohimbine (1–3 mg·kg−1; i.p.).
CONCLUSIONS AND IMPLICATIONS
These results show that levetiracetam produced antihyperalgesia which is at least in part mediated by GABAA, opioid, 5-HT and α2-adrenergic receptors, in an inflammatory model of pain. The efficacy of levetiracetam in this animal model of inflammatory pain suggests that it could be a potentially important agent for treating inflammatory pain conditions in humans.
Keywords: levetiracetam, inflammatory hyperalgesia, GABAA receptors, opioid receptors, 5-HT receptors, α2-adrenoceptors
Introduction
Antiepileptic drugs are widely used to treat multiple non-epileptic disorders such as neuropathic and inflammatory pain, migraine, essential tremors and psychiatric disorders. These pathophysiological processes disturb neuronal excitability by modulating ion channels, receptors and intracellular signalling pathways, all of which serve as targets for pharmacological actions of different antiepileptic drugs (Guindon et al., 2007; Johannessen Landmark, 2008).
Levetiracetam ([S]-α-ethyl-2-oxo-1-pyrrolidine acetamide) is a novel antiepileptic drug with a broad spectrum of anticonvulsant activity and an unusually high safety margin (De Smedt et al., 2007). Its analgesic properties are also a point of interest. Increasing evidence for antinociceptive effects of levetiracetam has been obtained in different animal models of pain. Experiments performed in rats show that levetiracetam exerts antihyperalgesic effects in a painful diabetic neuropathy (Ardid et al., 2003), in anaesthetic-induced hyperalgesia (Archer et al., 2007) and against postoperative pain (Silva et al., 2008). There is also preliminary clinical evidence for the efficacy of levetiracetam in treating trigeminal neuralgia (Jorns et al., 2009) and chronic pain associated with multiple sclerosis (Rossi et al., 2009). Despite this information, the mechanism of the antinociceptive effects of levetiracetam has not been elucidated.
Numerous studies have demonstrated antinociceptive effects of antiepileptic drugs in animal models of inflammatory pain (Field et al., 1997; Tomićet al., 2004; Stöhr et al., 2006; Vučkovićet al., 2006; Stepanović-Petrovićet al., 2008). In the present study we examined whether levetiracetam, administered by the clinically preferred oral route, had an antihyperalgesic effect in the carrageenan-induced model of inflammatory pain in the rat. In addition, the potential involvement of adrenoceptors, GABA, opioid and 5-hydroxytryptaminergic (5-HTergic) receptors in this antihyperalgesic effect was investigated.
Methods
Animals
Experiments were performed on male Wistar rats (Military Academy Breeding Farm, Belgrade, Serbia) that weighed between 180 and 220 g. The animals were housed in groups of four per cage (42.5 × 27 × 19 cm) and maintained on a 12/12 h light/dark cycle (lights were switched on at 06 h 00 min) at 22 ± 1°C and 60% relative humidity. Food and water were available ad libitum except during the experimental procedure. After arrival, the animals were allowed to acclimatize for at least 3 days before testing commenced. All experiments were carried out at the same time of day between 8 h 00 min and 16 h 00 min to avoid diurnal variations in behavioural tests. All experiments adhered to the guidelines of the Committee for Research and Ethical Issues of IASP (Zimmermann, 1983). Efforts were made to minimize the number of animals used. The total number of animals used in this study was 161.
Paw pressure test
Antihyperalgesic activity was assessed by a modified ‘paw pressure’ test (Randall and Selitto, 1957; Tomićet al., 2004). The rat was placed with its hind paws on two force transducer platforms of the apparatus (Hugo Sachs Elektronik, March – Hugstetten, Germany) and pushed slowly and smoothly downwards. Pressure was applied until one of the paws received a force that exceeded the trigger level set at 100 g. At this point an audible click by the apparatus was heard and measurement was stopped automatically. The rat used its non-inflamed paw as the weight-bearing limb in an attempt to spare the inflamed paw. Thereby agents capable of reducing the difference (d) between the pressure applied to the non-inflamed vs. the inflamed paw were recognized as possessing antihyperalgesic activity. The forces applied to the paws were read on the display and the difference (d) was calculated from the following equation:
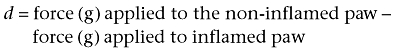 |
The mean from four consecutive measurements at each time point was used for further calculations. The pretreatment d-value was obtained before induction of inflammation by carrageenan as described previously (Morris, 2003). The post-treatment d-value was measured 30, 60, 90, 120, 180 and 240 min after drug administration. The experimenter was blind to the treatment of the animals.
The differences in force were expressed as the % of antihyperalgesic activity (%AA) and calculated according to the following formula (Tomićet al., 2004):
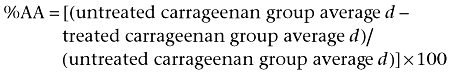 |
If the treated carrageenan group average d was greater than the untreated carrageenan group average d, a value of 0%AA was assigned.
