Abstract
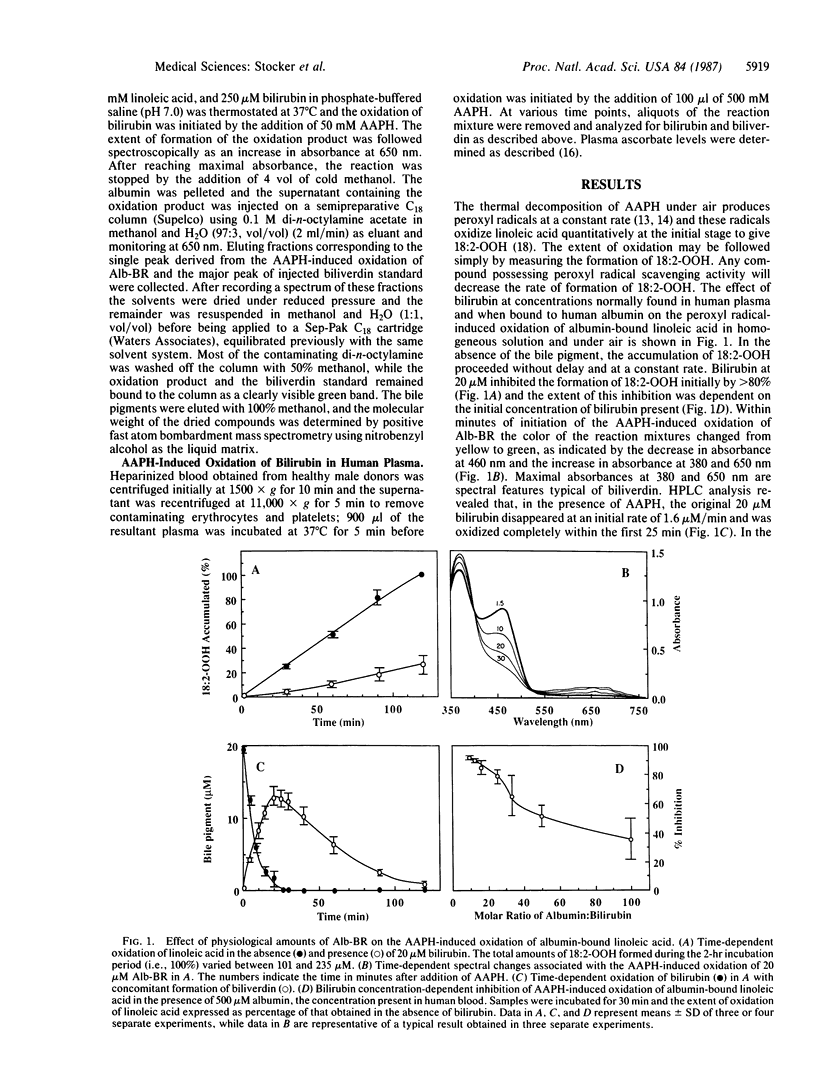
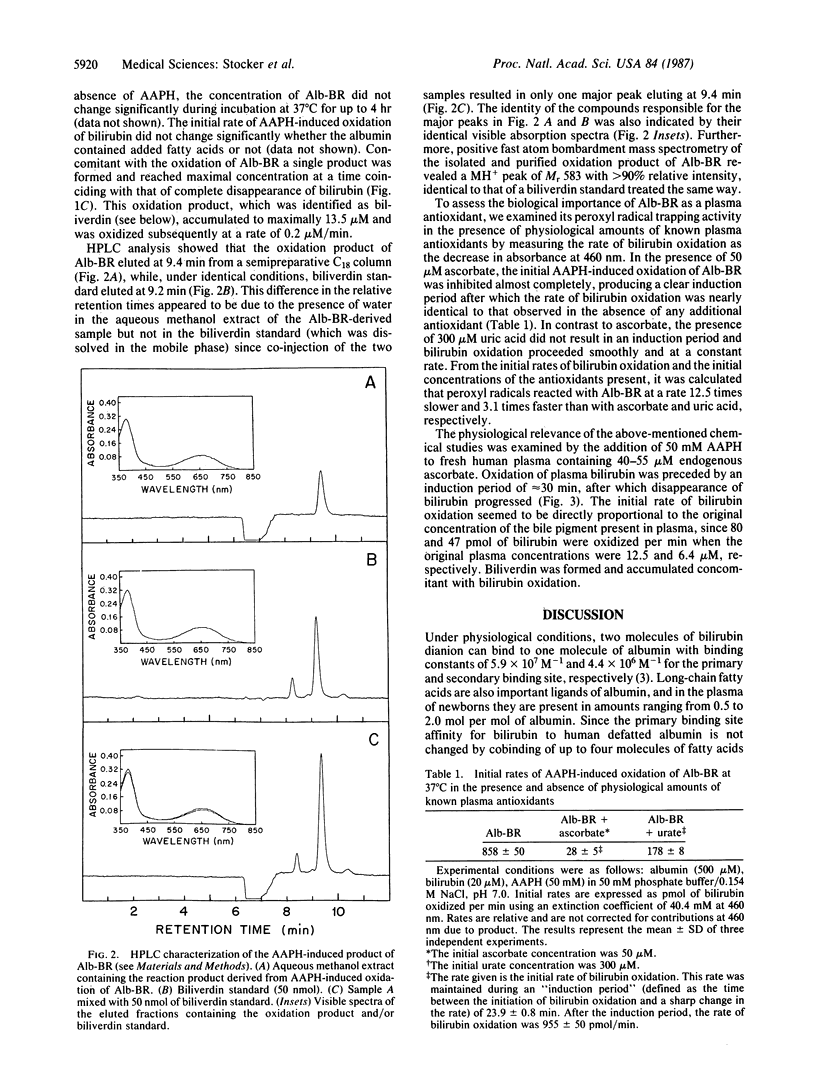
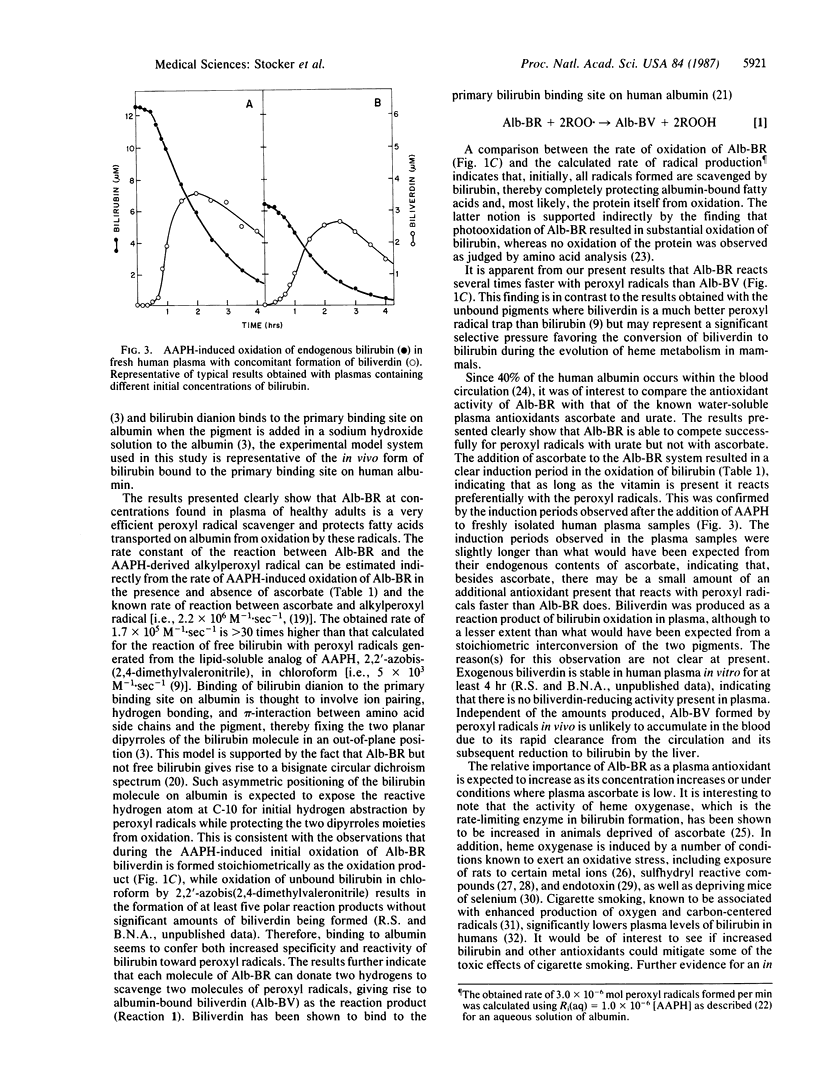
Bilirubin, when bound to human albumin and at concentrations present in normal human plasma, protects albumin-bound linoleic acid from peroxyl radical-induced oxidation in vitro. Initially, albumin-bound bilirubin (Alb-BR) is oxidized at the same rate as peroxyl radicals are formed and biliverdin is produced stoichiometrically as the oxidation product. On an equimolar basis, Alb-BR successfully competes with uric acid for peroxyl radicals but is less efficient in scavenging these radicals than vitamin C. These results show that 1 mol of Alb-BR can scavenge 2 mol of peroxyl radicals and that small amounts of plasma bilirubin are sufficient to prevent oxidation of albumin-bound fatty acids as well as of the protein itself. The data indicate a role for Alb-BR as a physiological antioxidant in plasma and the extravascular space.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahlfors C. E. Competitive interaction of biliverdin and bilirubin only at the primary bilirubin binding site on human albumin. Anal Biochem. 1981 Jan 15;110(2):295–307. doi: 10.1016/0003-2697(81)90195-0. [DOI] [PubMed] [Google Scholar]
- Blauer G., King T. E. Interactions of bilirubin with bovine serum albumin in aqueous solution. J Biol Chem. 1970 Jan 25;245(2):372–381. [PubMed] [Google Scholar]
- Bliuger A. F., Dudnik L. B., Maiore A. Ia, Mieze I. E. Rol' bilirubina kak prirodnogo antioksidanta v reguliatsii intensivnosti perekisnogo okisleniia lipidov pri ostrom virusnom gepatite. Biull Eksp Biol Med. 1985 Feb;99(2):166–168. [PubMed] [Google Scholar]
