Abstract
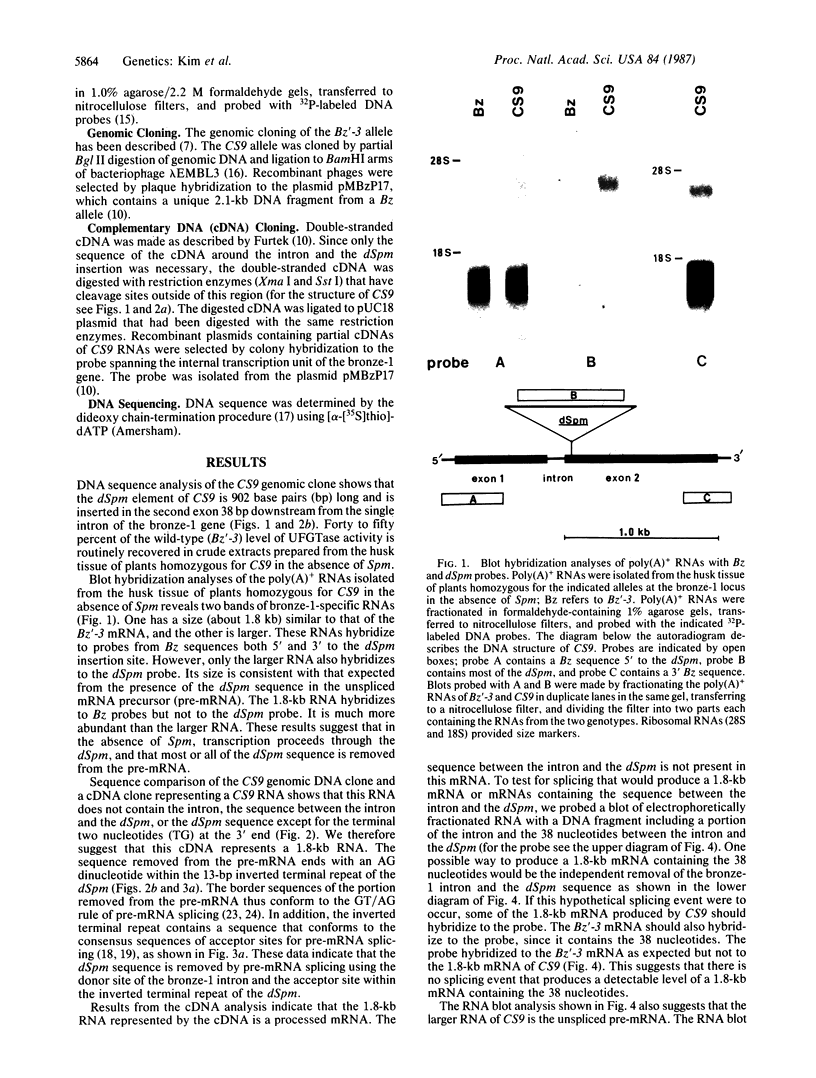
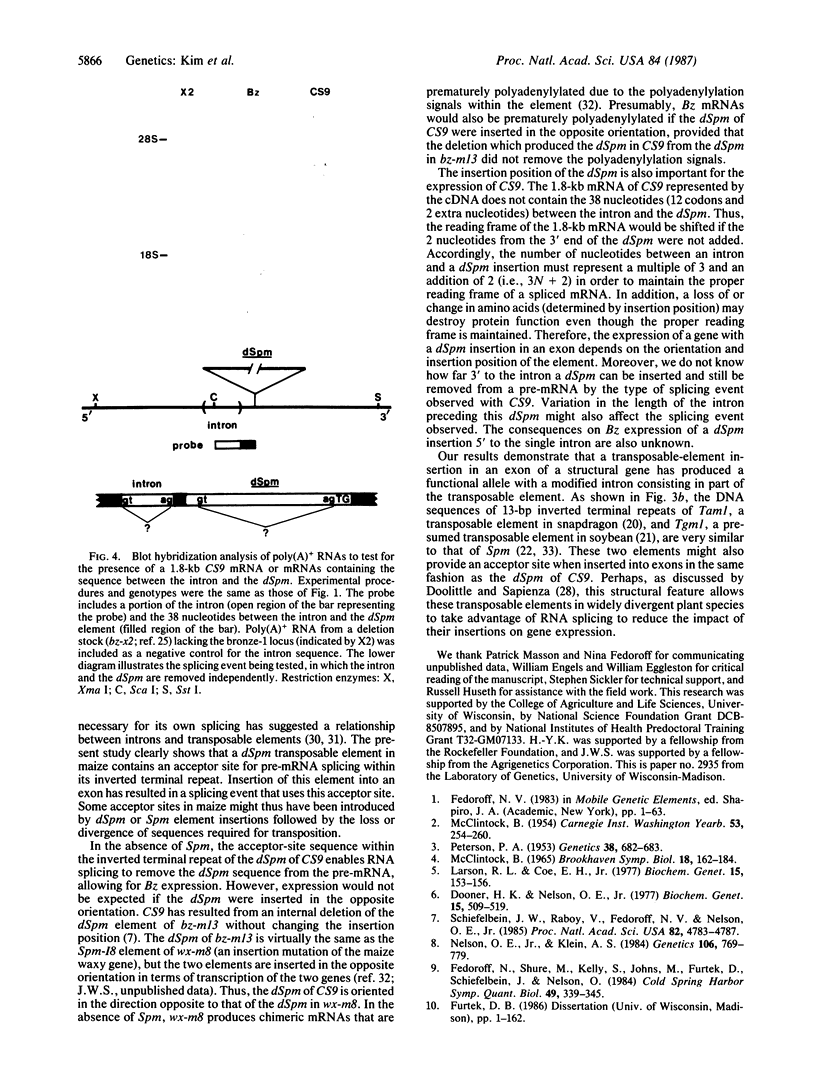
The bz-m13CS9 allele of the bronze-1 gene in maize contains a 902-base-pair defective Suppressor-mutator (dSpm) transposable element in the second exon. Nevertheless, 40-50% of the enzymatic activity conditioned by a nonmutant allele at the bronze-1 locus is routinely recovered in crude extracts prepared from plants carrying bz-m13CS9 in the absence of an autonomous Suppressor-mutator element. Analyses of RNAs produced by such plants show that transcription proceeds through the dSpm. The dSpm sequence of the messenger RNA precursor is then removed by RNA splicing using the donor site of the single bronze-1 intron and an acceptor site within the inverted terminal repeat of the dSpm. This results in a messenger RNA with the proper reading frame that could produce a functional enzyme. These data demonstrate that this dSpm insertion in an exon of a structural gene has produced a functional allele with a novel intron consisting, in part, of the dSpm. This mechanism appears to allow dSpm elements to reduce the impact of their insertions on gene expression.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonas U., Sommer H., Saedler H. The 17-kb Tam1 element of Antirrhinum majus induces a 3-bp duplication upon integration into the chalcone synthase gene. EMBO J. 1984 May;3(5):1015–1019. doi: 10.1002/j.1460-2075.1984.tb01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breathnach R., Benoist C., O'Hare K., Gannon F., Chambon P. Ovalbumin gene: evidence for a leader sequence in mRNA and DNA sequences at the exon-intron boundaries. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4853–4857. doi: 10.1073/pnas.75.10.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. W., Feix G., Frendewey D. Accurate in vitro splicing of two pre-mRNA plant introns in a HeLa cell nuclear extract. EMBO J. 1986 Nov;5(11):2749–2758. doi: 10.1002/j.1460-2075.1986.tb04563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle W. F., Sapienza C. Selfish genes, the phenotype paradigm and genome evolution. Nature. 1980 Apr 17;284(5757):601–603. doi: 10.1038/284601a0. [DOI] [PubMed] [Google Scholar]