The values for %AA were calculated after each measurement of d (30, 60, 90, 120, 180 and 240 min after levetiracetam administration) to establish the time of the peak effect. The ED50 (the dose that expected to result in 50%AA) was estimated from the corresponding log dose-response curves (Tallarida, 2000).
In this study, six series of experiments were performed (Figure 1). For each set of experiments, control animals received the same volume of carrageenan intraplantarly (i.pl.) in the right hind paw (Figure 1A). We first examined the antihyperalgesic effects of levetiracetam (Figure 1B), followed by the influence of bicuculline (Figure 1C line I), naloxone (Figure 1D line I), methysergide (Figure 1E line I) and yohimbine (Figure 1F line I) on the antihyperalgesic actions of levetiracetam. All antagonists were injected i.p. immediately before levetiracetam (p.o.), except for yohimbine, which was administered i.p. 15 min before levetiracetam. To exclude possible intrinsic effects of the antagonists the highest dose of each antagonist was tested separately (Figure 1C–F lines II). Matching control groups of animals received carrageenan (i.pl.), levetiracetam (p.o.) and the same volume of saline (i.p.) instead of the antagonists.
Figure 1.
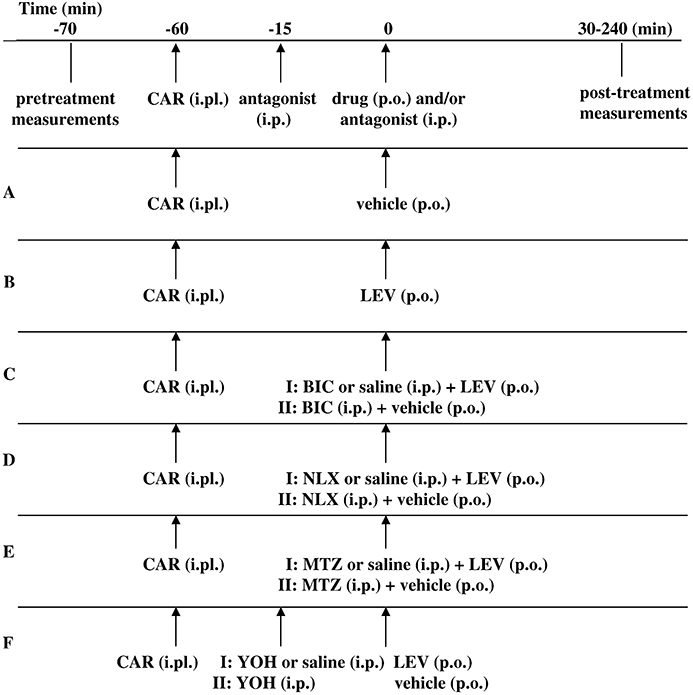
Experimental protocol used in evaluation of hyperalgesia induced by injection of carrageenan (CAR) into the right hind paw (A), the anti-hyperalgesic effect of levetiracetam (LEV) (B), the effects of bicuculline (BIC) (C), naloxone (NLX) (D), methysergide (MTZ) (E) and yohimbine (YOH) (F) on levetiracetam-induced anti hyperalgesia. i.pl., intraplantar.
% inhibition (%I) of the antihyperalgesic effect of levetiracetam by antagonists (bicuculline, naloxone, methysergide and yohimbine) was calculated according to the following formula (Tomićet al., 2004):
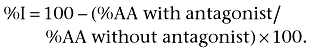 |
The ID50 (the dose that was expected to result in 50%I) was estimated from the corresponding log dose-response curves (Tallarida, 2000).
Drug administration
Levetiracetam (Kepra, UCB Pharma AG, 1630 Bulle, Belgium) was suspended in distilled water and sonicated for 15 min for proper drug distribution. A homogeneous suspension of levetiracetam was administered to rats by oral gavage (p.o.) in a volume of 2 mL·kg−1 body weight. Bicuculline hydrochloride (Sigma-Aldrich Chemie, Germany) was dissolved in saline by addition of one drop of 10N HCl. Naloxone hydrochloride (Sigma-Aldrich Chemie, Germany), methysergide maleate (Sigma-Aldrich Chemie, Germany) and yohimbine hydrochloride (Sigma-Aldrich Chemie, Germany) were dissolved in saline. All antagonists were injected i.p. in a volume of 2 mL·kg−1 body weight. Carrageenan λ (Sigma-Aldrich Chemie, Germany) was suspended in saline and injected i.pl. into the right hind paw of all animals, in a volume of 0.1 mL per paw, using a 1 mL syringe and 24 gauge (0.55 × 25 mm) needle.
Drug and molecular target nomenclature conforms to the British Journal of Pharmacology's Guide to Receptors and Channels (Alexander et al., 2008).
