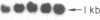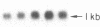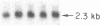Abstract
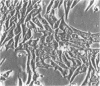
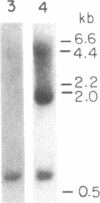
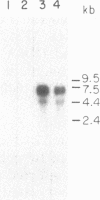
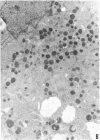
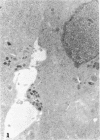
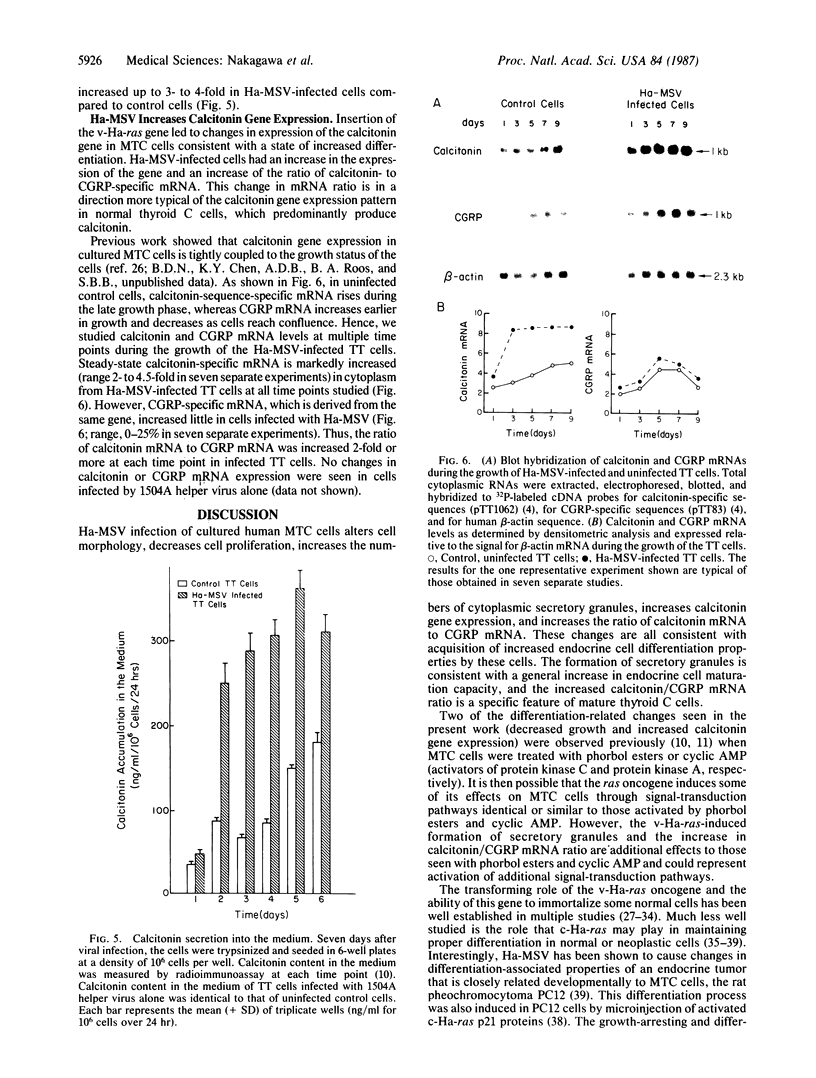
Medullary thyroid carcinoma (MTC) is an endocrine tumor of the thyroid C cells that expresses high levels of the neuroendocrine peptide hormone calcitonin. During tumor progression in the host, there is an apparent loss of differentiation in MTC cells that involves a consistent decrease in calcitonin content of the tumor cells associated with decreased expression of the calcitonin gene and/or changes in a mRNA alternative-processing pattern away from that characteristic of the parent thyroid C cell. We now report that introduction of the viral Harvey ras (v-Ha-ras) oncogene into cultured human MTC cells can reverse such changes in gene expression and can induce endocrine differentiation of the tumor cells. The expression of v-Ha-ras is associated with decreased cellular proliferation and DNA synthesis. There is a marked increase in the number of cytoplasmic secretory granules that are a classic feature of differentiated thyroid C cells. v-Ha-ras expression induces increased expression of the calcitonin gene and the processing of the primary gene transcript is shifted to favor calcitonin mRNA rather than calcitonin-gene-related peptide (CGRP) mRNA production. These studies with cultured human MTC cells provide a model system to study the role of Ha-ras and related genes in neuroendocrine differentiation. The findings suggest an important approach for identifying genes in solid tumors whose altered expression may play a role in the impaired maturational capacity characteristic of cancer cells during tumor progression.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amara S. G., Jonas V., Rosenfeld M. G., Ong E. S., Evans R. M. Alternative RNA processing in calcitonin gene expression generates mRNAs encoding different polypeptide products. Nature. 1982 Jul 15;298(5871):240–244. doi: 10.1038/298240a0. [DOI] [PubMed] [Google Scholar]
- Bar-Sagi D., Feramisco J. R. Microinjection of the ras oncogene protein into PC12 cells induces morphological differentiation. Cell. 1985 Oct;42(3):841–848. doi: 10.1016/0092-8674(85)90280-6. [DOI] [PubMed] [Google Scholar]
- Berger C. L., de Bustros A., Roos B. A., Leong S. S., Mendelsohn G., Gesell M. S., Baylin S. B. Human medullary thyroid carcinoma in culture provides a model relating growth dynamics, endocrine cell differentiation, and tumor progression. J Clin Endocrinol Metab. 1984 Aug;59(2):338–343. doi: 10.1210/jcem-59-2-338. [DOI] [PubMed] [Google Scholar]