- Dooner H. K. Genetic Fine Structure of the BRONZE Locus in Maize. Genetics. 1986 Aug;113(4):1021–1036. doi: 10.1093/genetics/113.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooner H. K., Nelson O. E. Controlling element-induced alterations in UDPglucose:flavonoid glucosyltransferase, the enzyme specified by the bronze locus in maize. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5623–5627. doi: 10.1073/pnas.74.12.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooner H. K., Nelson O. E. Genetic control of UDPglucose:flavonol 3-O-glucosyltransferase in the endosperm of maize. Biochem Genet. 1977 Jun;15(5-6):509–519. doi: 10.1007/BF00520194. [DOI] [PubMed] [Google Scholar]
- Fedoroff N., Shure M., Kelly S., Johns M., Furtek D., Schiefelbein J., Nelson O. Isolation of Spm controlling elements from maize. Cold Spring Harb Symp Quant Biol. 1984;49:339–345. doi: 10.1101/sqb.1984.049.01.040. [DOI] [PubMed] [Google Scholar]
- Frischauf A. M., Lehrach H., Poustka A., Murray N. Lambda replacement vectors carrying polylinker sequences. J Mol Biol. 1983 Nov 15;170(4):827–842. doi: 10.1016/s0022-2836(83)80190-9. [DOI] [PubMed] [Google Scholar]
- Fyrberg E. A., Bond B. J., Hershey N. D., Mixter K. S., Davidson N. The actin genes of Drosophila: protein coding regions are highly conserved but intron positions are not. Cell. 1981 Apr;24(1):107–116. doi: 10.1016/0092-8674(81)90506-7. [DOI] [PubMed] [Google Scholar]
- Gierl A., Schwarz-Sommer Z., Saedler H. Molecular interactions between the components of the En-I transposable element system of Zea mays. EMBO J. 1985 Mar;4(3):579–583. doi: 10.1002/j.1460-2075.1985.tb03669.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green M. R. Pre-mRNA splicing. Annu Rev Genet. 1986;20:671–708. doi: 10.1146/annurev.ge.20.120186.003323. [DOI] [PubMed] [Google Scholar]
- Jacquier A., Dujon B. An intron-encoded protein is active in a gene conversion process that spreads an intron into a mitochondrial gene. Cell. 1985 Jun;41(2):383–394. doi: 10.1016/s0092-8674(85)80011-8. [DOI] [PubMed] [Google Scholar]
- Klein A. S., Nelson O. E. Biochemical consequences of the insertion of a suppressor-mutator (Spm) receptor at the bronze-1 locus in maize. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7591–7595. doi: 10.1073/pnas.80.24.7591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson R. L., Coe E. H., Jr Gene-dependent flavonoid glucosyltransferase in maize. Biochem Genet. 1977 Feb;15(1-2):153–156. doi: 10.1007/BF00484558. [DOI] [PubMed] [Google Scholar]