Statistical analysis
All computations were performed according to Tallarida (2000), using computer program Pharm Tools Pro. Statistic analysis was performed according to Dawson-Saunders and Trapp (1994), using SPSS 15 for Windows. The results are presented as mean values ± SEM obtained from groups of 6–8 animals. Differences between the corresponding means were verified by analysis of variance with repeated measures (one-way anova), followed by Bonferroni test. A P-value of less than 0.05 was considered statistically significant.
Results
The effect of levetiracetam on carrageenan-induced hyperalgesia
In the paw pressure test in rats we observed that administration of levetiracetam (10–200 mg·kg−1; p.o.) caused a significant dose-dependent reduction of carrageenan-induced hyperalgesia (Figure 2). The antihyperalgesic effect of levetiracetam reached its maximum 120 min after p.o. application (small graph in Figure 2). The corresponding ED50± SEM at the peak effect point was 26.7 ± 2.2 mg·kg−1.
Figure 2.
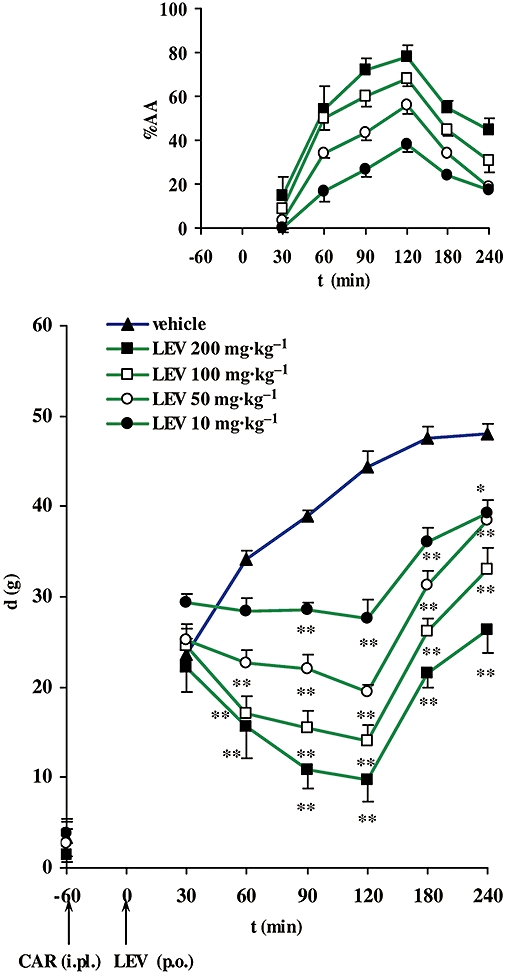
Time-course of the antihyperalgesic effect of levetiracetam (LEV) expressed as the difference in pressure g (d) applied to non-inflamed and inflamed [carrageenan (CAR)-injected] rat hind paws. Pretreatment d (plotted on the vertical axis) was obtained before CAR injection (i.pl.). Levetiracetam (p.o.) was given 60 min after induction of inflammation (denoted by arrows). Each point represents the mean ± SEM of paw pressure differences (d) of 6–8 animals. Statistical significance (*P < 0.05, **P < 0.01; one-way anova with repeated measures followed by Bonferroni test) was determined by comparing the curve with that for the vehicle. Upper (small) graph: time course of the antihyperalgesic effect of LEV expressed as a % of the antihyperalgesic activity (%AA). For symbols see the larger graph. i.pl., intraplantar.
The influence of bicuculline on the antihyperalgesic effect of levetiracetam
Bicuculline (0.5–2 mg·kg−1; i.p.) caused a significant decrease in the antihyperalgesic effect of levetiracetam (100 mg·kg−1; p.o.) (Figure 3A). The maximal inhibitory effects of bicuculline on levetiracetam-induced antihyperalgesia were achieved 60 min after i.p. administration; the corresponding values are shown in Figure 4. The estimated ID50± SEM for bicuculline at the peak effect point was 0.5 ± 0.4 mg·kg−1. Bicuculline itself (2 mg·kg−1; i.p.) did not have an intrinsic effect in this experimental model (Figure 3A).
Figure 3.