- Birchmeier C., Broek D., Wigler M. ras proteins can induce meiosis in Xenopus oocytes. Cell. 1985 Dec;43(3 Pt 2):615–621. doi: 10.1016/0092-8674(85)90233-8. [DOI] [PubMed] [Google Scholar]
- Broek D., Samiy N., Fasano O., Fujiyama A., Tamanoi F., Northup J., Wigler M. Differential activation of yeast adenylate cyclase by wild-type and mutant RAS proteins. Cell. 1985 Jul;41(3):763–769. doi: 10.1016/s0092-8674(85)80057-x. [DOI] [PubMed] [Google Scholar]
- Ellis R. W., DeFeo D., Maryak J. M., Young H. A., Shih T. Y., Chang E. H., Lowy D. R., Scolnick E. M. Dual evolutionary origin for the rat genetic sequences of Harvey murine sarcoma virus. J Virol. 1980 Nov;36(2):408–420. doi: 10.1128/jvi.36.2.408-420.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman L. F., Chahwala S. B., Cantley L. ras-transformed cells: altered levels of phosphatidylinositol-4,5-bisphosphate and catabolites. Science. 1986 Jan 24;231(4736):407–410. doi: 10.1126/science.3001936. [DOI] [PubMed] [Google Scholar]
- HARVEY J. J. AN UNIDENTIFIED VIRUS WHICH CAUSES THE RAPID PRODUCTION OF TUMOURS IN MICE. Nature. 1964 Dec 12;204:1104–1105. doi: 10.1038/2041104b0. [DOI] [PubMed] [Google Scholar]
- Hagag N., Halegoua S., Viola M. Inhibition of growth factor-induced differentiation of PC12 cells by microinjection of antibody to ras p21. Nature. 1986 Feb 20;319(6055):680–682. doi: 10.1038/319680a0. [DOI] [PubMed] [Google Scholar]
- Hankins W. D., Scolnick E. M. Harvey and Kirsten sarcoma viruses promote the growth and differentiation of erythroid precursor cells in vitro. Cell. 1981 Oct;26(1 Pt 1):91–97. doi: 10.1016/0092-8674(81)90036-2. [DOI] [PubMed] [Google Scholar]
- Jonas V., Lin C. R., Kawashima E., Semon D., Swanson L. W., Mermod J. J., Evans R. M., Rosenfeld M. G. Alternative RNA processing events in human calcitonin/calcitonin gene-related peptide gene expression. Proc Natl Acad Sci U S A. 1985 Apr;82(7):1994–1998. doi: 10.1073/pnas.82.7.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly R. B. Pathways of protein secretion in eukaryotes. Science. 1985 Oct 4;230(4721):25–32. doi: 10.1126/science.2994224. [DOI] [PubMed] [Google Scholar]
- Lacal J. C., Santos E., Notario V., Barbacid M., Yamazaki S., Kung H., Seamans C., McAndrew S., Crowl R. Expression of normal and transforming H-ras genes in Escherichia coli and purification of their encoded p21 proteins. Proc Natl Acad Sci U S A. 1984 Sep;81(17):5305–5309. doi: 10.1073/pnas.81.17.5305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman S. M., Mendelsohn G., Trump D. L., Wells S. A., Jr, Baylin S. B. The prognostic and biological significance of cellular heterogeneity in medullary thyroid carcinoma: a study of calcitonin, L-dopa decarboxylase, and histaminase. J Clin Endocrinol Metab. 1982 Feb;54(2):233–240. doi: 10.1210/jcem-54-2-233. [DOI] [PubMed] [Google Scholar]