- Leicht M., Long G. L., Chandra T., Kurachi K., Kidd V. J., Mace M., Jr, Davie E. W., Woo S. L. Sequence homology and structural comparison between the chromosomal human alpha 1-antitrypsin and chicken ovalbumin genes. Nature. 1982 Jun 24;297(5868):655–659. doi: 10.1038/297655a0. [DOI] [PubMed] [Google Scholar]
- Macreadie I. G., Scott R. M., Zinn A. R., Butow R. A. Transposition of an intron in yeast mitochondria requires a protein encoded by that intron. Cell. 1985 Jun;41(2):395–402. doi: 10.1016/s0092-8674(85)80012-x. [DOI] [PubMed] [Google Scholar]
- Mottinger J. P. The effects of X rays on the bronze and shrunken loci in maize. Genetics. 1970 Feb;64(2):259–271. doi: 10.1093/genetics/64.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mount S. M. A catalogue of splice junction sequences. Nucleic Acids Res. 1982 Jan 22;10(2):459–472. doi: 10.1093/nar/10.2.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson O. E., Klein A. S. Characterization of an spm-controlled bronze-mutable allele in maize. Genetics. 1984 Apr;106(4):769–779. doi: 10.1093/genetics/106.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira A., Cuypers H., Gierl A., Schwarz-Sommer Z., Saedler H. Molecular analysis of the En/Spm transposable element system of Zea mays. EMBO J. 1986 May;5(5):835–841. doi: 10.1002/j.1460-2075.1986.tb04292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes P. R., Vodkin L. O. Highly structured sequence homology between an insertion element and the gene in which it resides. Proc Natl Acad Sci U S A. 1985 Jan;82(2):493–497. doi: 10.1073/pnas.82.2.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiefelbein J. W., Raboy V., Fedoroff N. V., Nelson O. E., Jr Deletions within a defective suppressor-mutator element in maize affect the frequency and developmental timing of its excision from the bronze locus. Proc Natl Acad Sci U S A. 1985 Jul;82(14):4783–4787. doi: 10.1073/pnas.82.14.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharp P. A. On the origin of RNA splicing and introns. Cell. 1985 Sep;42(2):397–400. doi: 10.1016/0092-8674(85)90092-3. [DOI] [PubMed] [Google Scholar]