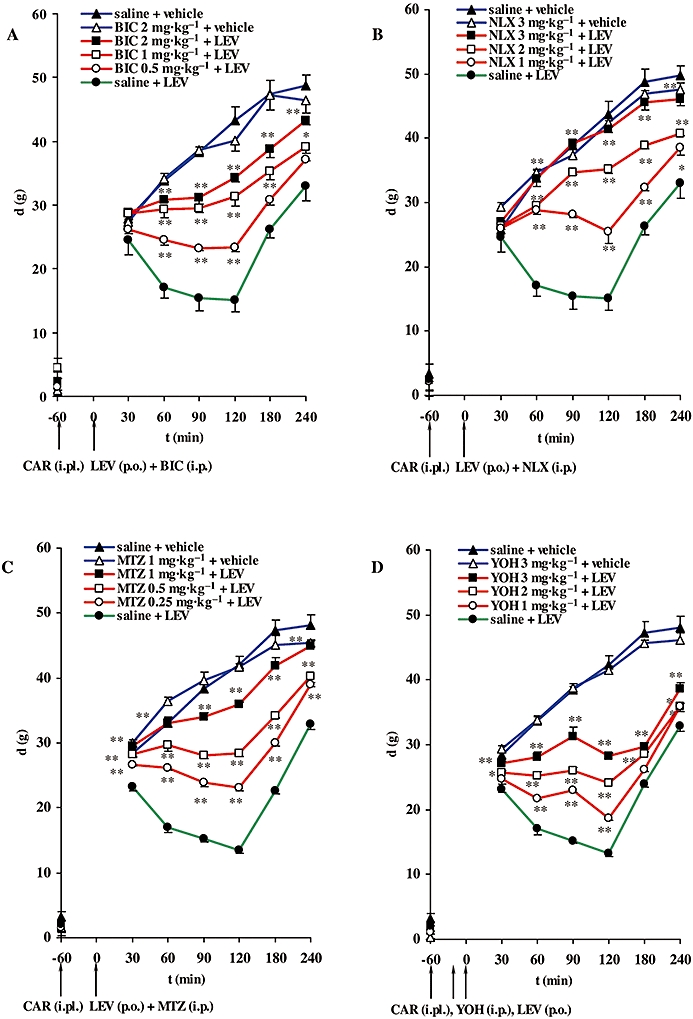
Time course of the inhibitory effects of bicuculline (BIC) (A), naloxone (NLX) (B), methysergide (MTZ) (C) and yohimbine (YOH) (D) on the antihyperalgesic effect of levetiracetam (LEV), expressed as the difference in pressure g (d) applied to non-inflamed and inflamed (CAR-injected) rat hind paws. Pretreatment d (plotted on the vertical axis) was obtained before induction of inflammation. BIC (i.p.) (A), NLX (i.p.) (B), MTZ (i.p.) (C) were injected immediately prior to LEV administration (p.o.) and 60 min after injection of CAR; only YOH (i.p.) (D) was injected 15 min before LEV and 45 min after CAR (denoted by arrows). Each point represents the mean ± SEM of paw pressure differences (d) of 6–8 animals. Statistical significance (*P < 0.05, **P < 0.01; one-way anova with repeated measures followed by Bonferroni test) was determined by comparison of the curves obtained in the presence of the receptor antagonists with the curve for saline + LEV. i.pl., intraplantar.
Figure 4.
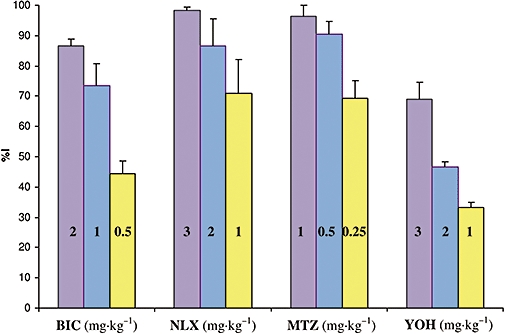
Inhibitory effects of bicuculline (BIC), naloxone (NLX), methysergide (MTZ) and yohimbine (YOH) on the antihyperalgesic effect of levetiracetam, expressed as a % of inhibition (%I), obtained at the time of maximal effect of antagonists.
The influence of naloxone on the antihyperalgesic effect of levetiracetam
Naloxone (1–3 mg·kg−1; i.p.) significantly decreased the antihyperalgesic effect of levetiracetam (100 mg·kg−1; p.o.) (Figure 3B). The maximal inhibitory effects of naloxone were achieved 90 min after i.p. application; the corresponding values are presented in Figure 4. The estimated ID50± SEM for naloxone at the peak effect point was 0.7 ± 0.3 mg·kg−1. The highest dose of naloxone tested did not induce a significant change in the hyperalgesia induced by carrageenan (Figure 3B).
The influence of methysergide on the antihyperalgesic effect of levetiracetam
Methysergide (0.25–1 mg·kg−1; i.p.) significantly decreased the antihyperalgesic effect of levetiracetam (100 mg·kg−1; p.o.) (Figure 3C). The peak effects of methysergide were observed 60 min after i.p. administration; the values of maximal %I are shown in Figure 4. The estimated ID50± SEM for methysergide at the maximal effect point was 0.1 ± 0.2 mg·kg−1. The highest dose of methysergide used (1 mg·kg−1; i.p.) did not affect carrageenan-induced hyperalgesia in this experimental model (Figure 3C).
The influence of yohimbine on the antihyperalgesic effect of levetiracetam
Pretreatment with yohimbine (1–3 mg·kg−1; i.p.) significantly decreased the antihyperalgesic effect of levetiracetam (100 mg·kg−1; p.o.) (Figure 3D). The maximal inhibitory effects of yohimbine were achieved 90 min after i.p. injection; the corresponding values are presented in Figure 4. The estimated ID50± SEM for yohimbine at the peak effect point was 2.0 ± 0.1 mg·kg−1. The highest dose of yohimbine tested did not affect carrageenan-induced hyperalgesia (Figure 3D).