- Bloomer J. R., Berk P. D., Howe R. B., Berlin N. I. Interpretation of plasma bilirubin levels based on studies with radioactive bilirubin. JAMA. 1971 Oct 11;218(2):216–220. [PubMed] [Google Scholar]
- Brodersen R. Binding of bilirubin to albumin. CRC Crit Rev Clin Lab Sci. 1980 Jan;11(4):305–399. [PubMed] [Google Scholar]
- Brodersen R. Localization of bilirubin pools in the non-jaundiced rat, with a note on bilirubin dynamics in normal human adults and in Gilbert's syndrome. Scand J Clin Lab Invest. 1972 Sep;30(1):95–106. doi: 10.3109/00365517209081097. [DOI] [PubMed] [Google Scholar]
- Chan-Yeung M., Ferreira P., Frohlich J., Schulzer M., Tan F. The effects of age, smoking, and alcohol on routine laboratory tests. Am J Clin Pathol. 1981 Mar;75(3):320–326. doi: 10.1093/ajcp/75.3.320. [DOI] [PubMed] [Google Scholar]
- Church D. F., Pryor W. A. Free-radical chemistry of cigarette smoke and its toxicological implications. Environ Health Perspect. 1985 Dec;64:111–126. doi: 10.1289/ehp.8564111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fantone J. C., Ward P. A. Role of oxygen-derived free radicals and metabolites in leukocyte-dependent inflammatory reactions. Am J Pathol. 1982 Jun;107(3):395–418. [PMC free article] [PubMed] [Google Scholar]
- Gemsa D., Woo C. H., Fudenberg H. H., Schmid R. Stimulation of heme oxygenase in macrophages and liver by endotoxin. J Clin Invest. 1974 Feb;53(2):647–651. doi: 10.1172/JCI107599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi G., Yoshida T. Function and induction of the microsomal heme oxygenase. Mol Cell Biochem. 1983;53-54(1-2):163–183. doi: 10.1007/BF00225252. [DOI] [PubMed] [Google Scholar]