- Nelkin B. D., Rosenfeld K. I., de Bustros A., Leong S. S., Roos B. A., Baylin S. B. Structure and expression of a gene encoding human calcitonin and calcitonin gene related peptide. Biochem Biophys Res Commun. 1984 Sep 17;123(2):648–655. doi: 10.1016/0006-291x(84)90278-x. [DOI] [PubMed] [Google Scholar]
- Noda M., Ko M., Ogura A., Liu D. G., Amano T., Takano T., Ikawa Y. Sarcoma viruses carrying ras oncogenes induce differentiation-associated properties in a neuronal cell line. Nature. 1985 Nov 7;318(6041):73–75. doi: 10.1038/318073a0. [DOI] [PubMed] [Google Scholar]
- Pierce J. H., Aaronson S. A. BALB- and Harvey-murine sarcoma virus transformation of a novel lymphoid progenitor cell. J Exp Med. 1982 Sep 1;156(3):873–887. doi: 10.1084/jem.156.3.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Keller J., Schultz A. M., Holmes K. L., Medicus R., Ihle J. N. Infection of immune mast cells by Harvey sarcoma virus: immortalization without loss of requirement for interleukin-3. Mol Cell Biol. 1985 Sep;5(9):2257–2264. doi: 10.1128/mcb.5.9.2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rein A., Schultz A. M., Bader J. P., Bassin R. H. Inhibitors of glycosylation reverse retroviral interference. Virology. 1982 May;119(1):185–192. doi: 10.1016/0042-6822(82)90075-7. [DOI] [PubMed] [Google Scholar]
- Rhim J. S., Jay G., Arnstein P., Price F. M., Sanford K. K., Aaronson S. A. Neoplastic transformation of human epidermal keratinocytes by AD12-SV40 and Kirsten sarcoma viruses. Science. 1985 Mar 8;227(4691):1250–1252. doi: 10.1126/science.2579430. [DOI] [PubMed] [Google Scholar]
- Rosenfeld M. G., Amara S. G., Evans R. M. Alternative RNA processing: determining neuronal phenotype. Science. 1984 Sep 21;225(4668):1315–1320. doi: 10.1126/science.6089345. [DOI] [PubMed] [Google Scholar]
- Ruppert J. M., Eggleston J. C., deBustros A., Baylin S. B. Disseminated calcitonin-poor medullary thyroid carcinoma in a patient with calcitonin-rich primary tumor. Am J Surg Pathol. 1986 Jul;10(7):513–518. doi: 10.1097/00000478-198607000-00009. [DOI] [PubMed] [Google Scholar]
- Saad M. F., Ordonez N. G., Guido J. J., Samaan N. A. The prognostic value of calcitonin immunostaining in medullary carcinoma of the thyroid. J Clin Endocrinol Metab. 1984 Nov;59(5):850–856. doi: 10.1210/jcem-59-5-850. [DOI] [PubMed] [Google Scholar]