Discussion
In the present study we showed for the first time, the antihyperalgesic effect of levetiracetam and its mechanisms of action in a model of inflammatory pain. Similar to our results, it has been demonstrated that systemic carbamazepine, oxcarbazepine and lamotrigine also inhibit hyperalgesia, induced by pro-inflammatory agents, in the paw pressure test in rats (Nakamura-Craig and Follenfant, 1995; Tomićet al., 2004; Vučkovićet al., 2006; Stepanović-Petrovićet al., 2008). In contrast to our results, Munro et al. (2007) did not observe any antihyperalgesic activity of levetiracetam in their experimental model of formalin-induced inflammatory pain in rats, despite the use of higher doses of levetiracetam than in our study (100–600 mg·kg−1 vs. 10–200 mg·kg−1). The reason for this discrepancy might be due to the use of different pro-inflammatory compounds (formalin vs. carrageenan in our study) and/or due to different routes of levetiracetam administration (i.p. vs. p.o. in our study). The dose-range (10–200 mg·kg−1) used in our study is comparable to the doses effective in animal models of epilepsy (Klitgaard et al., 1998).
The finding that bicuculline (0.5–2 mg·kg−1; i.p.), a selective GABAA receptor antagonist, significantly decreased the antihyperalgesic effect of levetiracetam, indicates that GABAA receptors are involved in its action. This could be interpreted in two ways. Levetiracetam could either act on GABAA receptors directly or indirectly by enhancing GABAergic neurotransmission. There is evidence that levetiracetam does not have a significant affinity for GABA receptors (Gower et al., 1992; Noyer et al., 1995; De Smedt et al., 2007). On the other hand, several indirect effects of levetiracetam on GABAergic neurotransmission have been reported. Hence, levetiracetam could potentiate GABA-mediated inhibition, possibly via changes in GABA metabolism and turnover that are secondary to the postsynaptic effects of the drug (Löscher et al., 1996) or through regulation of GABA transporters (Ueda et al., 2007). Levetiracetam has been shown to reverse the action of negative allosteric modulators on GABAA ionotropic receptors and facilitate GABA-mediated inhibition (Rigo et al., 2002). Taken together with our results, this suggests that levetiracetam acts on GABAA receptors indirectly, by augmenting GABAergic neurotransmission.
The possible involvement of the opioidergic system in the antihyperalgesic effect of levetiracetam in the inflammatory paw pressure test was investigated by pretreatment with naloxone, a non-selective opioid receptor antagonist. Naloxone (1–3 mg·kg−1; i.p.) decreased the antihyperalgesic effect of levetiracetam. Although a binding study of levetiracetam on opioid receptors has yet to be carried out, several in vitro studies have revealed that levetiracetam selectively inhibits the N-type Ca2+ channels and reduces Ca2+ conductance (Niespodziany et al., 2001; Lukyanetz et al., 2002). Opioid receptor agonists also inhibit N-type Ca2+ channels in a voltage-dependent manner (Wu et al., 2004). Hence, levetiracetam, by influencing N-type Ca2+ channels, might incorporate opioid-induced antinociception in its effects.
To elucidate the antinociceptive profile of levetiracetam in more detail, we tested the extent of its dependence on the 5-HT system. 5-HT plays an important role in modulating nociceptive transmission through both inhibitory and facilatory signalling pathways. This dual action can be the result of the mediation of different effects through different 5-HT receptors. Results obtained from electrophysiological studies suggest that 5-HT1 receptors most probably mediate a depressant action and antinociceptive effects, while 5-HT2 receptors usually mediate membrane depolarization and pronociceptive effects (Millan, 2002; Lopez-Garsia, 2006). Methysergide (0.25–1 mg·kg−1; i.p.), a non-selective 5-HT receptor antagonist, significantly reduced the antihyperalgesic effect of levetiracetam in the inflammatory paw pressure test. There are no data available on the binding properties of levetiracetam to 5-HT receptors, nor on its ability to influence 5-HT release in nociceptive transmission. The results obtained in the present study indicate that 5-HT receptors (most probably 5-HT1) are involved in the antihyperalgesic effect of levetiracetam. Subsequent studies are needed to identify the receptor subtype.