- Lamola A. A., Eisinger J., Blumberg W. E., Patel S. C., Flores J. Flurorometric study of the partition of bilirubin among blood components: basis for rapid microassays of bilirubin and bilirubin binding capacity in whole blood. Anal Biochem. 1979 Nov 15;100(1):25–42. doi: 10.1016/0003-2697(79)90105-2. [DOI] [PubMed] [Google Scholar]
- Levi A. J., Gatmaitan Z., Arias I. M. Two hepatic cytoplasmic protein fractions, Y and Z, and their possible role in the hepatic uptake of bilirubin, sulfobromophthalein, and other anions. J Clin Invest. 1969 Nov;48(11):2156–2167. doi: 10.1172/JCI106182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Metals as regulators of heme metabolism. Science. 1977 Dec 23;198(4323):1215–1221. doi: 10.1126/science.337492. [DOI] [PubMed] [Google Scholar]
- Maines M. D., Kappas A. Regulation of heme pathway enzymes and cellular glutathione content by metals that do not chelate with tetrapyrroles: blockade of metal effects by thiols. Proc Natl Acad Sci U S A. 1977 May;74(5):1875–1878. doi: 10.1073/pnas.74.5.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonagh A. F., Assisi F. The ready isomerization of bilirubin IX- in aqueous solution. Biochem J. 1972 Sep;129(3):797–800. doi: 10.1042/bj1290797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odell G. B. Influence of binding on the toxicity of bilirubin. Ann N Y Acad Sci. 1973 Nov 26;226:225–237. doi: 10.1111/j.1749-6632.1973.tb20484.x. [DOI] [PubMed] [Google Scholar]
- Pedersen A. O., Schonheyder F., Brodersen R. Photooxidation of human serum albumin and its complex with bilirubin. Eur J Biochem. 1977 Jan;72(2):213–221. doi: 10.1111/j.1432-1033.1977.tb11242.x. [DOI] [PubMed] [Google Scholar]