- Scher C. D., Scolnick E. M., Siegler R. Induction of erythroid leukaemia by Harvey and Kirsten sarcoma viruses. Nature. 1975 Jul 17;256(5514):225–226. doi: 10.1038/256225a0. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Papageorge A. G., Stokes P. E., Weeks M. O., Scolnick E. M. Guanine nucleotide-binding and autophosphorylating activities associated with the p21src protein of Harvey murine sarcoma virus. Nature. 1980 Oct 23;287(5784):686–691. doi: 10.1038/287686a0. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Teitlebaum S. L., Moore K. E., Shieber W. Parafollicular cells in the normal human thyroid. Nature. 1971 Apr 2;230(5292):334–335. doi: 10.1038/230334a0. [DOI] [PubMed] [Google Scholar]
- Toda T., Uno I., Ishikawa T., Powers S., Kataoka T., Broek D., Cameron S., Broach J., Matsumoto K., Wigler M. In yeast, RAS proteins are controlling elements of adenylate cyclase. Cell. 1985 Jan;40(1):27–36. doi: 10.1016/0092-8674(85)90305-8. [DOI] [PubMed] [Google Scholar]
- Trump D. L., Mendelsohn G., Baylin S. B. Discordance between plasma calcitonin and tumor-cell mass in medullary thyroid carcinoma. N Engl J Med. 1979 Aug 2;301(5):253–255. doi: 10.1056/NEJM197908023010507. [DOI] [PubMed] [Google Scholar]
- Weissman B. E., Aaronson S. A. BALB and Kirsten murine sarcoma viruses alter growth and differentiation of EGF-dependent balb/c mouse epidermal keratinocyte lines. Cell. 1983 Feb;32(2):599–606. doi: 10.1016/0092-8674(83)90479-8. [DOI] [PubMed] [Google Scholar]
- Wolfman A., Macara I. G. Elevated levels of diacylglycerol and decreased phorbol ester sensitivity in ras-transformed fibroblasts. Nature. 1987 Jan 22;325(6102):359–361. doi: 10.1038/325359a0. [DOI] [PubMed] [Google Scholar]
- Yoakum G. H., Lechner J. F., Gabrielson E. W., Korba B. E., Malan-Shibley L., Willey J. C., Valerio M. G., Shamsuddin A. M., Trump B. F., Harris C. C. Transformation of human bronchial epithelial cells transfected by Harvey ras oncogene. Science. 1985 Mar 8;227(4691):1174–1179. doi: 10.1126/science.3975607. [DOI] [PubMed] [Google Scholar]
- Yuspa S. H., Kilkenny A. E., Stanley J., Lichti U. Keratinocytes blocked in phorbol ester-responsive early stage of terminal differentiation by sarcoma viruses. Nature. 1985 Apr 4;314(6010):459–462. doi: 10.1038/314459a0. [DOI] [PubMed] [Google Scholar]
- de Bustros A., Baylin S. B., Berger C. L., Roos B. A., Leong S. S., Nelkin B. D. Phorbol esters increase calcitonin gene transcription and decrease c-myc mRNA levels in cultured human medullary thyroid carcinoma. J Biol Chem. 1985 Jan 10;260(1):98–104. [PubMed] [Google Scholar]
- deBustros A., Baylin S. B., Levine M. A., Nelkin B. D. Cyclic AMP and phorbol esters separately induce growth inhibition, calcitonin secretion, and calcitonin gene transcription in cultured human medullary thyroid carcinoma. J Biol Chem. 1986 Jun 15;261(17):8036–8041. [PubMed] [Google Scholar]