The finding that yohimbine (1–3 mg·kg−1; i.p.), a selective α2-adrenoceptor antagonist, decreased significantly the antihyperalgesic effect of levetiracetam implicates the involvement of α2-adrenoceptors in its action. At present, there is no information concerning the binding properties of levetiracetam to α2-adrenoceptors and its ability to influence brain noradrenaline levels. The α2-adrenoceptors are widely distributed in the peripheral and central nervous systems and could mediate analgesia or hyperalgesia, depending on the subtype of α2-adrenoceptor involved (Millan, 2002; Sawynok, 2003). The α2-adrenoceptors are located on primary afferent terminals, on the spinal dorsal horn neurones and within several brainstem nuclei that are implicated in analgesia (Petrovaara, 2006; Chen et al., 2007). After systemic administration of levetiracetam, the interaction with α2-adrenoceptors that results in analgesia can occur at all of these sites, either directly or indirectly. Further experiments are needed to clarify the site and mode of interaction between levetiracetam and α2-adrenoceptors.
Interplay between opioidergic, 5-HT and noradrenergic systems in pain modulation has been proposed previously (Millan, 2002) and may be necessary for providing maximal pain relief. Therefore, activation of the opioidergic system, in addition to temporary activation of noradrenergic and 5-HT systems, might indicate that the analgesic effect of levetiracetam depends on the interaction of the descending inhibitory system, via 5-HT and noradrenaline release, with the opioid inhibitory system. It should be mentioned that the functional status of opioid receptors (Zollner et al., 2003; Vanegas and Schaible, 2004), 5-HT receptors (Zhang et al., 2002) and α2-adrenoceptors (Vanegas and Schaible, 2004) could be altered following peripheral inflammation. These changes lead to an increase in the number and sensitivity of these receptors, resulting in an increase in the antinociceptive efficacy mediated by them. Accordingly, Ardid et al. (2003) provided evidence that levetiracetam (540 mg·kg−1; i.p.) has only a weak antinocieptive effect in the paw pressure test in naive (carrageenan untreated) rats. It is possible that levetiracetam activates the opioidergic antinociceptive pathway, which in turn interacts with other pain inhibitory systems. Moreover, Jasmin et al. (2003) showed that GABAA receptor stimulation from the cerebral cortex produces analgesia by enhancing the descending inhibition of spinal nociceptive neurones. The possible implication of this is that enhancement of GABA transmission by levetiracetam also increases the activity of descending inhibitory, noradrenergic and 5-HT pathways.
As levetiracetam modulates GABAAergic, opioidergic, 5-HT and α2-adrenergic systems through its antihyperalgesic effect, a broad spectrum of side effects could be expected. However, this antiepileptic drug, which has been in use in humans for more than 10 years is generally well-tolerated and in this respect better than many other antiepileptic drugs (De Smedt et al., 2007). Still, some of levetiracetam side effects could be related to its non-specific actions: nausea and vomiting could be the result of its effects on the opioid and 5-HT systems; anorexia could be due to its effect on 5-HT receptors; amnesia and ataxia could be due to its effect on the GABAA receptors; and headache could be a result of its effect on α2-adrenoceptors.
In conclusion, the results of the present study show that levetiracetam induces antihyperalgesia in an inflammatory model of pain and that this effect is at least in part, mediated by α2-adrenoceptors, GABAA, opioid and 5-HT receptors. Levetiracetam's efficacy in this animal model of inflammatory pain suggests that it could be a potentially important agent for treating inflammatory pain in humans.
Acknowledgments
This work was supported by the Serbian Ministry of Science and Technological Development, Grant no. 145 030.
Glossary
Abbreviations
- d (g)
difference between the pressure applied to the non-inflamed vs. the inflamed paw
- %AA
% antihyperalgesic activity
- %I
% inhibition of antihyperalgesic activity; i.pl., intraplantar
Conflicts of interest
The authors have no conflicts of interest that are relevant to the content of this article.