- ROTHSCHILD M. A., BAUMAN A., YALOW R. S., BERSON S. A. Tissue distribution of I131 labeled human serum albumin following intravenous administration. J Clin Invest. 1955 Sep;34(9):1354–1358. doi: 10.1172/JCI103183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter R., Wendel A. Selenium and drug metabolism--I. Multiple modulations of mouse liver enzymes. Biochem Pharmacol. 1983 Oct 15;32(20):3063–3067. doi: 10.1016/0006-2952(83)90250-2. [DOI] [PubMed] [Google Scholar]
- Schmid R., McDonagh A. F. The enzymatic formation of bilirubin. Ann N Y Acad Sci. 1975 Apr 15;244:533–552. doi: 10.1111/j.1749-6632.1975.tb41553.x. [DOI] [PubMed] [Google Scholar]
- Stocker R., Yamamoto Y., McDonagh A. F., Glazer A. N., Ames B. N. Bilirubin is an antioxidant of possible physiological importance. Science. 1987 Feb 27;235(4792):1043–1046. doi: 10.1126/science.3029864. [DOI] [PubMed] [Google Scholar]
- Tögl-Leimüller A., Egger G., Porta S. Albumin as one-way transport vehicle into sites of inflammation. Exp Pathol. 1986;30(2):91–96. doi: 10.1016/s0232-1513(86)80066-4. [DOI] [PubMed] [Google Scholar]
- Walsch S., Degkwitz E. Activity of microsomal heme oxygenase in liver and spleen of ascorbic acid-deficient guinea pigs. Hoppe Seylers Z Physiol Chem. 1980 Aug;361(8):1243–1249. doi: 10.1515/bchm2.1980.361.2.1243. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Brodsky M. H., Baker J. C., Ames B. N. Detection and characterization of lipid hydroperoxides at picomole levels by high-performance liquid chromatography. Anal Biochem. 1987 Jan;160(1):7–13. doi: 10.1016/0003-2697(87)90606-3. [DOI] [PubMed] [Google Scholar]