Supplemental material
Supporting Information: Teaching Materials; Figs 1–4 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (3rd edn) 2008;153(Suppl. 2):S1–S209. doi: 10.1038/sj.bjp.0707746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Archer DP, Lamberty Y, Wang B, Davis MJ, Samanani N, Roth SH. Levetiracetam reduces anesthetic-induced hyperalgesia in rats. Anesth Analg. 2007;104:180–185. doi: 10.1213/01.ane.0000247788.57318.1f. [DOI] [PubMed] [Google Scholar]
- Ardid D, Lamberty Y, Alloui A, Coudore-Civiale MA, Klitgaard H, Eschalier A. Antihyperalgesic effect of levetiracetam in neuropathic pain models in rats. Eur J Pharmacol. 2003;473:27–33. doi: 10.1016/s0014-2999(03)01933-2. [DOI] [PubMed] [Google Scholar]
- Chen SR, Pan HM, Richardson TE, Pan HL. Potentiation of spinal alpha(2)-adrenoceptor analgesia in rats deficient in TRPV1-expressing afferent neurons. Neuropharmacology. 2007;52:1624–1630. doi: 10.1016/j.neuropharm.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson-Saunders B, Trapp RG. Basic & Clinical Biostatistics. 2nd edn. Norwalk: Appleton & Lange; 1994. [Google Scholar]
- De Smedt T, Raedt R, Vonck K, Boon P. Levetiracetam: the profile of a novel anticonvulsant drug-part I: preclinical data. CNS Drug Rev. 2007;13:43–56. doi: 10.1111/j.1527-3458.2007.00004.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field MJ, Oles RJ, Lewis AS, McCleary S, Hughes J, Singh L. Gabapentin (neurontin) and S-(+)-3-isobutylgaba represent a novel class of selective antihyperalgesic agents. Br J Pharmacol. 1997;121:1513–1522. doi: 10.1038/sj.bjp.0701320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gower AJ, Noyer M, Verloes R, Gobert J, Wülfert E. ucb L059, a novel anticonvulsant drug: pharmacological profile in animals. Eur J Pharmacol. 1992;222:193–203. doi: 10.1016/0014-2999(92)90855-x. [DOI] [PubMed] [Google Scholar]
- Guindon J, Walczak JS, Beaulieu P. Recent advances in the pharmacological management of pain. Drugs. 2007;67:2121–2133. doi: 10.2165/00003495-200767150-00002. [DOI] [PubMed] [Google Scholar]
- Jasmin L, Rabkin SD, Granato A, Boudah A, Ohara PT. Analgesia and hyperalgesia from GABA-mediated modulation of the cerebral cortex. Nature. 2003;424:316–320. doi: 10.1038/nature01808. [DOI] [PubMed] [Google Scholar]
- Johannessen Landmark C. Antiepileptic drugs in non-epilepsy disorders: relations between mechanisms of action and clinical efficacy. CNS Drugs. 2008;22:27–47. doi: 10.2165/00023210-200822010-00003. [DOI] [PubMed] [Google Scholar]
- Jorns TP, Johnston A, Zakrzewska JM. Pilot study to evaluate the efficacy and tolerability of levetiracetam (Keppra) in treatment of patients with trigeminal neuralgia. Eur J Neurol. 2009;16:740–744. doi: 10.1111/j.1468-1331.2009.02585.x. [DOI] [PubMed] [Google Scholar]
- Klitgaard H, Matagne A, Gobert J, Wülfert E. Evidence for a unique profile of levetiracetam in rodent models of seizures and epilepsy. Eur J Pharmacol. 1998;353:191–206. doi: 10.1016/s0014-2999(98)00410-5. [DOI] [PubMed] [Google Scholar]
- Lopez-Garsia JA. Serotonergic modulation of spinal sensory circuits. Curr Top Med Chem. 2006;6:1987–1996. doi: 10.2174/156802606778522159. [DOI] [PubMed] [Google Scholar]
- Löscher W, Hönack D, Bloms-Funke P. The novel antiepileptic drug levetiracetam (ucb L059) induces alterations in GABA metabolism and turnover in discrete areas of rat brain and reduces neuronal activity in substantia nigra pars reticulata. Brain Res. 1996;735:208–216. doi: 10.1016/0006-8993(96)00587-2. [DOI] [PubMed] [Google Scholar]
- Lukyanetz EA, Shkryl VM, Kostyuk PG. Selective blockade of N-type calcium channels by levetiracetam. Epilepsia. 2002;43:9–18. doi: 10.1046/j.1528-1157.2002.24501.x. [DOI] [PubMed] [Google Scholar]
- Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- Morris CJ. Carrageenan-induced paw edema in the rat and mouse. In: Winyard PG, Willoughby DA, editors. Inflammation Protocols (Methods in Molecular Biology) Vol. 225. New Jersey, Totowa: Humana Press Inc; 2003. pp. 115–123. [DOI] [PubMed] [Google Scholar]
- Munro G, Erichsen HK, Mirza NR. Pharmacological comparison of anticonvulsant drugs in animal models of persistent pain and anxiety. Neuropharmacology. 2007;53:609–618. doi: 10.1016/j.neuropharm.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Nakamura-Craig M, Follenfant RL. Effect of lamotrigine in the acute and chronic hyperalgesia induced by PGE2 and in the chronic hyperalgesia in rats with streptozotocin-induced diabetes. Pain. 1995;63:33–37. doi: 10.1016/0304-3959(95)00016-L. [DOI] [PubMed] [Google Scholar]
- Niespodziany I, Klitgaard H, Margineanu DG. Levetiracetam inhibits the high-voltage-activated Ca2+ cuurent in pyramidal neurones of rat hippocampal slices. Neurosci Lett. 2001;306:5–8. doi: 10.1016/s0304-3940(01)01884-5. [DOI] [PubMed] [Google Scholar]
- Noyer M, Gillard M, Matagne A, Hénichart JP, Wülfert E. The novel antiepileptic drug levetiracetam (ucb L059) appears to act via a specific binding site in CNS membranes. Eur J Pharmacol. 1995;286:137–146. doi: 10.1016/0014-2999(95)00436-o. [DOI] [PubMed] [Google Scholar]
- Petrovaara A. Noradrenergic pain modulation. Prog Neurobiol. 2006;80:53–83. doi: 10.1016/j.pneurobio.2006.08.001. [DOI] [PubMed] [Google Scholar]
- Randall LO, Selitto JJ. A method for measurement of analgesic activity on inflamed tissue. Arch Int Pharmacodyn Ther. 1957;111:409–419. [PubMed] [Google Scholar]
- Rigo JM, Hans G, Nguyen L, Rocher V, Belachew S, Malgrange B, et al. The anti-epileptic drug levetiracetam reverses the inhibition by negative allosteric modulators of neuronal GABA- and glycine-gated currents. Br J Pharmacol. 2002;136:659–672. doi: 10.1038/sj.bjp.0704766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi S, Mataluni G, Codecà C, Fiore S, Buttari F, Musella A, et al. Effects of levetiracetam on chronic pain in multiple sclerosis: results of a pilot, randomized, placebo-controlled study. Eur J Neurol. 2009;16:360–366. doi: 10.1111/j.1468-1331.2008.02496.x. [DOI] [PubMed] [Google Scholar]
- Sawynok J. Topical and peripherally acting analgesics. Pharmacol Rev. 2003;55:1–20. doi: 10.1124/pr.55.1.1. [DOI] [PubMed] [Google Scholar]
- Silva J, Dolezal T, Prochazkova M, Votava M, Krsiak M. Preemptive levetiracetam decreases postoperative pain in rats. Neuro Endocrinol Lett. 2008;29:953–957. [PubMed] [Google Scholar]
- Stepanović-Petrović RM, Tomić MA, Vučković SM, Kocev N, Ugrešić ND, Prostran MS, et al. GABAergic mechanisms are involved in the antihyperalgesic effects of carbamazepine and oxcarbazepine in a rat model of inflammatory hyperalgesia. Pharmacology. 2008;82:53–58. doi: 10.1159/000127841. [DOI] [PubMed] [Google Scholar]
- Stöhr T, Krause E, Selve N. Lacosamide displays potent antinociceptive effects in animal models for inflammatory pain. Eur J Pain. 2006;10:241–249. doi: 10.1016/j.ejpain.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Tallarida RJ. Drug Synergism and Dose-Effect Data Analysis. 1st edn. London, New York, Washington: Chapman & Hall/CRC, Boca Raton; 2000. [Google Scholar]
- Tomić MA, Vučković SM, Stepanović-Petrović RM, Ugrešić N, Prostran MS, Bošković B. The anti-hyperalgesic effects of carbamazepine and oxcarbazepine are attenuated by treatment with adenosine receptor antagonists. Pain. 2004;111:253–260. doi: 10.1016/j.pain.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Ueda Y, Doi T, Nagatomo K, Tokumaru J, Takaki M, Willmore LJ. Effect of levetiracetam on molecular regulation of hippocampal glutamate and GABA transporters in rats with chronic seizures induced by amygdalar FeCl3 injection. Brain Res. 2007;1151:55–61. doi: 10.1016/j.brainres.2007.03.021. [DOI] [PubMed] [Google Scholar]
- Vanegas H, Schaible HG. Descending control of persistent pain: inhibitory or facilitatory? Brain Res Brain Res Rev. 2004;46:295–309. doi: 10.1016/j.brainresrev.2004.07.004. [DOI] [PubMed] [Google Scholar]
- Vučković SM, Tomić MA, Stepanović-Petrović RM, Ugrešić N, Prostran MS, Bošković B. The effects of alpha2-adrenoceptor agents on anti-hyperalgesic effects of carbamazepine and oxcarbazepine in a rat model of inflammatory pain. Pain. 2006;125:10–19. doi: 10.1016/j.pain.2006.04.023. [DOI] [PubMed] [Google Scholar]
- Wu ZZ, Chen SR, Pan HL. Differential sensitivity of N- and P/Q-type Ca2+ channel currents to a mu opioid in isolectin B4-positive and -negative dorsal root ganglion neurons. J Pharmacol Exp Ther. 2004;311:939–947. doi: 10.1124/jpet.104.073429. [DOI] [PubMed] [Google Scholar]
- Zhang YQ, Gao X, Ji GC, Huang YL, Wu GC, Zhao ZQ. Expression of of 5-HT1A receptor mRNA in rat lumbar spinal dorsal horn neurons after peripheral inflammation. Pain. 2002;98:287–295. doi: 10.1016/S0304-3959(02)00026-X. [DOI] [PubMed] [Google Scholar]
- Zimmermann M. Ethical guidelines for investigations of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]
- Zollner C, Shaqura MA, Bopaiah CP, Mousa S, Stein C, Schafer M. Painful inflammation-induced increase in mu-opioid receptor binding and G-protein coupling in primary afferent neurons. Mol Pharmacol. 2003;64:202–210. doi: 10.1124/mol.64.2.202. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


